Simple Summary
Lactic acidosis is a prominent feature of the tumour microenvironment and a key player in cancer metabolism. This review is aimed at combining the mechanisms through which lactic acidosis alters the metabolism of cancer cells, and determining how this effect could bring valuable contribution to the current understanding of the metabolism of whole tumours. This work also highlights the therapeutic perspectives that advances in lactic acidosis understanding open up.
Abstract
Lactic acidosis, a hallmark of solid tumour microenvironment, originates from lactate hyperproduction and its co-secretion with protons by cancer cells displaying the Warburg effect. Long considered a side effect of cancer metabolism, lactic acidosis is now known to play a major role in tumour physiology, aggressiveness and treatment efficiency. Growing evidence shows that it promotes cancer cell resistance to glucose deprivation, a common feature of tumours. Here we review the current understanding of how extracellular lactate and acidosis, acting as a combination of enzymatic inhibitors, signal, and nutrient, switch cancer cell metabolism from the Warburg effect to an oxidative metabolic phenotype, which allows cancer cells to withstand glucose deprivation, and makes lactic acidosis a promising anticancer target. We also discuss how the evidence about lactic acidosis’ effect could be integrated in the understanding of the whole-tumour metabolism and what perspectives it opens up for future research.
1. Introduction
Lactic acidosis is a hallmark of the tumour microenvironment, one that has been shown to promote cancer resistance to chemotherapy [1]. It results from the intensive secretion of lactate and protons in the presence of glucose by cells displaying the Warburg effect, a characteristic anomaly of proliferating, and, particularly, cancer cells. Cells harbouring the Warburg effect perform high-rate glycolysis, lactic fermentation, and co-excretion of lactate and protons [2]. This enables them to proliferate at a high rate in the presence of glucose, which they consume avidly. However, the rapid consumption of glucose leads to its exhaustion, and an energetic dead-end and paradox. Interestingly, lactic acidosis has been shown to help cancer cells withstand glucose deprivation [3]. In media conditioned with high lactate concentration and acidity, cancer cell lines avoid apoptosis and survive 10 times longer in the absence of glucose. Further studies have demonstrated that cancer cells resist glucose starvation by reprogramming their metabolism [4,5]. In this review, we focus on the essential literature addressing how lactic acidosis affects energy metabolism and preserves homeostasis in glucose-deprived cancer cells, and what therapeutic prospects it opens up. We then discuss how this effect at the cellular scale could help understand the metabolism of whole tumours.
2. Defining the Experimental Conditions of the Presented Studies
In this work, we review a series of studies relevant to address how lactic acidosis helps cells resist glucose deficiency. These studies are performed in varying conditions (Table 1). In order to clarify the various experimental conditions, we emphasise the following definitions [3]. “Lactosis” refers to an in vitro condition in which extracellular lactate concentration exceeds 15 mM. It must be noted that most presented studies were performed with culture media containing 10% foetal bovine serum, which brings ~1.5 mM lactate to the medium [6]. At pH 6.7, >15 mM added lactate helps cancer cells resist glucose deprivation [3]. “Acidosis” refers to an extracellular pH of 5.8–6.7. Under pH 6.7 normal cells suffer from acidosis, and tumour pH can drop down to 5.8 [4]. ”Lactic acidosis” refers to the combination of both lactosis and acidosis. Lactic acidosis and acidosis are frequently encountered in tumours [7]. Both originate from the co-secretion of lactate and protons, and acidosis is also caused by the mitochondrial production of CO2 and its dissociation into HCO3− and H+ [8]. Lactosis is a condition virtually absent in vivo, but one that can be achieved easily in vitro to study the effect of lactate independently from acidification by adding buffered sodium lactate to the medium.
“Glucose deprivation” or “depletion” refers to conditions where glucose is scarce, but not necessarily absent from the milieu. Intratumoral glucose concentration can drop to 0.1–0.4 mM, while its level in healthy tissues is ~1 mM [9]. In vitro studies recreate glucose deprivation with culture media that contain, initially, up to 3 mM glucose, the amount that cancer cells typically deplete in one day [3,6].

Table 1.
The presented studies addressing lactic acidosis’ impact on cell energy metabolism are performed under various conditions. For each reference, the tested cell line or cancer type and medium conditions (glucose concentration, lactate concentration, and pH) are specified. When unspecified, the pH value was assumed to equal 7.4.
Table 1.
The presented studies addressing lactic acidosis’ impact on cell energy metabolism are performed under various conditions. For each reference, the tested cell line or cancer type and medium conditions (glucose concentration, lactate concentration, and pH) are specified. When unspecified, the pH value was assumed to equal 7.4.
| Reference | Glucose Concentration (mM) | Lactate Concentration (mM) | pH | Cell Lines or Tumour Origin |
|---|---|---|---|---|
| [3] | 3 | 20 | 6.7 | 4T1, Bcap37, RKO, SGC7901 |
| [10] | Unspecified | 25 | 6 to 6.7 | HMEC, DU145, SiHa, WiDr |
| [4] | 10 | 10 | 6.5 | MCF-7, MDA-MB-468, MDA-MB-231, SkBr3 |
| [11] | 5 and 25 | 5 to 30 | 6.7 | U251 and glioblastoma |
| [12] | Unspecified | 10 or 20 | 7.4 | A549, H1299 |
| [13] | 10 | 5 to 30 | 7.4 | A549, H1299 |
| [14] | Unspecified | 10 or 30 | 7.4 | SiHa and mouse xenograft |
| [5] | 6 | 25 | 6.5 | 4T1, Bcap37, HeLa, A549 |
| [15] | Unspecified | 4 to 40 | 5 to 8 | MCF7, T47D |
| [16] | Unspecified | 5 or 10 | 7.4 | A549, H1299, BEAS-2B |
| [17] | 10 | 3 to 40 | 6.2 | A549, A427, MCF7, MRC5 |
| [18] | Unspecified | 0 | 6.5 | A549, H1299, MRC5 |
| [19] | 5 | 10 | 7.4 | SiHa, HeLa |
| [20] | 10 | 10 or 25 | 6.7 | MCF-7, ZR-75-1, T47D, MDA-MB-231, MDA-MB-157 |
| [21] | 5.6 | 10 or 20 | 6.7 | LS174T, HCT116, MCT4 |
| [22] | Unspecified | 20 | 7.4 | MCF7 |
| [23] | Unspecified | 10 | 7.4 | MDA-MB-231 |
| [24] | 0 | 28 | 6.2 | A549, A427 |
| [25] | Unspecified | 20 | 7.4 | U87-MG, A172, U251 |
| [1] | Unspecified | 20 | 7.4 | 92.1 |
| [26] | 0 | 10 | 7.4 | MDA436 and mouse xenograft |
| [27] | 10 | 2 to 20 | 7.4 | Human myeloid cell lines |
| [28] | 0.175 | 4 | 7.4 | glioma stem cells |
| [29] | 2.5 or 25 | 10 | 7.4 | Colo205, Ls174T, Mosers, HT29 |
| [30] | 1 to 2.5 | 25 | 7.4 | MCF-7 |
| [31] | Unspecified | 20 | 7.4 | Huh-7, Hep3B |
| [32] | 0 | 20 | 6.8 | A549 |
| [33] | 0 | 20 | 6.7 | 4T1, HeLa, NCI–H460 |
| [34] | Unspecified | 0 | 6.5 | PANC-1, SW1990 |
| [35] | Unspecified | 12 | 6.8 | PaTu-8902, HeLa, HepG2, HDF |
3. Lactic Acidosis Seen by Cancer Research: A Brief History
In the 2000s, cancer research took a renewed interest in the Warburg effect, a hallmark of cancer discovered a century ago [2,36,37]. As a consequence, views on lactic acidosis changed drastically.
Acidosis had been known to promote tumour aggressiveness by exerting a selective pressure. Some cancer cells had been shown to survive acidosis by maintaining an alkaline intracellular pH, while other cells—cancerous or healthy—underwent hydrolysis and death [38,39,40]. The proliferation of those selected cells, which are more resistant to unfavourable environments, had been known to increase tumour malignancy [41,42]. As for lactate, it had been considered more of a by-product of glycolysis until the 1980s, when its use as a nutrient in non-cancerous tissues was discovered [43,44]. The role of extracellular lactate in cancer was investigated only later, in the 2000s [45,46], when it was found to correlate with tumour malignancy [47,48,49,50]. Two explanations for this were initially proposed. First, lactate promotes relaxation of the tissue surrounding the tumour, which would make room for its development and metastasis [48]. Second, lactate makes the cellular environment hostile, as does acidosis [38], which promotes angiogenesis [47].
The metabolic importance of extracellular lactate and lactic acidosis was first evidenced in 2008. Lactic acidosis was shown to alter the expression of metabolism genes [10] and, more importantly, lactate was proven to be, per se, a key source of energy for cancer cells [51]. In 2009, the term “reverse Warburg effect” was first used to describe cancer cells not showing the Warburg effect, but instead inducing it in neighbouring stromal fibroblasts and consuming the lactate produced by them [52]. These discoveries reappraised the paradigm of the Warburg effect, showing that it wasn’t compulsory in cancer since lactate could be metabolised rather than only produced. Following these works, in 2012, Wu et al., demonstrated that lactic acidosis allows cells to avoid glucose starvation [3]. Lactic acidosis rescues glucose-deprived cancer cells, but importantly, acidosis or lactosis alone have much more limited effects. After this pioneering work, lactic acidosis was further shown to reprogram cell metabolism [5]. Nowadays, extracellular lactate and acidosis are viewed as central players in cancer cell metabolism [53,54,55].
4. Lactic Acidosis’ Effect on Energy Metabolism
Lactic acidosis was shown to impact numerous aspects of energy metabolism. We focus here on nutrient import, glycolysis, the tricarboxylic acid (TCA) cycle, oxidative phosphorylation (OxPhos), and pathways generating reduced coenzymes (Figure 1).
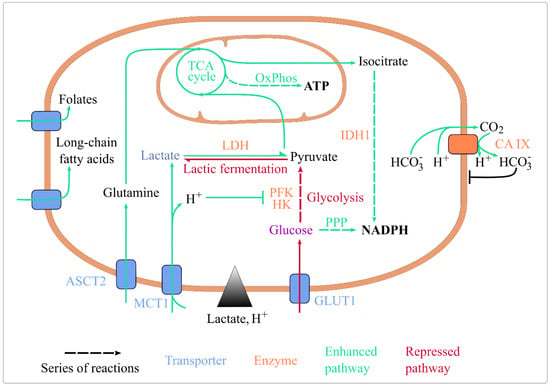
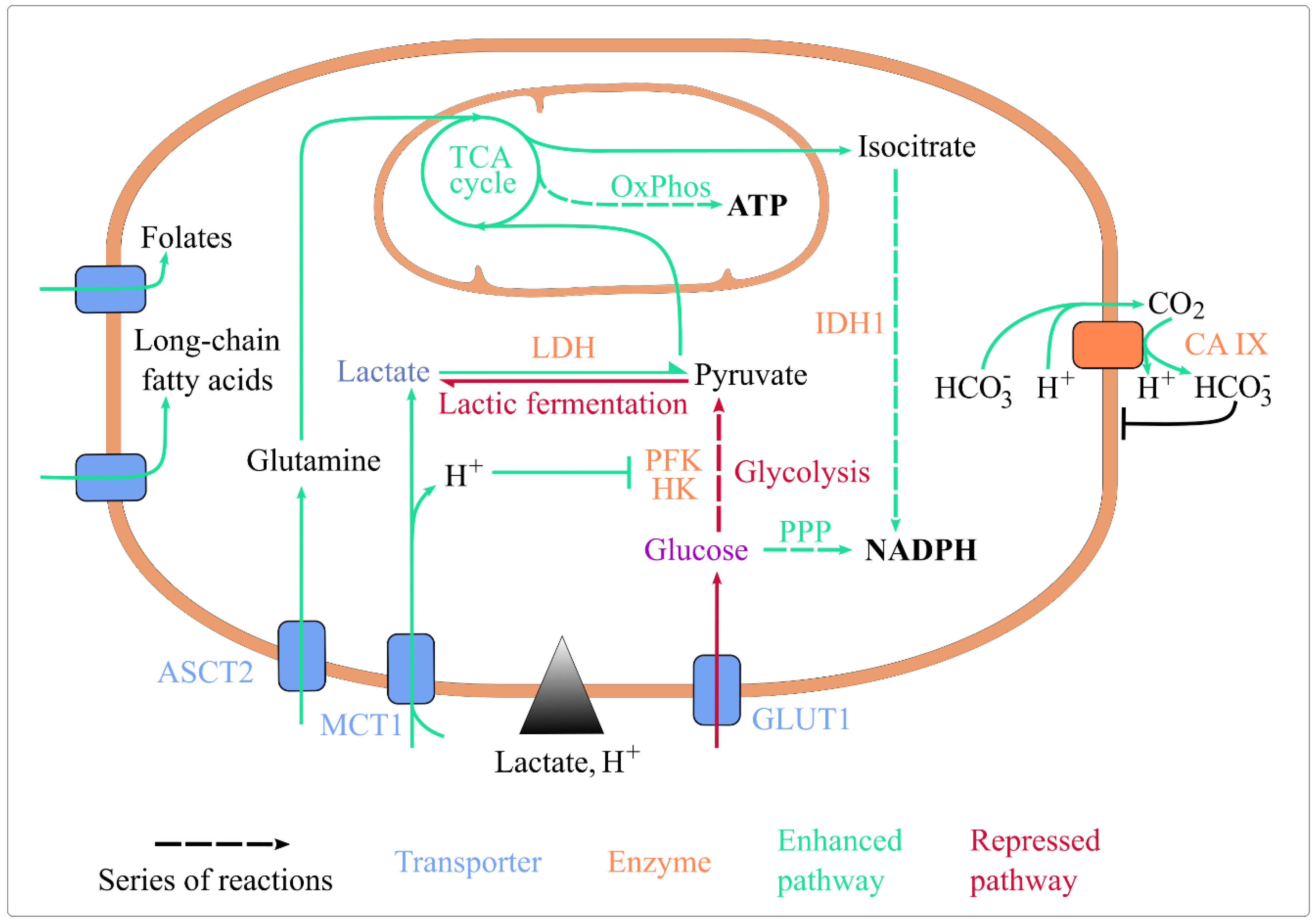
Figure 1.
Lactic acidosis rewires energy metabolism and maintains cellular homeostasis. Lactic acidosis enhances the uptake of folate, long-chain fatty acids, glutamine, and lactate. It represses glucose import, glycolysis (by inhibiting HK and PFK, its rate-limiting enzymes) and lactic fermentation. It enhances lactate conversion to pyruvate, routing of pyruvate and glutamine towards the TCA cycle, ATP generation by OxPhos, and coenzyme reduction by IDH1 and the oxidative PPP. It also upregulates CA IX expression, which basifies intracellular pH. Abbreviations: ASCT2: Alanine, Serine, Cysteine Transporter 2; CA IX: carbonate anhydrase 1; GLUT1: glucose transporter 1; HK: hexokinase; IDH1: isocitrate dehydrogenase 1; MCT1: monocarboxylate transporter 1; OxPhos: oxidative phosphorylation; PFK1: phosphofructokinase 1; PPP: pentose phosphate pathway; TCA: tricarboxylic acid.
4.1. Lactic Acidosis and Exchanges at the Plasma Membrane
In glucose deprivation, the capacity of cancer cells to uptake and metabolise alternative nutrients is key to their survival [56]. Extracellular acidosis and lactosis were shown to increase such capacity.
4.1.1. Acidosis Sustains the Activity of Proton-Nutrient Symporters
Extracellular acidosis has a direct impact on exchanges at the plasma membrane [57]. In healthy tissues, protons are more concentrated inside the cell than outside. In tumours, the contrary is true [58,59]. Extracellular acidosis inverts the transmembrane proton gradient in tumour cells, which may positively impact proton-nutrient symports. Of interest, lactate is imported in cancer cells via the monocarboxylate transporters (MCTs) [51]. Since MCTs co-transport lactate with a proton, lactate import should be sensitive to the proton gradient and facilitated under acidosis. This mechanism is expected to explain why cancer cells respond differently to lactosis and lactic acidosis [3], since a rise in extracellular lactate only increases intracellular lactate levels in acidic conditions [60]. The co-transport of extracellular lactate and protons probably underlies the synergy of their effects on intracellular metabolism (Figure 1).
Of note, protons are also co-imported with several other nutrients, such as Fe2+, folates, amino acids and peptides. Brown & Ganapathy suggested that acidosis may affect their uptake (Figure 1), but this hypothesis remains to be confirmed [61].
4.1.2. Lactate and Acidosis Indirectly Enhance Nutrient Uptake
Extracellular lactic acidosis indirectly promotes the uptake of several nutrients (Figure 1). The import of lactate itself is increased in lactic acidosis, in part due to MCT1 overexpression [11], which is a response to an extracellular lactate signal [62] that is potentiated by extracellular acidity [12]. Extracellular lactate induces MCT1 and MCT4 via the G-protein-coupled receptor 81 (GPR81) transduction pathway [62]. In acidosis, extracellular lactate can also induce GPR81 expression [12,13]. Extracellular lactate signal enhancing MCT-mediated lactate import is necessary to cancer cell survival in absence of glucose, glutamine and pyruvate [62]. This supports the idea that ‘lactate induces its own metabolism’, which doesn’t exclude other regulations of MCT expression [63].
Glutamine uptake is increased by extracellular lactate or acidosis, as both conditions increase the expression of the glutamine transporter ASCT2 (alanine, serine and cysteine transporter 2) [14,64]. Fatty acid uptake is enhanced by acidosis [65], and folate import is intensified by 10 mM extracellular lactic acid [15].
Finally, and importantly, lactic acidosis seems to minimise glucose uptake, but not in all cell lines and cancers. Lactic acidosis decreases glucose uptake in various cell lines [5], as lactate in lung cancer cell lines [16]. On the opposite, 10 mM lactate has no effect on glucose uptake in the T47D breast cancer cell line [15]. The expression of the glucose transporters GLUT1 and GLUT4 are decreased by 2 mM lactate and acidosis in lung and breast cancer cell lines [17], and by acidosis in cervix, pharynx and colon cancer cell lines [64] but not in lung cancer cell lines [18].
4.1.3. Lactic Acidosis and pH Homeostasis
Cell exposure to lactic acidosis is associated with a drop in intracellular pH from 7.3 to ~6.9 [5]. Behind this acidification, several probable effects may be discerned. On the one hand, as discussed earlier, acidosis enhances proton-nutrient co-import, which could contribute to cellular acidification. On the other hand, lactate as a signal can mitigate the drop in pH by favouring alkalinization. A level of 10 mM extracellular lactate induces Carbonic Anhydrase IX (CA IX) [19], a transmembrane enzyme supporting proton export and a key regulator of cell pH [66] (Figure 1).
4.2. Lactic Acidosis, Glycolysis, and Lactic Fermentation
Unlike cells showing the Warburg effect, in which glycolysis and lactate dehydrogenase (LDH)-catalysed lactic fermentation are known to be hyperactive, cells exposed to lactic acidosis show a reduction in these pathways’ activity.
In glucose abundance, acidosis and lactic acidosis lower glucose consumption and lactate secretion [4,5,20], which indicates that glycolysis and lactic fermentation are downregulated. More interestingly, lactic acidosis decreases cancer cell dependency on glucose catabolism [1]. Thus, in glucose sufficiency, lactic acidosis minimises glucose catabolism activity and its importance in cell survival (Figure 1).
Glycolysis and lactic fermentation are likely downregulated at the level of both gene expression and enzyme activity. The expression of glycolysis enzymes is reduced by lactic acidosis in breast cancer cell lines [10], and by extracellular lactate in lung cancer cell lines [16], but it is maintained by extracellular lactate in breast cancer cell lines [21,22]. The activity of glycolysis enzymes, especially the rate-limiting hexokinase and phosphofructokinase [67], is directly decreased by intracellular acidification [4] (Figure 1). In line, intracellular acidification has been predicted in silico to hinder the Warburg effect [68]. Intracellular lactate accumulation, in parallel, directly inhibits lactic fermentation [5] (Figure 1). The interconversion of lactate and pyruvate through LDH follows the mass action law, therefore a rise in lactate concentration inhibits its production from pyruvate and favours the reverse reaction. This thermodynamic effect leads to a complete stop of lactic fermentation at ~25 mM intracellular lactate [5]. This concentration is within the range resulting from lactic acidosis.
4.3. Lactic Acidosis and Mitochondrial Catabolism
4.3.1. Lactic Acidosis Intensifies Mitochondrial Catabolism
Lactic acidosis enhances mitochondrial metabolic activity, in particular the TCA cycle and OxPhos. Both lactic acidosis and lactosis enhance mitochondrial biogenesis [23,24] and the expression of the enzymes of the TCA cycle and OxPhos [11,25], which potentiates mitochondrial catabolism and ATP production. The reactivation of those pathways allows the maintenance of the cellular ATP concentration in glucose deprivation and increases resistance to starvation [11].
4.3.2. Lactic Acidosis Shapes TCA Cycle Alternative Fueling
In addition to glucose-derived pyruvate, the TCA cycle can be supplied with various substrates. This flexibility is particularly true of cancer cells [69]. In challenging nutritional contexts such as glucose deprivation, the TCA cycle of cancer cells can be sustained by alternative nutrients. Lactate and glutamine are its main substrate suppliers after glucose [70]. The use of both is promoted by lactic acidosis.
The pyruvate generated from lactate can directly sustain the TCA cycle [26,27,28,29,30,31,71] (Figure 1). This pathway depends on upstream lactate import by MCTs, whose enhancement in lactic acidosis is discussed in Section 4.1.2. In line, extracellular lactate increases the mitochondrial membrane potential, and hence ATP production efficiency in OxPhos [21,72], and could even be necessary to pro-tumoural cell proliferation [32]. In more detail, the routing of lactate to mitochondria is debated. In the classical view, lactate is converted to pyruvate in the cytosol, then pyruvate is shuttled to mitochondria [73,74]. In addition to this classical way, Brooks et al. proposed an alternative model in which lactate would be shuttled to mitochondria via the mitochondrial lactate oxidation complex (mLOC), that includes MCT1 [75]. The controversy raised by this model has been well-reviewed in [76,77], that summarized the evidence for and against it in non-cancer cells. In cancer cells supplied with sufficient glucose, lactate’s contribution to the TCA cycle over glucose remains under debate: some studies suggest that lactate shuttled to mitochondria is preferred [71], while others question this [78]. Either way, under glucose deprivation, we can hypothesise that lactate’s contribution to the TCA cycle is of significant importance.
Glutamine is a major nutrient for cancer cells. It undergoes oxidative glutaminolysis in mitochondria, where it is processed by glutaminase 1 or 2 (GLS1/2) and then glutamate dehydrogenase 1 (GDH1) to sustain the TCA cycle. Lactic acidosis [20], acidosis [20,64], and lactate [14] upregulate GLS1 and GLS2 and stimulate oxidative glutaminolysis. Lactic acidosis, however, doesn’t necessarily promote glutamine consumption compared to lactosis [23]. In summary, either extracellular lactate, acidosis or lactic acidosis enhance glutamine utilisation by inducing glutaminase expression (Figure 1).
4.4. Lactic Acidosis and Redox Homeostasis
Cell survival requires redox homeostasis, i.e., controlled levels of reactive oxygen species (ROS) and redox coenzymes. The former lead to cell death when they accumulate, and the latter support the entire metabolism and cellular antioxidant defences.
Particularly, a high NADPH/NADP+ ratio kinetically favours anabolic reactions and helps keep ROS levels low. In cancer cells this ratio is abnormally high and sustains hyperactive anabolism [70]. High NADPH levels are supported by the oxidation of nutrients, such as lactate and glutamine via the TCA cycle and then oxidation of glutamine- and lactate-derived malate and isocitrate by the malic enzyme 1 (ME1) and Isocitrate Dehydrogenase 1 (IDH1), and mainly glucose via the pentose phosphate pathway (PPP). Redox homeostasis in cancer cells is therefore particularly sensitive to nutritional stress such as glucose deprivation.
In this condition, lactic acidosis helps stabilise the NADPH/NADP+ ratio at ~50% of its level in glucose sufficiency [3]. Likely, the gatekeepers of the NADPH/NADP+ ratio in glucose abundance have reduced efficiency under glucose deprivation and lactic acidosis, whereas new control mechanisms gain importance. On the one hand, glutamine use via ME1 is not necessary to the maintenance of the NADPH/NADP+ ratio under lactic acidosis [33]. Glutamine would indeed be completely degraded in mitochondrial catabolism instead of sustaining ME1 activity [20]. On the other hand, the glucose directed away from glycolysis towards the PPP would prevail more in NADPH/NADP+ maintenance under lactic acidosis. Lactic acidosis [20] and acidosis [34] respectively increase the expression and activity of glucose-6-phosphate dehydrogenase (G6PD), the first enzyme of the PPP, and lactic acidosis makes G6PD activity necessary to NADPH/NADP+ ratio maintenance and cell survival [20] in glucose sufficiency. However in glucose deprivation, the PPP alone cannot maintain redox balance [33]. Alternatively, lactate would become a key player in NADPH/NADP+ ratio maintenance, via the TCA cycle [35], and IDH1 [33].
Whether, in glucose abundance, such reprogramming strengthens cell defences against ROS level increase is uncertain. Acidosis increases ROS levels [35] and cell sensitivity to oxidative stress, but cell adaptation to acidosis decreases them [34]. Lactate import through MCT1 is key to maintain low ROS levels [79]. Lactic acidosis was found to either increase ROS levels, as does acidosis [20], or to rescue acidosis’ negative effect [35]. At any rate, in glucose deprivation, lactic acidosis mainly prevents increased ROS levels by providing IDH1 with its substrate [33].
A high NADH/NAD+ ratio supports ATP production. Lactic acidosis impact on the NADH/NAD+ ratio has not been directly investigated. However lactate use by the TCA cycle increases the NADH/NAD+ ratio in glucose deprivation [30]. This increase could contribute to the inhibition of glycolysis by lactic acidosis: a high NADH/NAD+ ratio would inhibit glycolysis according to the mass action law. Yet this hypothesis remains to be tested.
4.5. Section Summary
In the energy metabolism of cancer cells, acidosis and extracellular lactate act as enzymatic inhibitors, and lactate as a signal and a nutrient. They mostly curb glycolysis and lactic fermentation and enhance the TCA cycle and OxPhos (Figure 1). Acidification and lactate accumulation in the tumour microenvironment would promote and sustain an oxidative phenotype, which is fitter than the fermenting phenotype in glucose deprivation, an adverse nutritional context that is common in tumours.
5. Therapeutic Strategies Targeting Lactic Acidosis
Nowadays, lactic acidosis per se is targeted in therapies directed against cancer. Of note, it is also a major target in the treatment of type 2 diabetes [80,81]. Neutralising acidosis in tumours has been proposed as a way to restore sensitivity of cancer cells to glucose starvation and increase the efficacy of regular treatments. The proof of principle of this approach has been established by combining transarterial chemoembolization (TACE) with the infusion of bicarbonate, a basifying agent that turns neoplastic lactic acidosis into lactosis [82,83]. Compared to TACE alone, TILA-TACE (Targeting-Intratumoural-Lactic-Acidosis TACE) presented a very significantly enhanced anticancer activity for patients with hepatocellular carcinoma. The mechanisms underlying this activity have been evaluated in detail by Ying et al. [84].
Modulating extracellular lactate availability in tumours by nanomedicine is another promising therapeutic strategy. The delivery by nanoparticles of a cocktail of lactate oxidases and catalases to colon carcinoma cells in vitro suppresses tumoural lactosis and stops cell proliferation [85]. The delivery by nanoparticles of a glucose catalase combined with a MCT1 inhibitor, that together prevent the use of both glucose and lactate by tumour cells, inhibits the proliferation of SiHa cell line xenografts in mice [86]. Conversely, lactate-loaded nanoparticles induce an overload of lactate and cytotoxicity in orthotopic glioblastoma models, although only in normoxic conditions and not in hypoxia [87].
Understanding the effects of lactic acidosis also helps reappraise the potential of already-existing targets. In particular, the lactate transporter MCT1 was formerly targeted to inhibit lactate secretion in cells showing the Warburg effect, and is now targeted to hamper lactate uptake [27,88,89,90,91]. Similarly the strategies targeting LDH isoforms were aimed historically at inhibiting lactate production from pyruvate. The LDHA isoform, that has a higher affinity for pyruvate than for lactate and catalyses preferentially lactate production, is a historical target that still attracts much attention [92]. However, with the discovery of lactic acidosis effect, the LDHB isoform that catalyses preferentially the conversion of lactate to pyruvate now rises as an alternative target [32].
6. Implications of Lactic Acidosis in the Whole-Tumour Metabolism
Deciphering how lactic acidosis impacts cancer cells enlightens important aspects of the metabolism of the whole tumour, and raises new perspectives to complement its understanding.
From the belief that cancer cells have a unique metabolic signature, i.e., the Warburg effect, research has progressively recognized intratumoural heterogeneity as the metabolic hallmark of cancer [55]. The main metabolic heterogeneity in tumours is now suggested to be mitochondrial activity [93,94], that is promoted and sustained by lactic acidosis. To describe this heterogeneity, tumours have traditionally been modelled as the coexistence of two metabolic populations: oxidative cells relying on OxPhos and fermenting cells showing the Warburg effect and relying on glycolysis and lactic fermentation [17,28,51,91,95,96]. Oxidative cells would be located in normoxic regions, in perivascular compartments [7,11,97], and fermenting cells in hypoxic regions farther from blood vessels [11,51,97] (Figure 2). Each population would thrive on different energy sources, fermenting cells glucose and oxidative ones lactate, and lactate would be transferred from fermenting to oxidative cells [95]. This model is supported by the coexistence in tumours of cells overexpressing MCT4, a preferential lactate exporter, and cells overexpressing MCT1, a preferential lactate importer [11,51,95,98]. Interestingly, a possible lactate transport via gap junctions has been evidenced recently [99,100,101]. This lactate transfer supports the idea of a metabolic symbiosis between both populations within tumours [17,28,46,51,61,91,95,96,101,102,103]. In this model, a central question is how the metabolic phenotypes of both populations are determined [46]. Hypoxia is thought to be the major promoter of the fermenting phenotype [102,104]. Lactic acidosis, according to the evidence presented in this work, is likely the promoter of the oxidative phenotype [5,11].
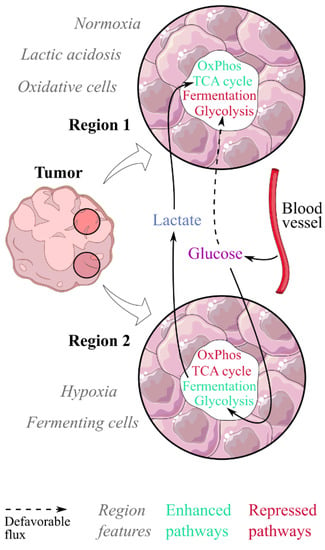
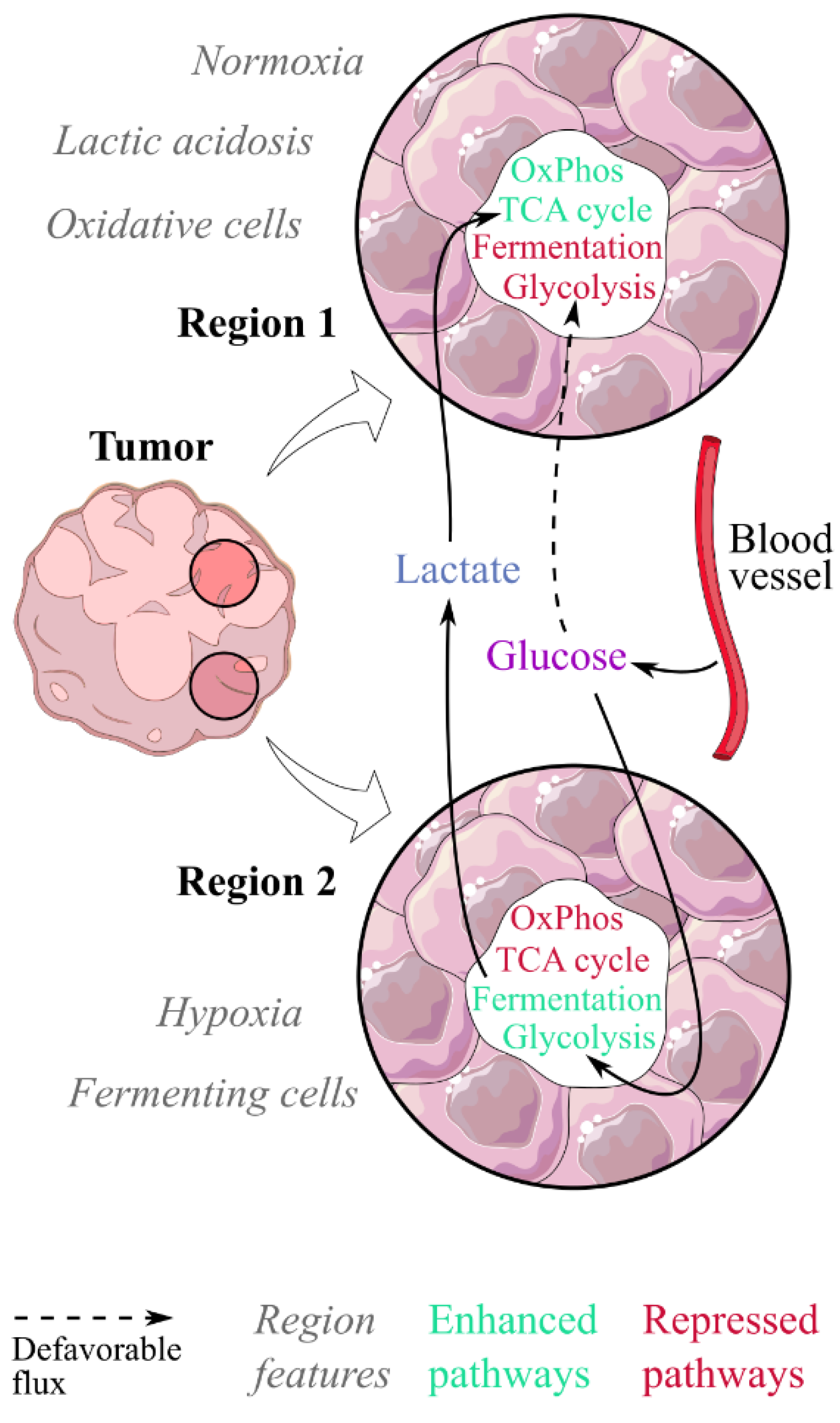
Figure 2.
Lactic acidosis would contribute to a metabolic symbiosis between fermenting and oxidative cells within tumours. In this model, two populations coexist in tumours: fermenting cells in hypoxic regions, and oxidative cells in normoxic regions where lactic acidosis would exert its effect. Fermenting cells would consume the glucose spared by oxidative cells and generate the lactate fueling them, both being in a metabolic symbiosis. Lactic acidosis promotes the switch from a fermenting to an oxidative phenotype.
However this hypothetical scenario raises a paradox: the oxidative phenotype, that derives from lactic acidosis, i.e., from the fermenting phenotype that is promoted by hypoxia, cannot thrive in hypoxic conditions. Two hypotheses could solve this paradox. In the first hypothesis, fermenting cells would induce the oxidative phenotype in their neighbours, located in better-perfused regions. However lactic acidosis intensity, maximal around secretory cells, decreases with the distance [51,105], which raises the question of the minimal level of lactic acidosis necessary to promote the oxidative phenotype. In the second hypothesis, lactic acidosis would feedback the Warburg effect in fermenting cells by switching them to an oxidative phenotype, which questions the minimal oxygen level necessary for the oxidative phenotype to survive. A possible answer to this question is that in the meantime, lactic acidosis could promote angiogenesis [38,47,61]. This questions the timeline of lactic acidosis action, in the promotion of both oxidative phenotype and angiogenesis. A third perspective to answer the paradox is to address how, earlier, hypoxia and lactic acidosis may interplay in the promotion of metabolic phenotypes, which has caught little attention until now [18,20,23,106].
7. Conclusions
Lactic acidosis associated with tumour progression allows cancer cells to survive in unfavourable environments. In the last decade, the influence of neoplastic lactic acidosis on the energy metabolism of cancer cells has been deciphered. Lactic reduces glycolysis and lactic fermentation, stimulates the TCA cycle and OxPhos, and promotes the use of alternative nutrients. All in all, it contributes to cell resistance to glucose deprivation. Cancelling lactic acidosis’ effect is therefore a relevant anticancer strategy that restores cancer cell sensitivity to glucose deprivation, a common feature of the tumour microenvironment. In the future, clarifying how lactic acidosis action is integrated in the whole-tumour metabolism would be of high interest.
Author Contributions
Writing—original draft preparation, Z.D.; writing—review and editing, Z.D., A.B., G.J.P.R. and B.P.; supervision, G.J.P.R. and B.P.; funding acquisition, G.J.P.R. and B.P. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from ITMO Cancer of Aviesan within the framework of the 2021–2030 Cancer Control Strategy, on funds administered by Inserm.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barnes, E.M.E.; Xu, Y.; Benito, A.; Herendi, L.; Siskos, A.P.; Aboagye, E.O.; Nijhuis, A.; Keun, H.C. Lactic acidosis induces resistance to the pan-Akt inhibitor uprosertib in colon cancer cells. Br. J. Cancer 2020, 122, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Wu, H.; Ding, Z.; Hu, D.; Sun, F.; Dai, C.; Xie, J.; Hu, X. Central role of lactic acidosis in cancer cell resistance to glucose deprivation-induced cell death. J. Pathol. 2012, 227, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, Z.; Cheng, J.; Sun, B.; Yang, J.; Li, H.; Wu, S.; Dong, F.; Yan, X. Differential metabolic responses in breast cancer cell lines to acidosis and lactic acidosis revealed by stable isotope assisted metabolomics. Sci. Rep. 2020, 10, 21967. [Google Scholar] [CrossRef]
- Xie, J.; Wu, H.; Dai, C.; Pan, Q.; Ding, Z.; Hu, D.; Ji, B.; Luo, Y.; Hu, X. Beyond Warburg effect—Dual metabolic nature of cancer cells. Sci. Rep. 2015, 4, 4927. [Google Scholar] [CrossRef]
- Lefevre, C.; Panthu, B.; Naville, D.; Guibert, S.; Pinteur, C.; Elena-Herrmann, B.; Vidal, H.; Rautureau, G.J.P.; Mey, A. Metabolic Phenotyping of Adipose-Derived Stem Cells Reveals a Unique Signature and Intrinsic Differences between Fat Pads. Stem Cells Int. 2019, 2019, e9323864. [Google Scholar] [CrossRef]
- Grasmann, G.; Mondal, A.; Leithner, K. Flexibility and Adaptation of Cancer Cells in a Heterogenous Metabolic Microenvironment. Int. J. Mol. Sci. 2021, 22, 1476. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Hirayama, A.; Kami, K.; Sugimoto, M.; Kami, K.; Sugimoto, M.; Sugawara, M.; Toki, N.; Onozuka, H.; Kinoshita, T.; Saito, N.; et al. Quantitative Metabolome Profiling of Colon and Stomach Cancer Microenvironment by Capillary Electrophoresis Time-of-Flight Mass Spectrometry. Cancer Res. 2009, 69, 4918–4925. [Google Scholar] [CrossRef]
- Chen, J.L.-Y.; Lucas, J.E.; Schroeder, T.; Mori, S.; Wu, J.; Nevins, J.; Dewhirst, M.; West, M.; Chi, J.-T. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet. 2008, 4, e1000293. [Google Scholar] [CrossRef]
- Duan, K.; Liu, Z.J.; Hu, S.Q.; Liu, Z.-J.; Hu, S.-Q.; Huo, H.-Y.; Xu, Z.-R.; Ruan, J.-F.; Sun, Y.; Dai, L.-P.; et al. Lactic acid induces lactate transport and glycolysis/OXPHOS interconversion in glioblastoma. Biochem. Biophys. Res. Commun. 2018, 503, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Z.; Zhang, Y.; Cao, Y.; Wei, H.; Wu, Z. Upregulation of lactate-inducible snail protein suppresses oncogene-mediated senescence through p16INK4a inactivation. J. Exp. Clin. Cancer Res. 2018, 37, 39. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhu, Z.; He, Y.; Zhang, Z.; Zhang, Y.; Wang, Y.; Luo, J.; Peng, T.; Cheng, F.; Gao, J.; et al. A lactate-induced Snail/STAT3 pathway drives GPR81 expression in lung cancer cells. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2020, 1866, 165576. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escuredo, J.; Dadhich, R.K.; Dhup, S.; Cacace, A.; Van Hée, V.F.; De Saedeleer, C.J.; Sboarina, M.; Rodriguez, F.; Fontenille, M.-J.; Brisson, L.; et al. Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle 2015, 15, 72–83. [Google Scholar] [CrossRef]
- Guedes, M.; Araújo, J.R.; Correia-Branco, A.; Gregório, I.; Martel, F.; Keating, E. Modulation of the uptake of critical nutrients by breast cancer cells by lactate: Impact on cell survival, proliferation and migration. Exp. Cell Res. 2016, 341, 111–122. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, D.; Jiang, Y.; Hou, J.; Tian, M.; Li, J.; Sun, L.; Zhang, Y.; Zhang, T.; Li, Z.; et al. Lactate Modulates Cellular Metabolism through Histone Lactylation-Mediated Gene Expression in Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 647559. [Google Scholar] [CrossRef]
- Prado-Garcia, H.; Campa-Higareda, A.; Romero-Garcia, S. Lactic Acidosis in the Presence of Glucose Diminishes Warburg Effect in Lung Adenocarcinoma Cells. Front. Oncol. 2020, 10, 807. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Liousia, M.; Arelaki, S.; Kalamida, D.; Pouliliou, S.; Mitrakas, A.; Tsolou, A.; Sivridis, E.; Koukourakis, M. Differential effect of hypoxia and acidity on lung cancer cell and fibroblast metabolism. Biochem. Cell Biol. 2017, 95, 428–436. [Google Scholar] [CrossRef]
- Panisova, E.; Kery, M.; Sedlakova, O.; Brisson, L.; Debreova, M.; Sboarina, M.; Sonveaux, P.; Pastorekova, S.; Svastova, E. Lactate stimulates CA IX expression in normoxic cancer cells. Oncotarget 2017, 8, 77819–77835. [Google Scholar] [CrossRef]
- Lamonte, G.; Tang, X.; Chen, J.L.-Y.; Wu, J.; Ding, C.-K.C.; Keenan, M.M.; Sangokoya, C.; Kung, H.-N.; Ilkayeva, O.; Boros, L.G.; et al. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab. 2013, 1, 23. [Google Scholar] [CrossRef]
- Jin, L.; Guo, Y.; Chen, J.; Wen, Z.; Jiang, Y.; Qian, J. Lactate receptor HCAR1 regulates cell growth, metastasis and maintenance of cancer-specific energy metabolism in breast cancer cells. Mol. Med. Rep. 2022, 26, 268. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Hata, K.; Hirose, K.; Okui, T.; Toyosawa, S.; Uzawa, N.; Nishimura, R.; Yoneda, T. The lactate sensor GPR81 regulates glycolysis and tumor growth of breast cancer. Sci. Rep. 2022, 12, 6261. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Prado-Garcia, H.; Valencia-Camargo, A.D.; Alvarez-Pulido, A. Lactic Acidosis Promotes Mitochondrial Biogenesis in Lung Adenocarcinoma Cells, Supporting Proliferation Under Normoxia or Survival Under Hypoxia. Front. Oncol. 2019, 9, 1053. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Vicario, N.; Tibullo, D.; Giallongo, C.; Broggi, G.; Caltabiano, R.; Barbagallo, G.M.V.; Altieri, R.; Baghini, M.; Di Rosa, M.; et al. Lactate Induces the Expressions of MCT1 and HCAR1 to Promote Tumor Growth and Progression in Glioblastoma. Front. Oncol. 2022, 12, 871798. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Giallongo, S.; Orlando, L.; Broggi, G.; Longo, A.; Russo, A.; Caltabiano, R.; Giallongo, C.; Barbagallo, I.; Di Rosa, M.; et al. Lactate Rewrites the Metabolic Reprogramming of Uveal Melanoma Cells and Induces Quiescence Phenotype. Int. J. Mol. Sci. 2022, 24, 24. [Google Scholar] [CrossRef]
- Park, S.; Chang, C.Y.; Safi, R.; Liu, X.; Baldi, R.; Jasper, J.S.; Anderson, G.R.; Liu, T.; Rathmell, J.C.; Dewhirst, M.W.; et al. ERRα-Regulated Lactate Metabolism Contributes to Resistance to Targeted Therapies in Breast Cancer. Cell. Rep. 2016, 15, 323–335. [Google Scholar] [CrossRef]
- Erdem, A.; Marin, S.; Pereira-Martins, D.A.; Geugien, M.; Cunningham, A.; Pruis, M.G.; Weinhäuser, I.; Gerding, A.; Bakker, B.M.; Wierenga, A.T.J.; et al. Inhibition of the succinyl dehydrogenase complex in acute myeloid leukemia leads to a lactate-fuelled respiratory metabolic vulnerability. Nat. Commun. 2022, 13, 2013. [Google Scholar] [CrossRef]
- Minami, N.; Tanaka, K.; Sasayama, T.; Kohmura, E.; Saya, H.; Sampetrean, O. Lactate Reprograms Energy and Lipid Metabolism in Glucose-Deprived Oxidative Glioma Stem Cells. Metabolites 2021, 11, 325. [Google Scholar] [CrossRef]
- Montal, E.D.; Bhalla, K.; Dewi, R.E.; Ruiz, C.F.; Haley, J.A.; Ropell, A.E.; Gordon, C.; Girnun, G.D.; Haley, J.D. Inhibition of phosphoenolpyruvate carboxykinase blocks lactate utilization and impairs tumor growth in colorectal cancer. Cancer Metab. 2019, 7, 8. [Google Scholar] [CrossRef]
- Otto, A.M.; Hintermair, J.; Janzon, C. NADH-linked metabolic plasticity of MCF-7 breast cancer cells surviving in a nutrient-deprived microenvironment. J. Cell. Biochem. 2015, 116, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, M.; Yao, X.; Fei, Y.; Lin, Z.; Li, Z.; Cai, K.; Zhao, Y.; Luo, Z. HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and Its Therapeutic Implications. Cell Rep. 2020, 33, 108487. [Google Scholar] [CrossRef]
- Deng, H.; Gao, Y.; Trappetti, V.; Hertig, D.; Karatkevich, D.; Losmanova, T.; Urzi, C.; Ge, H.; Geest, G.A.; Bruggmann, R.; et al. Targeting lactate dehydrogenase B-dependent mitochondrial metabolism affects tumor initiating cells and inhibits tumorigenesis of non-small cell lung cancer by inducing mtDNA damage. Cell. Mol. Life Sci. 2022, 79, 445. [Google Scholar] [CrossRef]
- Ying, M.; You, D.; Zhu, X.; Cai, L.; Zeng, S.; Hu, X. Lactate and glutamine support NADPH generation in cancer cells under glucose deprived conditions. Redox Biol. 2021, 46, 102065. [Google Scholar] [CrossRef]
- Chen, S.; Ning, B.; Song, J.; Yang, Z.; Zhou, L.; Chen, Z.; Mao, L.; Liu, H.; Wang, Q.; He, S.; et al. Enhanced pentose phosphate pathway activity promotes pancreatic ductal adenocarcinoma progression via activating YAP/MMP1 axis under chronic acidosis. Int. J. Biol. Sci. 2022, 18, 2304–2316. [Google Scholar] [CrossRef]
- Koncošová, M.; Vrzáčková, N.; Křížová, I.; Tomášová, P.; Rimpelová, S.; Dvořák, A.; Vítek, L.; Rumlová, M.; Ruml, T.; Zelenka, J. Inhibition of Mitochondrial Metabolism Leads to Selective Eradication of Cells Adapted to Acidic Microenvironment. Int. J. Mol. Sci. 2021, 22, 10790. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Urbano, A.M. Otto Warburg: The journey towards the seminal discovery of tumor cell bioenergetic reprogramming. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2021, 1867, 165965. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Laconi, E. The evolving concept of tumor microenvironments. BioEssays 2007, 29, 738–744. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. A microenvironmental model of carcinogenesis. Nat. Rev. Cancer 2008, 8, 56–61. [Google Scholar] [CrossRef]
- Damaghi, M.; Tafreshi, N.K.; Lloyd, M.C.; Sprung, R.; Estrella, V.; Wojtkowiak, J.W.; Morse, D.L.; Koomen, J.M.; Bui, M.M.; Gatenby, R.A.; et al. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Commun. 2015, 6, 8752. [Google Scholar] [CrossRef] [PubMed]
- Damaghi, M.; Gillies, R.J. Lysosomal protein relocation as an adaptation mechanism to extracellular acidosis. Cell Cycle 2016, 15, 1659–1660. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef] [PubMed]
- Cori, C.F. The Glucose-Lactic Acid Cycle and Gluconeogenesis. In Current Topics in Cellular Regulation; Elsevier: Amsterdam, The Netherlands, 1981; Volume 18, pp. 377–387. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Res. 1989, 18, 6449–6465. [Google Scholar]
- Nakajima, E.C.; Van Houten, B. Metabolic symbiosis in cancer: Refocusing the Warburg lens. Mol. Carcinog. 2013, 52, 329–337. [Google Scholar] [CrossRef]
- Walenta, S.; Wetterling, M.; Lehrke, M.; Schwickert, G.; Sundfør, K.; Rofstad, E.K.; Mueller-Klieser, W. High Lactate Levels Predict Likelihood of Metastases, Tumor Recurrence, and Restricted Patient Survival in Human Cervical Cancers. Cancer Res. 2000, 60, 916–921. [Google Scholar]
- Stern, R.; Shuster, S.; Neudecker, B.A.; Formby, B. Lactate stimulates fibroblast expression of hyaluronan and CD44: The Warburg effect revisited. Exp. Cell Res. 2002, 276, 24–31. [Google Scholar] [CrossRef]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Sonveaux, P.; Végran, F.; Schroeder, T.; Vegran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yan, C.; Ma, J.; Peng, P.; Ren, X.; Cai, S.; Shen, X.; Wu, Y.; Zhang, S.; Wang, X.; et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat. Metab. 2023, 5, 61–79. [Google Scholar] [CrossRef]
- Zhang, C.; Quinones, A.; Le, A. Metabolic reservoir cycles in cancer. Semin. Cancer Biol. 2022, 86, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Stéphanou, A. Searching for the Metabolic Signature of Cancer: A Review from Warburg’s Time to Now. Biomolecules 2022, 12, 1412. [Google Scholar] [CrossRef] [PubMed]
- Papalazarou, V.; Maddocks, O.D.K. Supply and demand: Cellular nutrient uptake and exchange in cancer. Mol. Cell 2021, 81, 3731–3748. [Google Scholar] [CrossRef] [PubMed]
- Elingaard-Larsen, L.O.; Rolver, M.G.; Sørensen, E.E.; Pedersen, S.F. How Reciprocal Interactions between the Tumor Microenvironment and Ion Transport Proteins Drive Cancer Progression. In From Malignant Transformation to Metastasis; Stock, C., Pardo, L.A., Eds.; Reviews of Physiology, Biochemistry and Pharmacology; Springer: Cham, Switzerland, 2020; Volume 182, pp. 1–38. [Google Scholar] [CrossRef]
- Gillies, R.J.; Raghunand, N.; Karczmar, G.S.; Bhujwalla, Z.M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging 2002, 16, 430–450. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, F.A.; Kettunen, M.I.; Day, S.E.; Hu, D.-E.; Ardenkjær-Larsen, J.H.; Zandt, R.I.; Jensen, P.R.; Karlsson, M.; Golman, K.; Lerche, M.H.; et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 2008, 453, 940–943. [Google Scholar] [CrossRef]
- Hu, X.; Chao, M.; Wu, H. Central role of lactate and proton in cancer cell resistance to glucose deprivation and its clinical translation. Signal Transduct. Target Ther. 2017, 2, 16047. [Google Scholar] [CrossRef]
- Brown, T.P.; Ganapathy, V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 2020, 206, 107451. [Google Scholar] [CrossRef]
- Roland, C.L.; Arumugam, T.; Deng, D.; Liu, S.H.; Philip, B.; Gomez, S.; Burns, W.R.; Ramachandran, V.; Wang, H.; Cruz-Monserrate, Z.; et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 2014, 74, 5301–5310. [Google Scholar] [CrossRef]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.; Draoui, N.; Polet, F.; Pinto, A.; Drozak, X.; Riant, O.; Feron, O. The SIRT1/HIF2α axis drives reductive glutamine metabolism under chronic acidosis and alters tumor response to therapy. Cancer Res. 2014, 74, 5507–5519. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.; Bastien, E.; de Jesus, J.P.S.; Dierge, E.; Martherus, R.; Linden, C.V.; Doix, B.; Degavre, C.; Guilbaud, C.; Petit, L.; et al. TGFβ2-induced formation of lipid droplets supports acidosis-driven EMT and the metastatic spreading of cancer cells. Nat. Commun. 2020, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Queen, A.; Bhutto, H.N.; Yousuf, M.; Syed, M.A.; Hassan, I. Carbonic anhydrase IX: A tumor acidification switch in heterogeneity and chemokine regulation. Semin. Cancer Biol. 2022, 86, 899–913. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Dai, C.; Hu, X. Evidence That Does Not Support Pyruvate Kinase M2 (PKM2)-catalyzed Reaction as a Rate-limiting Step in Cancer Cell Glycolysis. J. Biol. Chem. 2016, 291, 8987–8999. [Google Scholar] [CrossRef] [PubMed]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Cancer cell metabolism and mitochondria: Nutrient plasticity for TCA cycle fueling. Biochim. Biophys. Acta BBA-Rev. Cancer 2017, 1868, 7–15. [Google Scholar] [CrossRef]
- Inigo, M.; Deja, S.; Burgess, S.C. Ins and Outs of the TCA Cycle: The Central Role of Anaplerosis. Annu. Rev. Nutr. 2021, 41, 19–47. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate metabolism in human lung tumors. Cell 2017, 171, 358–371. [Google Scholar] [CrossRef]
- Chen, L.B. Mitochondrial Membrane Potential in Living Cells. Annu. Rev. Cell Biol. 1988, 4, 155–181. [Google Scholar] [CrossRef]
- Herzig, S.; Raemy, E.; Montessuit, S.; Veuthey, J.-L.; Zamboni, N.; Westermann, B.; Kunji, E.R.S.; Martinou, J.-C. Identification and functional expression of the mitochondrial pyruvate carrier. Science 2012, 337, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Bricker, D.K.; Taylor, E.B.; Schell, J.C.; Orsak, T.; Boutron, A.; Chen, Y.-C.; Cox, J.E.; Cardon, C.M.; Van Vranken, J.G.; Dephoure, N.; et al. A Mitochondrial Pyruvate Carrier Required for Pyruvate Uptake in Yeast, Drosophila, and Humans. Science 2012, 337, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hussien, R.; Brooks, G.A. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: Evidence of a mitochondrial lactate oxidation complex. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1237–E1244. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Ying, M.; Guo, C.; Hu, X. The quantitative relationship between isotopic and net contributions of lactate and glucose to the tricarboxylic acid (TCA) cycle. J. Biol. Chem. 2019, 294, 9615–9630. [Google Scholar] [CrossRef]
- Tasdogan, A.; Faubert, B.; Ramesh, V.; Ubellacker, J.M.; Shen, B.; Solmonson, A.; Murphy, M.M.; Gu, Z.; Gu, W.; Martin, M.; et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 2020, 577, 115. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Hosogi, S.; Niisato, N.; Yokoyama, N.; Hayata, H.; Miyazaki, H.; Kusuzaki, K.; Fukuda, T.; Fukui, M.; Nakamura, N.; et al. Improvement of insulin resistance, blood pressure and interstitial pH in early developmental stage of insulin resistance in OLETF rats by intake of propolis extracts. Biochem. Biophys. Res. Commun. 2013, 432, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Pilot, C.; Marunaka, Y.; Fais, S. Targeting Acidity in Cancer and Diabetes. Biochim. Biophys. Acta Rev. Cancer. 2019, 1871, 273–280. [Google Scholar] [CrossRef]
- Chao, M.; Wu, H.; Jin, K.; Li, B.; Wu, J.; Zhang, G.; Yang, G.; Hu, X. A nonrandomized cohort and a randomized study of local control of large hepatocarcinoma by targeting intratumoral lactic acidosis. eLife 2016, 5, e15691. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhou, C.; Ren, H.; Zhang, Z.; Cheng, F.; Xiong, Z.; Chen, C.; Yang, J.; Gao, J.; Zhang, Y.; et al. Lactate-induced MRP1 expression contributes to metabolism-based etoposide resistance in non-small cell lung cancer cells. Cell Commun. Signal 2020, 18, 167. [Google Scholar] [CrossRef] [PubMed]
- Ying, C.; Jin, C.; Zeng, S.; Chao, M.; Hu, X. Alkalization of cellular pH leads to cancer cell death by disrupting autophagy and mitochondrial function. Oncogene 2022, 41, 3886–3897. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yeo, M.; Kang, Y.; Kim, H.J.; Park, S.G.; Jang, E.; Park, S.H.; Kim, E.; Kang, S. Lactate oxidase/catalase-displaying nanoparticles efficiently consume lactate in the tumor microenvironment to effectively suppress tumor growth. J. Nanobiotechnol. 2023, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wei, Z.; Li, Q.; Wan, F.; Chao, Z.; Zhang, X.; Lin, L.; Meng, H.; Tian, L. Advanced Cancer Starvation Therapy by Simultaneous Deprivation of Lactate and Glucose Using a MOF Nanoplatform. Adv. Sci. 2021, 8, e2101467. [Google Scholar] [CrossRef] [PubMed]
- Chavarria, V.; Ortiz-Islas, E.; Salazar, A.; la Cruz, V.P.-D.; Espinosa-Bonilla, A.; Figueroa, R.; Ortíz-Plata, A.; Sotelo, J.; Sánchez-García, F.J.; Pineda, B. Lactate-Loaded Nanoparticles Induce Glioma Cytotoxicity and Increase the Survival of Rats Bearing Malignant Glioma Brain Tumor. Pharmaceutics 2022, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Rodriguez-Cruz, V.; Morris, M.E. Cellular Uptake of MCT1 Inhibitors AR-C155858 and AZD3965 and Their Effects on MCT-Mediated Transport of L-Lactate in Murine 4T1 Breast Tumor Cancer Cells. AAPS J. 2019, 21, 13. [Google Scholar] [CrossRef]
- Beloueche-Babari, M.; Galobart, T.C.; Delgado-Goni, T.; Wantuch, S.; Parkes, H.G.; Tandy, D.; Harker, J.A.; Leach, M.O. Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br. J. Cancer 2020, 122, 895–903. [Google Scholar] [CrossRef]
- Ma, R.; Li, X.; Gong, S.; Ge, X.; Zhu, T.; Ge, X.; Weng, L.; Tao, Q.; Guo, J. Dual Roles of Lactate in EGFR-TKI-Resistant Lung Cancer by Targeting GPR81 and MCT1. J. Oncol. 2022, 2022, 3425841. [Google Scholar] [CrossRef]
- Pisarsky, L.; Bill, R.; Fagiani, E.; Dimeloe, S.; Goosen, R.W.; Hagmann, J.; Hess, C.; Christofori, G. Targeting Metabolic Symbiosis to Overcome Resistance to Anti-Angiogenic Therapy. Cell Rep. 2016, 15, 1161–1174. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 2022, 87, 184–195. [Google Scholar] [CrossRef]
- Xiao, Z.; Dai, Z.; Locasale, J.W. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat. Commun. 2019, 10, 3763. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J. Uncovering the Underlying Mechanisms of Cancer Metabolism through the Landscapes and Probability Flux Quantifications. IScience 2020, 23, 101002. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.M.; Tuluc, M.; Whitaker-Menezes, D.; Ames, J.A.; Anantharaman, A.; Butera, A.; Leiby, B.; Cognetti, D.; Sotgia, F.; Lisanti, M.P.; et al. Cancer metabolism, stemness and tumor recurrence. Cell Cycle 2013, 12, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Phipps, C.; Molavian, H.; Kohandel, M. A microscale mathematical model for metabolic symbiosis: Investigating the effects of metabolic inhibition on ATP turnover in tumors. J. Theor. Biol. 2015, 366, 103–114. [Google Scholar] [CrossRef]
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L.; et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef]
- Pereira-Nunes, A.; Afonso, J.; Granja, S.; Baltazar, F. Lactate and Lactate Transporters as Key Players in the Maintenance of the Warburg Effect. Adv. Exp. Med. Biol. 2020, 1219, 51–74. [Google Scholar] [CrossRef]
- Swietach, P.; Monterisi, S. A Barter Economy in Tumors: Exchanging Metabolites through Gap Junctions. Cancers 2019, 11, 117. [Google Scholar] [CrossRef]
- Dovmark, T.H.; Saccomano, M.; Hulikova, A.; Alves, F.; Swietach, P. Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene 2017, 36, 4538–4550. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, M.; Zhou, X.; Tian, S.; Yang, X.; Ning, Y.; Li, Y.; Zhang, S. The roles of connexins and gap junctions in the progression of cancer. Cell Commun. Signal 2023, 21, 8. [Google Scholar] [CrossRef]
- Guillaumond, F.; Leca, J.; Olivares, O.; Lavaut, M.-N.; Vidal, N.; Berthezène, P.; Dusetti, N.J.; Loncle, C.; Calvo, E.; Turrini, O.; et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, 3919–3924. [Google Scholar] [CrossRef]
- Martinez, C.A.; Scafoglio, C. Heterogeneity of Glucose Transport in Lung Cancer. Biomolecules 2020, 10, 868. [Google Scholar] [CrossRef]
- Shibao, S.; Minami, N.; Koike, N.; Fukui, N.; Yoshida, K.; Saya, H.; Sampetrean, O. Metabolic heterogeneity and plasticity of glioma stem cells in a mouse glioblastoma model. Neuro-Oncology 2018, 20, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Kubelt, C.; Peters, S.; Ahmeti, H.; Huhndorf, M.; Huber, L.; Cohrs, G.; Hövener, J.-B.; Jansen, O.; Synowitz, M.; Held-Feindt, J. Intratumoral Distribution of Lactate and the Monocarboxylate Transporters 1 and 4 in Human Glioblastoma Multiforme and Their Relationships to Tumor Progression-Associated Markers. Int. J. Mol. Sci. 2020, 21, 6254. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Lucas, J.E.; Chen, J.L.-Y.; LaMonte, G.; Wu, J.; Wang, M.C.; Koumenis, C.; Chi, J.-T. Functional interaction between responses to lactic acidosis and hypoxia regulates genomic transcriptional outputs. Cancer Res. 2012, 72, 491–502. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).