Tolerability of BRAF and MEK Inhibitors for Metastasized Melanoma after Intra-Class Switch: A Multicenter, Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Treatment
2.3. Data Collection
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
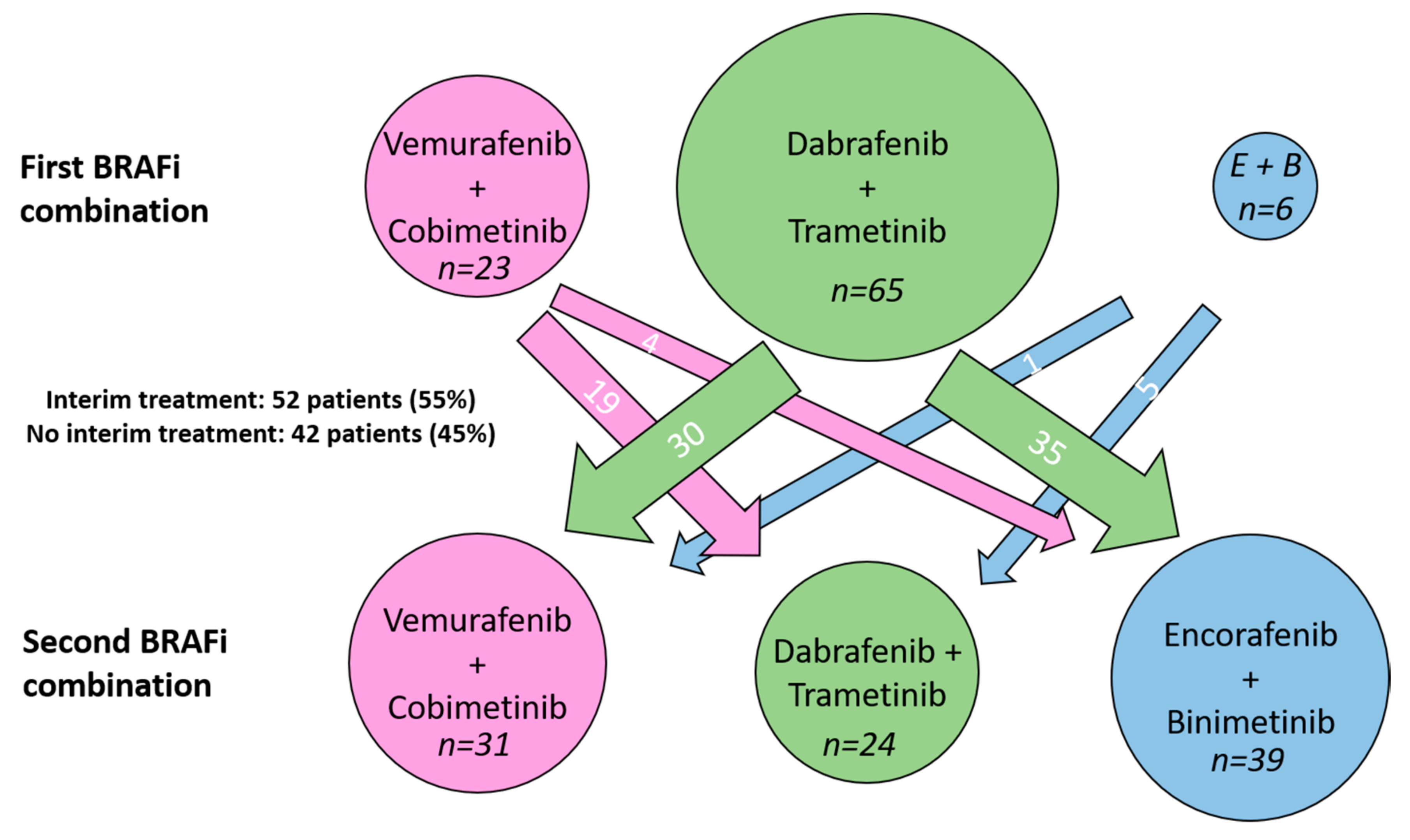
3.2. Treatment Characteristics
3.3. Adverse Events
3.3.1. Patients with a Second BRAFi+MEKi Combination Due to Adverse Events
3.3.2. Compound-Specific Toxic Effects
3.3.3. Patients with the Second BRAFi+MEKi Combination after Progressive Disease with the First Combination
3.4. Treatment Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Dummer, R.; Schadendorf, D.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Longer Follow-Up Confirms Relapse-Free Survival Benefit With Adjuvant Dabrafenib Plus Trametinib in Patients With Resected BRAF V600–Mutant Stage III Melanoma. J. Clin. Oncol. 2018, 36, 3441–3449. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Flaherty, K.T.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Schreuer, M.; Jansen, Y.; Planken, S.; Chevolet, I.; Seremet, T.; Kruse, V.; Neyns, B. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAF(V600)-mutant melanoma: An open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol. 2017, 18, 464–472. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Weber, J.S.; Patel, S.P.; Dummer, R.; Miller, W.H.; Cosgrove, D.; Carlino, M.S.; Tan, D.S.-W.; Lebbe, C.; Cipani, T.; et al. A phase Ib/II study of BRAF inhibitor (BRAFi) encorafenib (ENCO) plus MEK inhibitor (MEKi) binimetinib (BINI) in cutaneous melanoma patients naive to BRAFi treatment. J. Clin. Oncol. 2015, 33, 9007. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1315–1327. [Google Scholar] [CrossRef]
- Heinzerling, L.; Eigentler, T.K.; Fluck, M.; Hassel, J.C.; Heller-Schenck, D.; Leipe, J.; Pauschinger, M.; Vogel, A.; Zimmer, L.; Gutzmer, R. Tolerability of BRAF/MEK inhibitor combinations: Adverse event evaluation and management. ESMO Open 2019, 4, e000491. [Google Scholar] [CrossRef]
- Salzmann, M.; Benesova, K.; Buder-Bakhaya, K.; Papamichail, D.; Dimitrakopoulou-Strauss, A.; Lorenz, H.M.; Enk, A.H.; Hassel, J.C. Arthralgia Induced by BRAF Inhibitor Therapy in Melanoma Patients. Cancers 2020, 12, 3004. [Google Scholar] [CrossRef]
- Tahseen, A.I.; Patel, N.B. Successful dabrafenib transition after vemurafenib-induced toxic epidermal necrolysis in a patient with metastatic melanoma. JAAD Case Rep. 2018, 4, 930–933. [Google Scholar] [CrossRef]
- Ros, J.; Muñoz-Couselo, E. DRESS syndrome due to vemurafenib treatment: Switching BRAF inhibitor to solve a big problem. BMJ Case Rep. 2018. [Google Scholar] [CrossRef]
- Chic, N.; Mezquita, L.; Aldea, M.; Chebib, R.; Caramella, C.; Planchard, D.; Besse, B. Successful Switch to Vemurafenib Plus Cobimetinib After Dabrafenib Plus Trametinib Toxicity in BRAF(V600E)-Mutant Metastatic Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2021, 22, e54–e56. [Google Scholar] [CrossRef]
- Ibrahim, T.; Routier, E.; Weill, A.; Baz, M.; Robert, C. Successful re-challenge with anti-BRAF and anti-MEK in a patient with symptomatic melanoma flare. Eur. J. Cancer 2017, 82, 25–26. [Google Scholar] [CrossRef]
- Tietze, J.K.; Forschner, A.; Loquai, C.; Mitzel-Rink, H.; Zimmer, L.; Meiss, F.; Rafei-Shamsabadi, D.; Utikal, J.; Bergmann, M.; Meier, F.; et al. The efficacy of re-challenge with BRAF inhibitors after previous progression to BRAF inhibitors in melanoma: A retrospective multicenter study. Oncotarget 2018, 9, 34336–34346. [Google Scholar] [CrossRef]
- Cybulska-Stopa, B.; Rogala, P.; Czarnecka, A.M.; Galus, Ł.; Dziura, R.; Rajczykowski, M.; Kubiatowski, T.; Wiśniewska, M.; Gęga-Czarnota, A.; Teterycz, P.; et al. BRAF and MEK inhibitors rechallenge as effective treatment for patients with metastatic melanoma. Melanoma Res. 2020, 30, 465–471. [Google Scholar] [CrossRef]
- Valpione, S.; Carlino, M.S.; Mangana, J.; Mooradian, M.J.; McArthur, G.; Schadendorf, D.; Hauschild, A.; Menzies, A.M.; Arance, A.; Ascierto, P.A.; et al. Rechallenge with BRAF-directed treatment in metastatic melanoma: A multi-institutional retrospective study. Eur. J. Cancer 2018, 91, 116–124. [Google Scholar] [CrossRef]
- Desvignes, C.; Abi Rached, H.; Templier, C.; Drumez, E.; Lepesant, P.; Desmedt, E.; Mortier, L. BRAF inhibitor discontinuation and rechallenge in advanced melanoma patients with a complete initial treatment response. Melanoma Res. 2017, 27, 281–287. [Google Scholar] [CrossRef]
- Roux, J.; Pages, C.; Malouf, D.; Basset Seguin, N.; Madjlessi, N.; Baccard, M.; Comte, C.; Archimbaud, A.; Battistella, M.; Viguier, M.; et al. BRAF inhibitor rechallenge in patients with advanced BRAF V600-mutant melanoma. Melanoma Res. 2015, 25, 559–563. [Google Scholar] [CrossRef]
- Seghers, A.C.; Wilgenhof, S.; Lebbé, C.; Neyns, B. Successful rechallenge in two patients with BRAF-V600-mutant melanoma who experienced previous progression during treatment with a selective BRAF inhibitor. Melanoma Res. 2012, 22, 466–472. [Google Scholar] [CrossRef]
- Karoulia, Z.; Gavathiotis, E.; Poulikakos, P.I. New perspectives for targeting RAF kinase in human cancer. Nat. Rev. Cancer 2017, 17, 676–691. [Google Scholar] [CrossRef]
- National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0; US Department of Health and Human Services: Bethesda, MD, USA, 2017. [Google Scholar]
- Wagner, N.B.; Forschner, A.; Leiter, U.; Garbe, C.; Eigentler, T.K. S100B and LDH as early prognostic markers for response and overall survival in melanoma patients treated with anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies. Br. J. Cancer 2018, 119, 339–346. [Google Scholar] [CrossRef]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.M.; et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [CrossRef]
- Ben-Betzalel, G.; Baruch, E.N.; Boursi, B.; Steinberg-Silman, Y.; Asher, N.; Shapira-Frommer, R.; Schachter, J.; Markel, G. Possible immune adverse events as predictors of durable response to BRAF inhibitors in patients with BRAF V600-mutant metastatic melanoma. Eur. J. Cancer 2018, 101, 229–235. [Google Scholar] [CrossRef]


| Stage IV n = 87 | Adjuvant Stage III n = 7 | |
|---|---|---|
| Gender | ||
| Male | 43 (49%) | 5 (71%) |
| Female | 44 (51%) | 2 (29%) |
| Age | Median 55 years (range 19–85) | Median 62 years (range 43–83) |
| Previous systemic treatment lines | ||
| None | 37 (43%) | 6 (86%) |
| PD1 + CTLA4-inhibitors | 19 (22%) | |
| PD1-inhibitor monotherapy | 18 (21%) | 1 (14%) |
| Interferon α | 8 (9%) | |
| BRAF inhibitor monotherapy | 4 (5%) | |
| Dacarbazine | 1 (1%) | |
| Serum lactate dehydrogenase | ||
| Normal | 34/72 (47%) | 5 (71%) |
| Elevated | 38/72 (53%) | 2 (29%) |
| S100 serum levels | ||
| Normal | 27/68 (40%) | 5 (71%) |
| Elevated < 2 × ULN | 16/68 (24%) | |
| Elevated ≥ 2 ×ULN | 25/68 (37%) | 2 (29%) |
| Adverse Event (AE) | First BRAFi+MEKi Combination | Second BRAFi+MEKi Combination | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V+C (n = 23) | D+T (n = 65) | E+B (n = 6) | V+C (n = 31) | D+T (n = 24) | E+B (n = 39) | |||||||||||||||||||||||||||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |||||||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Highest grade AE | 4 | 17 | 6 | 26 | 7 | 30 | 9 | 14 | 17 | 26 | 23 | 35 | 4 | 67 | 1 | 17 | 1 | 17 | 6 | 19 | 9 | 29 | 11 | 36 | 5 | 21 | 7 | 30 | 6 | 25 | 5 | 13 | 6 | 15 | 13 | 33 |
| Pyrexia | 3 | 13 | 1 | 4 | 1 | 4 | 10 | 15 | 13 | 20 | 6 | 9 | 0 | 0 | 1 | 17 | 0 | 0 | 3 | 10 | 2 | 6 | 1 | 3 | 2 | 8 | 5 | 21 | 3 | 13 | 0 | 0 | 2 | 5 | 1 | 3 |
| Rash | 2 | 9 | 1 | 4 | 6 | 26 | 6 | 9 | 2 | 3 | 3 | 5 | 2 | 33 | 0 | 0 | 0 | 0 | 5 | 16 | 3 | 10 | 8 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 8 | 0 | 0 | 1 | 3 |
| Fatigue | 1 | 4 | 1 | 4 | 0 | 0 | 8 | 12 | 3 | 5 | 3 | 5 | 1 | 17 | 0 | 0 | 0 | 0 | 2 | 6 | 1 | 3 | 0 | 0 | 2 | 8 | 1 | 4 | 0 | 0 | 4 | 10 | 1 | 3 | 0 | 0 |
| Gastrointestinal | 1 | 4 | 2 | 9 | 0 | 0 | 10 | 15 | 3 | 5 | 5 | 8 | 5 | 83 | 0 | 0 | 0 | 0 | 3 | 10 | 3 | 10 | 1 | 3 | 1 | 4 | 3 | 13 | 0 | 0 | 1 | 3 | 2 | 5 | 4 | 10 |
| Elevated liver enzymes | 2 | 9 | 0 | 0 | 1 | 4 | 0 | 0 | 2 | 3 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 1 | 4 | 0 | 0 | 1 | 3 | 3 | 8 |
| Panniculitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 5 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac toxicity | 0 | 0 | 2 | 9 | 0 | 0 | 0 | 0 | 2 | 3 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elevated creatine kinase | 0 | 0 | 1 | 4 | 1 | 4 | 4 | 6 | 2 | 3 | 2 | 3 | 1 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 |
| Arthralgia | 1 | 4 | 0 | 0 | 1 | 4 | 7 | 11 | 5 | 8 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 3 | 10 | 1 | 3 | 1 | 4 | 1 | 4 | 1 | 4 | 0 | 0 | 2 | 5 | 1 | 3 |
| Pulmonal toxicity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ocular toxicity | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 8 |
| Peripheral edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thrombopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other * | 2 | 9 | 3 | 13 | 6 | 26 | 8 | 12 | 3 | 5 | 2 | 3 | 1 | 17 | 0 | 0 | 1 | 17 | 3 | 10 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 2 | 8 | 2 | 5 | 3 | 8 | 3 | 8 |
| Patients with Dose-Limiting Toxic Effects (DLT) during 1st BRAFi+MEKi Combination | n = 44 |
|---|---|
| DLT also occuring during the second BRAFi+MEKi combination | 17 (39%) |
| The same DLT occurred again | 5 (11%) |
| New DLT | 13 (30%) |
| No DLT during the second BRAFi+MEKi combination | 27 (61%) |
| Patients without DLT during the 1st BRAFi+MEKi combination | N = 50 |
| New DLT during the second BRAFi+MEKi combination | 16 (32%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzmann, M.; Wald, A.; Stege, H.; Loquai, C.; Zimmer, L.; Hayani, K.M.; Heinzerling, L.; Gutzmer, R.; Enk, A.H.; Hassel, J.C. Tolerability of BRAF and MEK Inhibitors for Metastasized Melanoma after Intra-Class Switch: A Multicenter, Retrospective Study. Cancers 2023, 15, 1426. https://doi.org/10.3390/cancers15051426
Salzmann M, Wald A, Stege H, Loquai C, Zimmer L, Hayani KM, Heinzerling L, Gutzmer R, Enk AH, Hassel JC. Tolerability of BRAF and MEK Inhibitors for Metastasized Melanoma after Intra-Class Switch: A Multicenter, Retrospective Study. Cancers. 2023; 15(5):1426. https://doi.org/10.3390/cancers15051426
Chicago/Turabian StyleSalzmann, Martin, Alexander Wald, Henner Stege, Carmen Loquai, Lisa Zimmer, Kinan M. Hayani, Lucie Heinzerling, Ralf Gutzmer, Alexander H. Enk, and Jessica C. Hassel. 2023. "Tolerability of BRAF and MEK Inhibitors for Metastasized Melanoma after Intra-Class Switch: A Multicenter, Retrospective Study" Cancers 15, no. 5: 1426. https://doi.org/10.3390/cancers15051426
APA StyleSalzmann, M., Wald, A., Stege, H., Loquai, C., Zimmer, L., Hayani, K. M., Heinzerling, L., Gutzmer, R., Enk, A. H., & Hassel, J. C. (2023). Tolerability of BRAF and MEK Inhibitors for Metastasized Melanoma after Intra-Class Switch: A Multicenter, Retrospective Study. Cancers, 15(5), 1426. https://doi.org/10.3390/cancers15051426








