Nutritional Intake, Hospital Readmissions and Length of Stay in Hospitalised Oncology Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
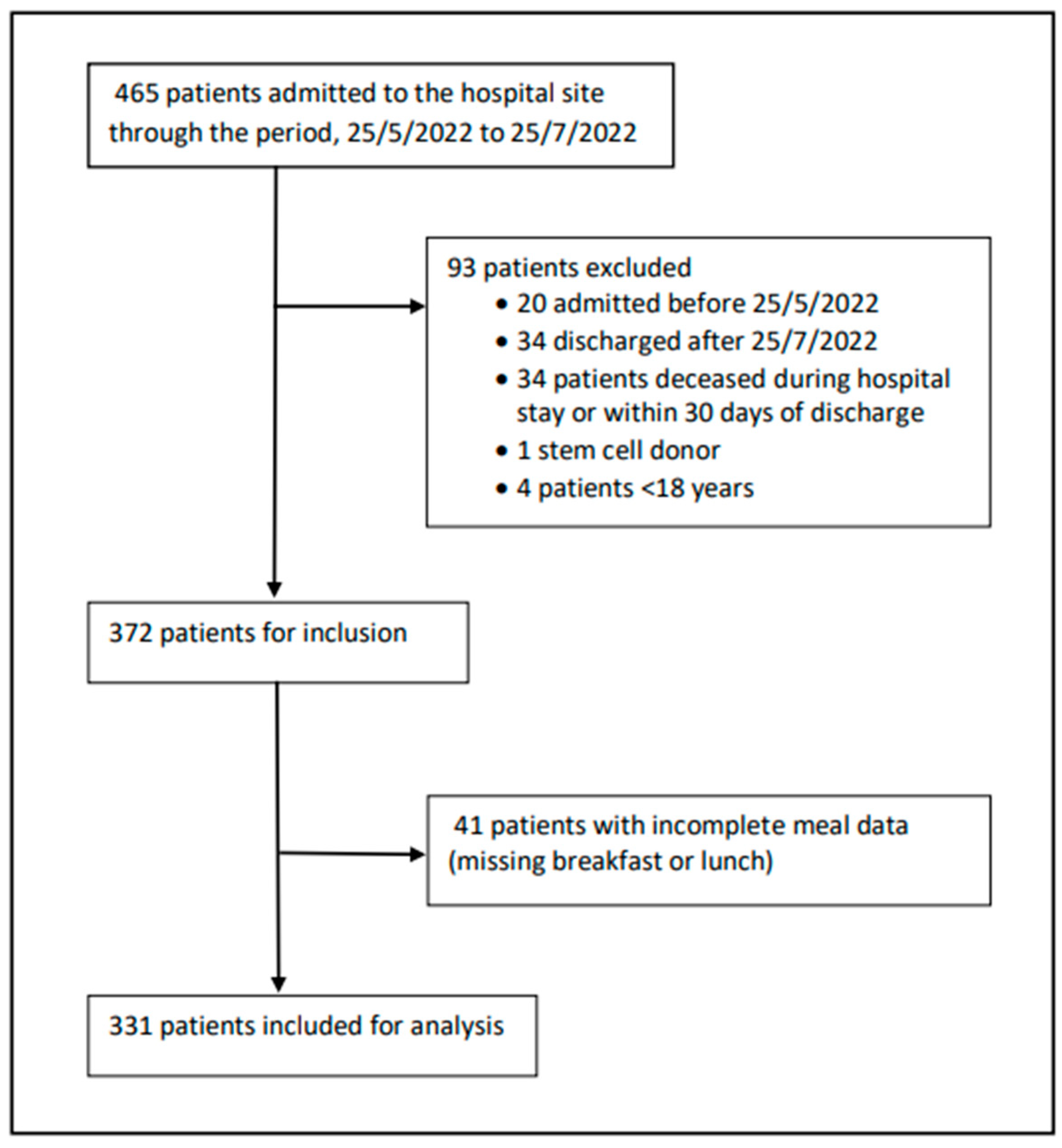
2.2. Data Collection and Study Population
2.3. Modelling of Missing Dinner Data
2.4. Statistical Analysis
3. Results
3.1. Participant Population Characteristics
3.2. Nutritional Intake Data
3.3. Length of Hospital Stay
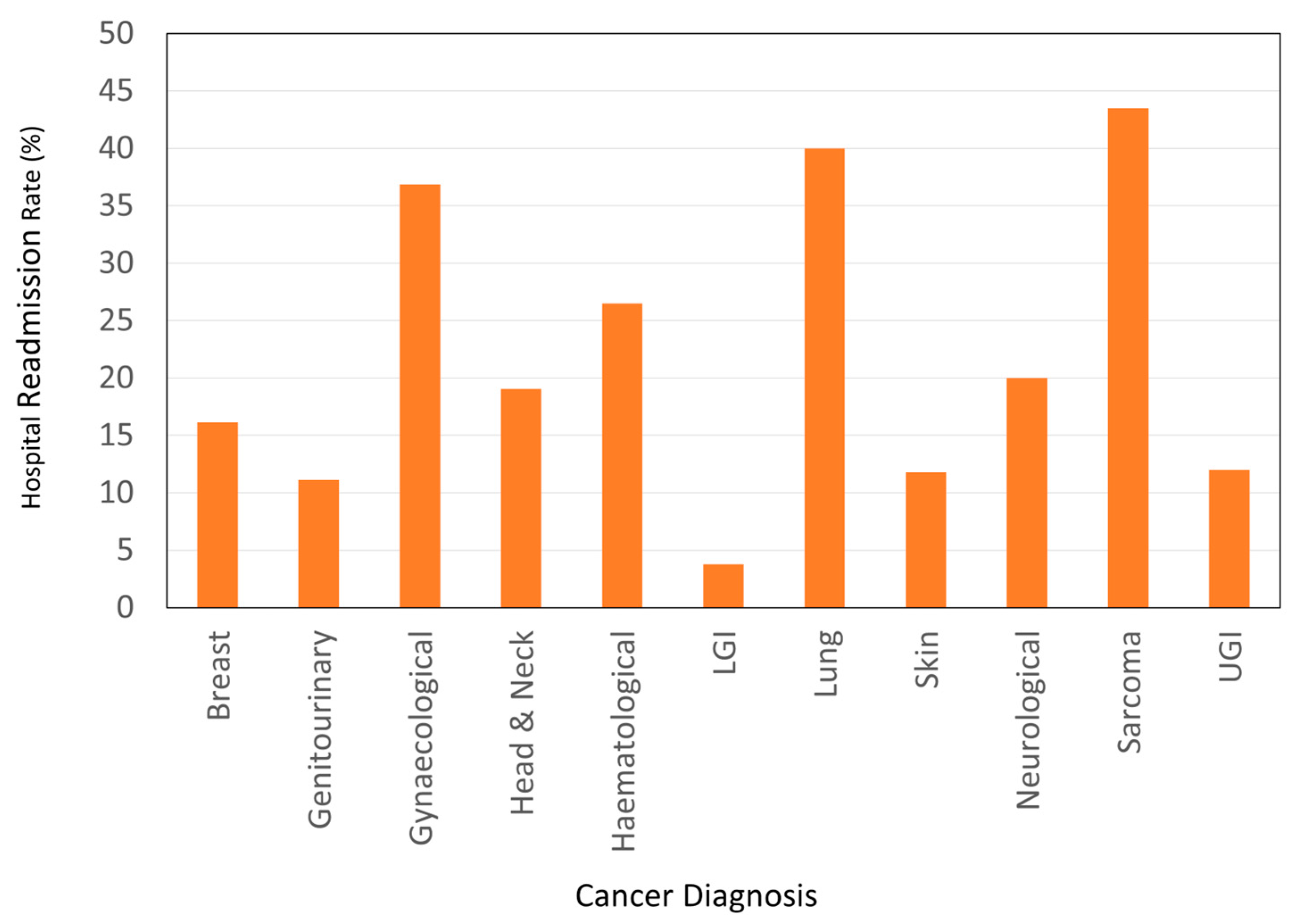
3.4. Hospital Readmissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Welfare. In Cancer; AIHW: Canberra, Australia, 2022. [Google Scholar]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Maunder, K.; Marshall, K.; Syed, K.; Smilevska, S.; Beck, E.; Mak, M.; Barrington, V. Validation of an electronic food intake tool and its usability and efficacy in the healthcare setting. J. Hum. Nutr. Diet. 2021, 35, 613–620. [Google Scholar] [CrossRef]
- Kiss, N.; Hiesmayr, M.; Sulz, I.; Bauer, P.; Heinze, G.; Mouhieddine, M.; Schuh, C.; Tarantino, S.; Simon, J. Predicting Hospital Length of Stay at Admission Using Global and Country-Specific Competing Risk Analysis of Structural, Patient, and Nutrition-Related Data from nutritionDay 2007–2015. Nutrients 2021, 13, 4111. [Google Scholar] [CrossRef]
- Bell, J.F.; Whitney, R.L.; Reed, S.C.; Poghosyan, H.; Lash, R.S.; Kim, K.K.; Davis, A.; Bold, R.J.; Joseph, J.G. Systematic Review of Hospital Readmissions Among Patients with Cancer in the United States. Oncol. Nurs. Forum 2017, 44, 176–191. [Google Scholar] [CrossRef]
- Burhenn, P.; Sun, C.L.; Scher, K.S.; Hsu, J.; Pandya, P.; Chui, C.Y.; Arsenyan, A.; Mitani, D.; Morrison, R.; Katheria, V.; et al. Predictors of hospital readmission among older adults with cancer. J. Geriatr. Oncol. 2020, 11, 1108–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deftereos, I.; Yeung, J.M.; Arslan, J.; Carter, V.M.; Isenring, E.; Kiss, N. Adherence to ESPEN guidelines and associations with postoperative outcomes in upper gastrointestinal cancer resection: Results from the multi-centre NOURISH point prevalence study. Clin. Nutr. ESPEN 2022, 47, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Meure, C.M.; Steer, B.; Porter, J. Interrelationships between Dietary Outcomes, Readmission Rates and Length of Stay in Hospitalised Oncology Patients: A Scoping Review. Nutrients 2023, 15, 400. [Google Scholar] [CrossRef]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Peter MacCallum Centre. Annual Report 2020–2021; Peter MacCallum Cancer Centre: Melbourne, Australia, 2021. [Google Scholar]
- Agarwal, E.; Ferguson, M.; Banks, M.; Bauer, J.; Capra, S.; Isenring, E. Nutritional status and dietary intake of acute care patients: Results from the Nutrition Care Day Survey 2010. Clin. Nutr. 2012, 31, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Wahed, S.; O’Dair, G.; Gemmell, L.; Hainsworth, P.; Horgan, A.F. A randomized controlled trial comparing a standard postoperative diet with low-volume high-calorie oral supplements following colorectal surgery. Color. Dis. 2013, 15, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Munk, T.; Beck, A.M.; Holst, M.; Rosenbom, E.; Rasmussen, H.H.; Nielsen, M.A.; Thomsen, T. Positive effect of protein-supplemented hospital food on protein intake in patients at nutritional risk: A randomised controlled trial. J. Hum. Nutr. Diet. 2014, 27, 122–132. [Google Scholar] [CrossRef]
- Maunder, K.; Lazarus, C.; Walton, K.; Williams, P.; Ferguson, M.; Beck, E. Energy and protein intake increases with an electronic bedside spoken meal ordering system compared to a paper menu in hospital patients. Clin. Nutr. ESPEN 2015, 10, e134–e139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Calleja Fernández, A.; Pintor de la Maza, B.; Vidal Casariego, A.; Villar Taibo, R.; López Gómez, J.J.; Cano Rodríguez, I.; Ballesteros Pomar, M.D. Food intake and nutritional status influence outcomes in hospitalized hematology-oncology patients. Nutr. Hosp. 2015, 31, 2598–2605. [Google Scholar] [CrossRef] [PubMed]
- Ramos Martínez, T.; Villar Taibo, R.; Vidal Casariego, A.; Pintor de la Maza, B.; Ramos, M.A.; García-Pérez, M.P.; Álvarez-Del-Campo, C.; Cano Rodríguez, I.; Ballesteros Pomar, M.D. The appearance of malnutrition in hematological inpatients prolongs hospital stay: The need for nutritional surveillance during hospitalization. Nutr. Hosp. 2019, 36, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Baumgartner, A.; Bounoure, L.; Bally, M.; Deutz, N.E.; Greenwald, J.L.; Stanga, Z.; Mueller, B.; Schuetz, P. Association of Nutritional Support with Clinical Outcomes Among Medical Inpatients Who Are Malnourished or at Nutritional Risk: An Updated Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1915138. [Google Scholar] [CrossRef] [Green Version]
- Villar-Taibo, R.; Calleja-Fernández, A.; Vidal-Casariego, A.; Pintor-de-la-Maza, B.; Álvarez-Del-Campo, C.; Arias-García, R.; Cano-Rodríguez, I.; Ballesteros-Pomar, M.D. A short nutritional intervention in a cohort of hematological inpatients improves energy and protein intake and stabilizes nutritional status. Nutr. Hosp. 2016, 33, 1347–1353. [Google Scholar] [CrossRef]
- Yeung, S.E.; Hilkewich, L.; Gillis, C.; Heine, J.A.; Fenton, T.R. Protein intakes are associated with reduced length of stay: A comparison between Enhanced Recovery After Surgery (ERAS) and conventional care after elective colorectal surgery. Am. J. Clin. Nutr. 2017, 106, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.-C.; Chang, P.-S.; Wu, P.-J.; Huang, Y.-T.; Lin, P.-T. An early moderate recommendation for energy intake based on nutritional status and clinical outcomes in patients with cancer: A retrospective study. Nutrition 2020, 79–80, 110997. [Google Scholar] [CrossRef] [PubMed]
- Jeejeebhoy, K.N.; Keller, H.; Gramlich, L.; Allard, J.P.; Laporte, M.; Duerksen, D.R.; Payette, H.; Bernier, P.; Vesnaver, E.; Davidson, B. Nutritional assessment: Comparison of clinical assessment and objective variables for the prediction of length of hospital stay and readmission. Am. J. Clin. Nutr. 2015, 101, 956–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, D.; Vashi, P.G.; Lammersfeld, C.A.; Braun, D.P. Role of nutritional status in predicting the length of stay in cancer: A systematic review of the epidemiological literature. Ann. Nutr. Metab. 2011, 59, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Munk, T.; Bruun, N.; Nielsen, M.A.; Thomsen, T. From Evidence to Clinical Practice: Positive Effect of Implementing a Protein-Enriched Hospital Menu in Conjunction with Individualized Dietary Counseling. Nutr. Clin. Pract. 2017, 32, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Johansen, N.; Kondrup, J.; Plum, L.M.; Bak, L.; Nørregaard, P.; Bunch, E.; Baernthsen, H.; Andersen, J.R.; Larsen, I.H.; Martinsen, A. Effect of nutritional support on clinical outcome in patients at nutritional risk. Clin. Nutr. 2004, 23, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Starke, J.; Schneider, H.; Alteheld, B.; Stehle, P.; Meier, R. Short-term individual nutritional care as part of routine clinical setting improves outcome and quality of life in malnourished medical patients. Clin. Nutr. 2011, 30, 194–201. [Google Scholar] [CrossRef]
- Kaegi-Braun, N.; Schuetz, P.; Mueller, B.; Kutz, A. Association of Nutritional Support with Clinical Outcomes in Malnourished Cancer Patients: A Population-Based Matched Cohort Study. Front. Nutr. 2020, 7, 603370. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | Values |
|---|---|
| Age: median, IQR (years) | 62 (48–62) |
| Sex: male, n (%) | 188 (56.8) |
| Primary Diagnosis: n (%) | |
| Breast | 29 (8.8) |
| Genitourinary | 27 (8.2) |
| Gynaecological | 19 (5.7) |
| Head & Neck | 20 (6.0) |
| Haematological | 68 (20.5) |
| Lower Gastrointestinal | 53 (16.0) |
| Lung | 25 (7.6) |
| Melanoma and Skin | 33 (10.0) |
| Neurological | 4 (1.2) |
| Sarcoma | 23 (6.9) |
| Upper Gastrointestinal | 26 (7.9) |
| Cancer of Unknown Primary | 4 (1,2) |
| Presence of Metastasis: n (%) | 142 (42.9) |
| Malnutrition Screening Tool a Score: n (%) | |
| 0 | 193 (58.3) |
| 1 | 44 (13.3) |
| 2 | 55 (16.6) |
| 3 | 31 (9.3) |
| 4 | 6 (1.8) |
| 5 | 2 (0.6) |
| Body Mass Index: kg/m2 (%) | |
| Underweight | 38 (11.5) |
| Normal | 106 (32.0) |
| Overweight | 79 (23.9) |
| Obese | 101 (30.5) |
| Unknown b | 7 (2.1) |
| Energy | Protein | |||||||
|---|---|---|---|---|---|---|---|---|
| Meets Estimated Nutrient Requirements a | 25% | 50% | 75% | 100% | 25% | 50% | 75% | 100% |
| n | 315 | 189 | 77 | 30 | 320 | 261 | 162 | 84 |
| % | 95.2% | 57.1% | 23.3% | 9.1% | 96.7% | 78.9% | 48.9% | 25.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meure, C.M.; Steer, B.; Porter, J. Nutritional Intake, Hospital Readmissions and Length of Stay in Hospitalised Oncology Patients. Cancers 2023, 15, 1488. https://doi.org/10.3390/cancers15051488
Meure CM, Steer B, Porter J. Nutritional Intake, Hospital Readmissions and Length of Stay in Hospitalised Oncology Patients. Cancers. 2023; 15(5):1488. https://doi.org/10.3390/cancers15051488
Chicago/Turabian StyleMeure, Cecelia MacFarling, Belinda Steer, and Judi Porter. 2023. "Nutritional Intake, Hospital Readmissions and Length of Stay in Hospitalised Oncology Patients" Cancers 15, no. 5: 1488. https://doi.org/10.3390/cancers15051488
APA StyleMeure, C. M., Steer, B., & Porter, J. (2023). Nutritional Intake, Hospital Readmissions and Length of Stay in Hospitalised Oncology Patients. Cancers, 15(5), 1488. https://doi.org/10.3390/cancers15051488







