B Cells in Breast Cancer Pathology
Abstract
:Simple Summary
Abstract
1. Introduction
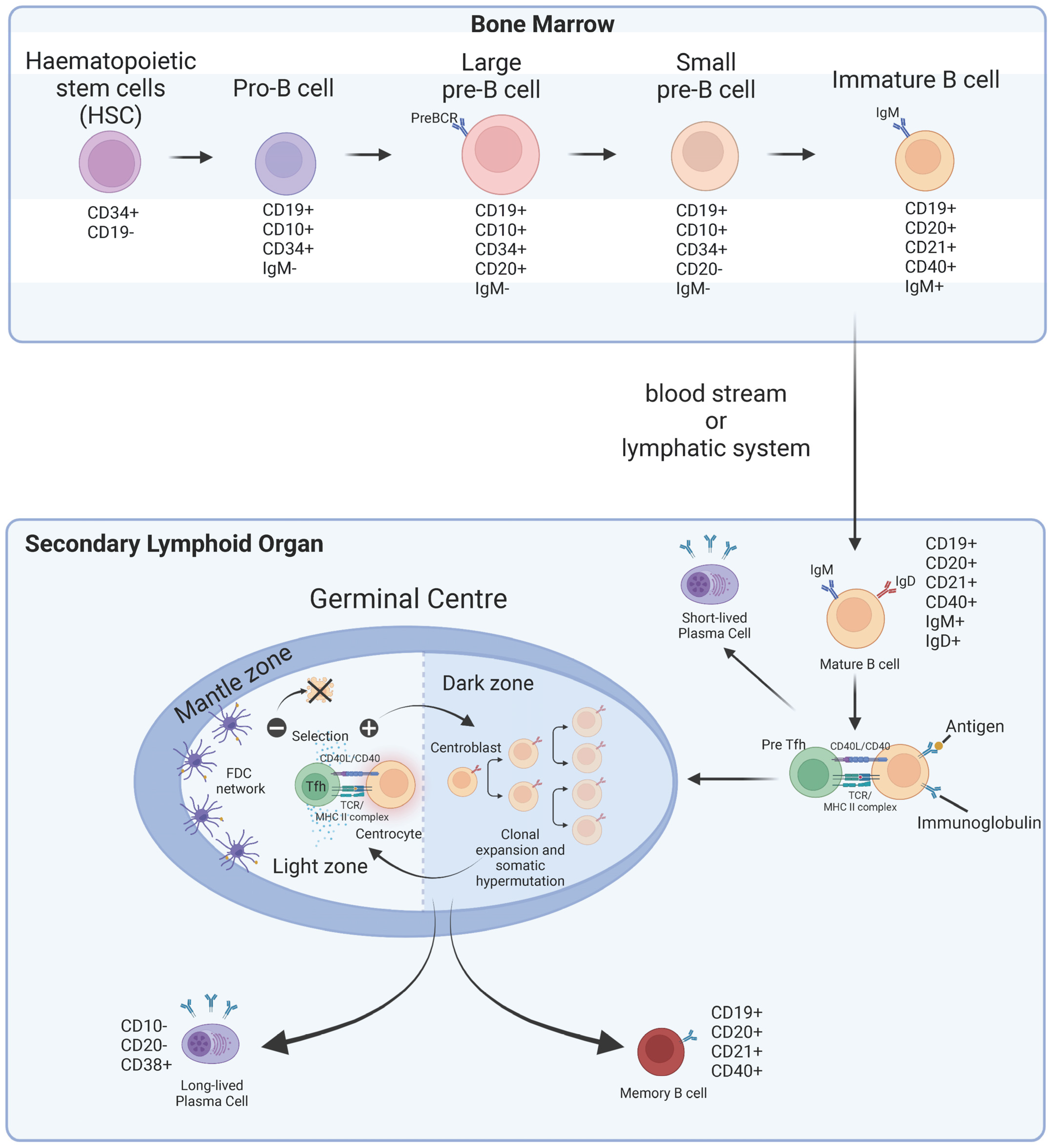
2. B Cells: Physiological Differentiation, Maturation, and Function
3. TIL-B and Adaptive Immunity against Breast Cancer
4. B Cells in Tertiary Lymphoid Structures
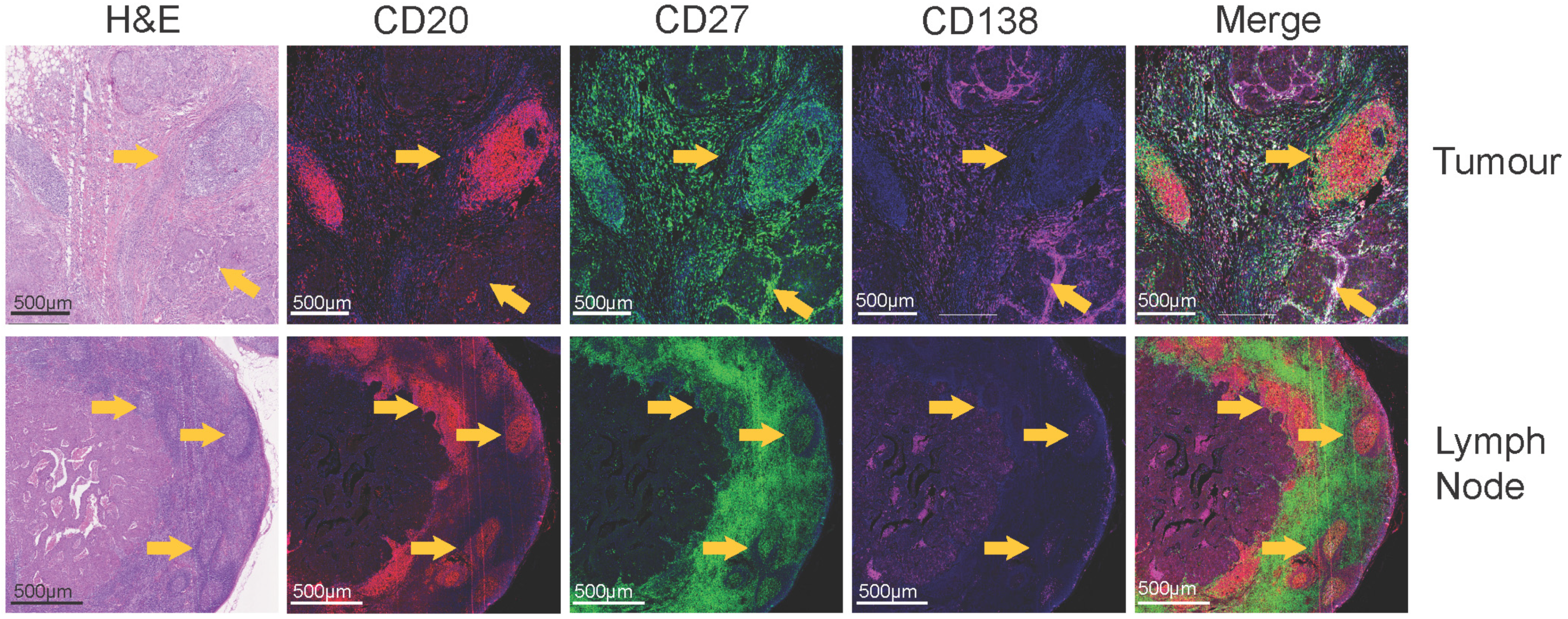
5. B Cells in Lymph Nodes of Breast Cancer Patients
6. B Cells Behaviour with Regards to Treatment Responses
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Onitilo, A.A.; Engel, J.M.; Greenlee, R.T.; Mukesh, B.N. Breast cancer subtypes based on ER/PR and Her2 expression: Comparison of clinicopathologic features and survival. Clin. Med. Res. 2009, 7, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef]
- Gao, Z.-h.; Li, C.-x.; Liu, M.; Jiang, J.-y. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: A meta-analysis. BMC Cancer 2020, 20, 1150. [Google Scholar] [CrossRef]
- Kolberg-Liedtke, C.; Gluz, O.; Heinisch, F.; Feuerhake, F.; Kreipe, H.; Clemens, M.; Nuding, B.; Malter, W.; Reimer, T.; Wuerstlein, R.; et al. Association of TILs with clinical parameters, Recurrence Score® results, and prognosis in patients with early HER2-negative breast cancer (BC)—A translational analysis of the prospective WSG PlanB trial. Breast Cancer Res. 2020, 22, 47. [Google Scholar] [CrossRef]
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Tiger—Grand Challenge. Available online: https://tiger.grand-challenge.org/ (accessed on 23 November 2022).
- Dieci, M.V.; Miglietta, F.; Guarneri, V. Immune infiltrates in breast cancer: Recent updates and clinical implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, Y.; Fu, L.; Zhai, L.; Zhu, J.; Han, Y.; Jiang, Y.; Zhang, Y.; Zhang, P.; Jiang, Z.; et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat. Med. 2019, 25, 312–322. [Google Scholar] [CrossRef]
- Blessin, N.C.; Li, W.; Mandelkow, T.; Jansen, H.L.; Yang, C.; Raedler, J.B.; Simon, R.; Buscheck, F.; Dum, D.; Luebke, A.M.; et al. Prognostic role of proliferating CD8(+) cytotoxic Tcells in human cancers. Cell. Oncol. 2021, 44, 793–803. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Cavallo, F.; Defilippi, P.; Conti, L. The crosstalk between tumor cells and the immune microenvironment in breast cancer: Implications for immunotherapy. Front. Oncol. 2021, 11, 610303. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Zhang, B.; Qiao, L.; Zhang, Y.; Zhang, Y. T Cell Dysfunction and Exhaustion in Cancer. Front. Cell Dev. Biol. 2020, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, H.; Jamiyan, T.; Yamaguchi, R.; Kakumoto, A.; Abe, A.; Harada, O.; Enkhbat, B.; Masunaga, A. Prognostic value of tumor-infiltrating B lymphocytes and plasma cells in triple-negative breast cancer. Breast Cancer 2021, 28, 904–914. [Google Scholar] [CrossRef]
- Garaud, S.; Buisseret, L.; Solinas, C.; Gu-Trantien, C.; de Wind, A.; Van den Eynden, G.; Naveaux, C.; Lodewyckx, J.N.; Boisson, A.; Duvillier, H.; et al. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight 2019, 5, e129641. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Hong, Y.; Qi, P.; Lu, G.; Mai, X.; Xu, S.; He, X.; Guo, Y.; Gao, L.; Jing, Z.; et al. Atlas of breast cancer infiltrated B-lymphocytes revealed by paired single-cell RNA-sequencing and antigen receptor profiling. Nat. Commun. 2021, 12, 2186. [Google Scholar] [CrossRef]
- Ruffell, B.; Au, A.; Rugo, H.S.; Esserman, L.J.; Hwang, E.S.; Coussens, L.M. Leukocyte composition of human breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 2796–2801. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, Z.M.; Going, J.J.; Edwards, J.; McMillan, D.C. The role of the tumour inflammatory cell infiltrate in predicting recurrence and survival in patients with primary operable breast cancer. Cancer Treat. Rev. 2012, 38, 943–955. [Google Scholar] [CrossRef]
- Fridman, W.H.; Petitprez, F.; Meylan, M.; Chen, T.W.-W.; Sun, C.-M.; Roumenina, L.T.; Sautès-Fridman, C. B cells and cancer: To B or not to B? J. Exp. Med. 2020, 218, e20200851. [Google Scholar] [CrossRef]
- Matsumoto, M.; Baba, A.; Yokota, T.; Nishikawa, H.; Ohkawa, Y.; Kayama, H.; Kallies, A.; Nutt, S.L.; Sakaguchi, S.; Takeda, K.; et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 2014, 41, 1040–1051. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.M.; Deriaud, E.; Leclerc, C.; Lo-Man, R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity 2005, 22, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Li, J.; Liu, Q.; Das, M.; Song, W.; Zhang, X.; Tiruthani, K.; Dorosheva, O.; Hu, H.; Lai, S.K.; et al. Nano-trapping CXCL13 reduces regulatory B cells in tumor microenvironment and inhibits tumor growth. J. Control. Release 2022, 343, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Lu, L.; Xia, Y.; Dai, F.; Wang, Y.; Bao, Y.; Lundy, S.K.; Ito, F.; Pan, Q.; Zhang, X.; et al. Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. Eur. J. Immunol. 2015, 45, 999–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calado, D.P.; Sasaki, Y.; Godinho, S.A.; Pellerin, A.; Kochert, K.; Sleckman, B.P.; de Alboran, I.M.; Janz, M.; Rodig, S.; Rajewsky, K. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat. Immunol. 2012, 13, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Sola, D.; Victora, G.D.; Ying, C.Y.; Phan, R.T.; Saito, M.; Nussenzweig, M.C.; Dalla-Favera, R. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat. Immunol. 2012, 13, 1083–1091. [Google Scholar] [CrossRef] [Green Version]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef]
- Sakkas, L.I. Regulatory B cells in autoimmune rheumatic diseases. Mediterr. J. Rheumatol. 2017, 28, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Stott, R.T.; Zhao, G.; SooHoo, J.; Xiong, W.; Lian, M.M.; Fitzgerald, L.; Shi, S.; Akrawi, E.; Lei, J.; et al. TGF-beta-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur. J. Immunol. 2014, 44, 1728–1736. [Google Scholar] [CrossRef] [Green Version]
- Schnellhardt, S.; Erber, R.; Buttner-Herold, M.; Rosahl, M.C.; Ott, O.J.; Strnad, V.; Beckmann, M.W.; King, L.; Hartmann, A.; Fietkau, R.; et al. Tumour-Infiltrating Inflammatory Cells in Early Breast Cancer: An Underrated Prognostic and Predictive Factor? Int. J. Mol. Sci. 2020, 21, 8238. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Wu, L.; Ye, J.; Tao, W. Cellular heterogeneity map of diverse immune and stromal phenotypes within breast tumor microenvironment. PeerJ 2020, 8, e9478. [Google Scholar] [CrossRef]
- Althobiti, M.; Aleskandarany, M.A.; Joseph, C.; Toss, M.; Mongan, N.; Diez-Rodriguez, M.; Nolan, C.C.; Ashankyty, I.; Ellis, I.O.; Green, A.R.; et al. Heterogeneity of tumour-infiltrating lymphocytes in breast cancer and its prognostic significance. Histopathology 2018, 73, 887–896. [Google Scholar] [CrossRef]
- Arias-Pulido, H.; Cimino-Mathews, A.; Chaher, N.; Qualls, C.; Joste, N.; Colpaert, C.; Marotti, J.D.; Foisey, M.; Prossnitz, E.R.; Emens, L.A.; et al. The combined presence of CD20 + B cells and PD-L1 + tumor-infiltrating lymphocytes in inflammatory breast cancer is prognostic of improved patient outcome. Breast Cancer Res. Treat. 2018, 171, 273–282. [Google Scholar] [CrossRef]
- Harris, R.J.; Cheung, A.; Ng, J.C.F.; Laddach, R.; Chenoweth, A.M.; Crescioli, S.; Fittall, M.; Dominguez-Rodriguez, D.; Roberts, J.; Levi, D.; et al. Tumor-Infiltrating B Lymphocyte Profiling Identifies IgG-Biased, Clonally Expanded Prognostic Phenotypes in Triple-Negative Breast Cancer. Cancer Res. 2021, 81, 4290–4304. [Google Scholar] [CrossRef]
- Mahmoud, S.M.; Lee, A.H.; Paish, E.C.; Macmillan, R.D.; Ellis, I.O.; Green, A.R. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res. Treat. 2012, 132, 545–553. [Google Scholar] [CrossRef]
- Pal, B.; Chen, Y.; Vaillant, F.; Capaldo, B.D.; Joyce, R.; Song, X.; Bryant, V.L.; Penington, J.S.; Di Stefano, L.; Tubau Ribera, N.; et al. A single-cell RNA expression atlas of normal, preneoplastic and tumorigenic states in the human breast. EMBO J. 2021, 40, e107333. [Google Scholar] [CrossRef]
- Peng, S.; Hebert, L.L.; Eschbacher, J.M.; Kim, S. Single-Cell RNA Sequencing of a Postmenopausal Normal Breast Tissue Identifies Multiple Cell Types That Contribute to Breast Cancer. Cancers 2020, 12, 3639. [Google Scholar] [CrossRef]
- Tsuda, B.; Miyamoto, A.; Yokoyama, K.; Ogiya, R.; Oshitanai, R.; Terao, M.; Morioka, T.; Niikura, N.; Okamura, T.; Miyako, H.; et al. B-cell populations are expanded in breast cancer patients compared with healthy controls. Breast Cancer 2018, 25, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Ishigami, E.; Sakakibara, M.; Sakakibara, J.; Masuda, T.; Fujimoto, H.; Hayama, S.; Nagashima, T.; Sangai, T.; Nakagawa, A.; Nakatani, Y.; et al. Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer 2019, 26, 180–189. [Google Scholar] [CrossRef]
- Liu, M.; Wei, F.; Wang, J.; Yu, W.; Shen, M.; Liu, T.; Zhang, D.; Wang, Y.; Ren, X.; Sun, Q. Myeloid-derived suppressor cells regulate the immunosuppressive functions of PD-1(-)PD-L1(+) Bregs through PD-L1/PI3K/AKT/NF-kappaB axis in breast cancer. Cell Death Dis. 2021, 12, 465. [Google Scholar] [CrossRef]
- Shen, M.; Wang, J.; Yu, W.; Zhang, C.; Liu, M.; Wang, K.; Yang, L.; Wei, F.; Wang, S.E.; Sun, Q.; et al. A novel MDSC-induced PD-1(-)PD-L1(+) B-cell subset in breast tumor microenvironment possesses immuno-suppressive properties. Oncoimmunology 2018, 7, e1413520. [Google Scholar] [CrossRef] [Green Version]
- Olkhanud, P.B.; Damdinsuren, B.; Bodogai, M.; Gress, R.E.; Sen, R.; Wejksza, K.; Malchinkhuu, E.; Wersto, R.P.; Biragyn, A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011, 71, 3505–3515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, P.A.; Norena, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Zhao, Q.; Liao, J.Y.; Song, E.; Xia, Q.; Pan, J.; Li, Y.; Li, J.; Zhou, B.; Ye, Y.; et al. Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity. Cell 2020, 180, 1081–1097.e1024. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Zhang, L.; Yang, X.; Li, H.; Ma, B.; Walton, S.; Wu, X.; Yuan, J.; Wang, T.; Liu, X. Targeting interleukin-10 signalling for cancer immunotherapy, a promising and complicated task. Hum. Vaccines Immunother. 2020, 16, 2328–2332. [Google Scholar] [CrossRef]
- Yeong, J.; Lim, J.C.T.; Lee, B.; Li, H.; Chia, N.; Ong, C.C.H.; Lye, W.K.; Putti, T.C.; Dent, R.; Lim, E.; et al. High Densities of Tumor-Associated Plasma Cells Predict Improved Prognosis in Triple Negative Breast Cancer. Front. Immunol. 2018, 9, 1209. [Google Scholar] [CrossRef]
- Brynjolfsson, S.F.; Persson Berg, L.; Olsen Ekerhult, T.; Rimkute, I.; Wick, M.J.; Martensson, I.L.; Grimsholm, O. Long-Lived Plasma Cells in Mice and Men. Front. Immunol. 2018, 9, 2673. [Google Scholar] [CrossRef] [Green Version]
- Hollern, D.P.; Xu, N.; Thennavan, A.; Glodowski, C.; Garcia-Recio, S.; Mott, K.R.; He, X.; Garay, J.P.; Carey-Ewend, K.; Marron, D.; et al. B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer. Cell 2019, 179, 1191–1206.e1121. [Google Scholar] [CrossRef]
- Li, Q.; Lao, X.; Pan, Q.; Ning, N.; Yet, J.; Xu, Y.; Li, S.; Chang, A.E. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin. Cancer Res. 2011, 17, 4987–4995. [Google Scholar] [CrossRef] [Green Version]
- Wortman, J.C.; He, T.F.; Solomon, S.; Zhang, R.Z.; Rosario, A.; Wang, R.; Tu, T.Y.; Schmolze, D.; Yuan, Y.; Yost, S.E.; et al. Spatial distribution of B cells and lymphocyte clusters as a predictor of triple-negative breast cancer outcome. NPJ Breast Cancer 2021, 7, 84. [Google Scholar] [CrossRef]
- Schettini, F.; Paré, L.; Marín-Aguilera, M.; Puig, S.; Mezquita, L.; Baste, N.; Pascual, T.; Martinez Saez, O.; Conte, B.; Jares, P.; et al. 14-gene immunoglobulin (IGG) and proliferation signatures and association with overall survival across cancer-types. J. Clin. Oncol. 2022, 40, 2636. [Google Scholar] [CrossRef]
- Prat, A.; Guarneri, V.; Pascual, T.; Braso-Maristany, F.; Sanfeliu, E.; Pare, L.; Schettini, F.; Martinez, D.; Jares, P.; Griguolo, G.; et al. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine 2022, 75, 103801. [Google Scholar] [CrossRef]
- Garaud, S.; Zayakin, P.; Buisseret, L.; Rulle, U.; Silina, K.; de Wind, A.; Van den Eyden, G.; Larsimont, D.; Willard-Gallo, K.; Line, A. Antigen Specificity and Clinical Significance of IgG and IgA Autoantibodies Produced in situ by Tumor-Infiltrating B Cells in Breast Cancer. Front. Immunol. 2018, 9, 2660. [Google Scholar] [CrossRef]
- Yang, B.; Ma, C.; Chen, Z.; Yi, W.; McNutt, M.A.; Wang, Y.; Korteweg, C.; Gu, J. Correlation of immunoglobulin G expression and histological subtype and stage in breast cancer. PLoS ONE 2013, 8, e58706. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zheng, J.; Guo, J.; Zhang, Q.; Du, J.; Zhao, X.; Wang, Z.; Liao, Q. SIA-IgG confers poor prognosis and represents a novel therapeutic target in breast cancer. Bioengineered 2022, 13, 10072–10087. [Google Scholar] [CrossRef]
- Kdimati, S.; Mullins, C.S.; Linnebacher, M. Cancer-Cell-Derived IgG and Its Potential Role in Tumor Development. Int. J. Mol. Sci. 2021, 22, 11597. [Google Scholar] [CrossRef]
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018, 174, 1293–1308.e36. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063. [Google Scholar] [CrossRef]
- Deng, J.; Thennavan, A.; Shah, S.; Bagdatlioglu, E.; Klar, N.; Heguy, A.; Marier, C.; Meyn, P.; Zhang, Y.; Labbe, K.; et al. Serial single-cell profiling analysis of metastatic TNBC during Nab-paclitaxel and pembrolizumab treatment. Breast Cancer Res. Treat. 2021, 185, 85–94. [Google Scholar] [CrossRef]
- Hu, L.; Su, L.; Cheng, H.; Mo, C.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; et al. Single-Cell RNA Sequencing Reveals the Cellular Origin and Evolution of Breast Cancer in BRCA1 Mutation Carriers. Cancer Res. 2021, 81, 2600–2611. [Google Scholar] [CrossRef]
- Wu, S.Z.; Al-Eryani, G.; Roden, D.L.; Junankar, S.; Harvey, K.; Andersson, A.; Thennavan, A.; Wang, C.; Torpy, J.R.; Bartonicek, N.; et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet. 2021, 53, 1334–1347. [Google Scholar] [CrossRef]
- Xu, K.; Wang, R.; Xie, H.; Hu, L.; Wang, C.; Xu, J.; Zhu, C.; Liu, Y.; Gao, F.; Li, X.; et al. Single-cell RNA sequencing reveals cell heterogeneity and transcriptome profile of breast cancer lymph node metastasis. Oncogenesis 2021, 10, 66. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Mo, H.; Hu, X.; Gao, R.; Zhao, Y.; Liu, B.; Niu, L.; Sun, X.; Yu, X.; et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell 2021, 39, 1578–1593.e1578. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhang, W.; Wang, C.; Hu, L.; Wang, R.; Wang, C.; Tang, L.; Zhou, G.; Zou, B.; Xie, H.; et al. Integrative analyses of scRNA-seq and scATAC-seq reveal CXCL14 as a key regulator of lymph node metastasis in breast cancer. Hum. Mol. Genet. 2021, 30, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Li, C.; Cao, L.; Chen, Y.; Ren, C.; Chen, X.; Mok, H.; Wen, L.; Li, K.; Wang, Y.; et al. Single-cell profile of tumor and immune cells in primary breast cancer, sentinel lymph node, and metastatic lymph node. Breast Cancer 2022, 30, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Xiong, Q.; Yan, M.; Zhan, X.; Yang, Z.; Peng, C.; Sun, B.; Pang, D.; Liu, T. Intratumor expanded T cell clones can be non-sentinel lymph node derived in breast cancer revealed by single-cell immune profiling. J. Immunother. Cancer 2022, 10, e003325. [Google Scholar] [CrossRef]
- Mao, X.; Zhou, D.; Lin, K.; Zhang, B.; Gao, J.; Ling, F.; Zhu, L.; Yu, S.; Chen, P.; Zhang, C.; et al. Single-cell and spatial transcriptome analyses revealed cell heterogeneity and immune environment alternations in metastatic axillary lymph nodes in breast cancer. Cancer Immunol. Immunother. 2022. [Google Scholar] [CrossRef]
- Liu, T.; Liu, C.; Yan, M.; Zhang, L.; Zhang, J.; Xiao, M.; Li, Z.; Wei, X.; Zhang, H. Single cell profiling of primary and paired metastatic lymph node tumors in breast cancer patients. Nat. Commun. 2022, 13, 6823. [Google Scholar] [CrossRef]
- Fu, T.; Dai, L.J.; Wu, S.Y.; Xiao, Y.; Ma, D.; Jiang, Y.Z.; Shao, Z.M. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J. Hematol. Oncol. 2021, 14, 98. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, L.; Li, S.; Gu, J.; Cui, X.; Zhou, Y. Pan-cancer analysis of genomic properties and clinical outcome associated with tumor tertiary lymphoid structure. Sci. Rep. 2020, 10, 21530. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Han, Y.; Deng, Y.; Li, J.; Jiang, Y. The Presence of Tertiary Lymphoid Structures Provides New Insight Into the Clinicopathological Features and Prognosis of Patients With Breast Cancer. Front. Immunol. 2022, 13, 868155. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, I.A.; Song, I.H.; Shin, S.J.; Kim, J.Y.; Yu, J.H.; Gong, G. Tertiary lymphoid structures: Prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J. Clin. Pathol. 2016, 69, 422–430. [Google Scholar] [CrossRef]
- Ukita, M.; Hamanishi, J.; Yoshitomi, H.; Yamanoi, K.; Takamatsu, S.; Ueda, A.; Suzuki, H.; Hosoe, Y.; Furutake, Y.; Taki, M.; et al. CXCL13-producing CD4+ T cells accumulate in the early phase of tertiary lymphoid structures in ovarian cancer. JCI Insight 2022, 7, e157215. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; de Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef] [Green Version]
- Jacquelot, N.; Tellier, J.; Sl, N.; Gt, B. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology 2021, 10, 1900508. [Google Scholar] [CrossRef]
- Martinet, L.; Garrido, I.; Filleron, T.; Le Guellec, S.; Bellard, E.; Fournie, J.J.; Rochaix, P.; Girard, J.P. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011, 71, 5678–5687. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Elson, C.O.; Cong, Y. Treg cell-IgA axis in maintenance of host immune homeostasis with microbiota. Int. Immunopharmacol. 2011, 11, 589–592. [Google Scholar] [CrossRef] [Green Version]
- Quintana, Á.; Peg, V.; Prat, A.; Moliné, T.; Villacampa, G.; Paré, L.; Galván, P.; Dientsmann, R.; Schmid, P.; Curigliano, G.; et al. Immune analysis of lymph nodes in relation to the presence or absence of tumor infiltrating lymphocytes in triple-negative breast cancer. Eur. J. Cancer 2021, 148, 134–145. [Google Scholar] [CrossRef]
- Weinstein, A.M.; Storkus, W.J. Therapeutic Lymphoid Organogenesis in the Tumor Microenvironment. Adv. Cancer Res. 2015, 128, 197–233. [Google Scholar] [CrossRef] [Green Version]
- Nzula, S.; Going, J.J.; Stott, D.I. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res. 2003, 63, 3275–3280. [Google Scholar]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Overview: Pembrolizumab for Untreated PD-L1-Positive Metastatic Non-Small-Cell Lung Cancer: Guidance. Available online: https://www.nice.org.uk/guidance/ta531 (accessed on 10 December 2022).
- Tonellotto, F.; Bergmann, A.; de Souza Abrahao, K.; de Aguiar, S.S.; Bello, M.A.; Thuler, L.C.S. Impact of Number of Positive Lymph Nodes and Lymph Node Ratio on Survival of Women with Node-Positive Breast Cancer. Eur. J. Breast Health 2019, 15, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hardiman, T.; Wu, K.; Quist, J.; Gazinska, P.; Ng, T.; Purushotham, A.; Salgado, R.; Guo, X.; Pinder, S.E.; et al. Systemic immune reaction in axillary lymph nodes adds to tumor-infiltrating lymphocytes in triple-negative breast cancer prognostication. NPJ Breast Cancer 2021, 7, 86. [Google Scholar] [CrossRef]
- Grigoriadis, A.; Gazinska, P.; Pai, T.; Irhsad, S.; Wu, Y.; Millis, R.; Naidoo, K.; Owen, J.; Gillett, C.E.; Tutt, A.; et al. Histological scoring of immune and stromal features in breast and axillary lymph nodes is prognostic for distant metastasis in lymph node-positive breast cancers. J. Pathol. Clin. Res. 2018, 4, 39–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verghese, G.; Li, M.; Lohan, A.; Cherian, N.; Rane, S.; Liu, F.; Shah, A.; Gazinska, P.; Thavaraj, S.; Sethi, A.; et al. Abstract 6233: A deep learning pipeline to capture the prognostic immune responses in lymph nodes of breast cancer patients. Cancer Res. 2022, 82, 6233. [Google Scholar] [CrossRef]
- Rakaee, M.; Adib, E.; Ricciuti, B.; Sholl, L.M.; Shi, W.; Alessi, J.V.M.; Cortellini, A.; Fulgenzi, C.A.M.; Pinato, D.J.J.; Hashemi, S.M.; et al. Artificial intelligence in digital pathology approach identifies the predictive impact of tertiary lymphoid structures with immune-checkpoints therapy in NSCLC. J. Clin. Oncol. 2022, 40, 9065. [Google Scholar] [CrossRef]
- Matos-Cruz, V.; Sargent, R.; Chinnaobireddy, V.; Pouryahya, M.; Lee, G.; Fahy, D.; Kirkup, C.; Sucipto, K.; Gullapally, S.; Brosnan-Cashman, J.; et al. 1291 A multi-tumor machine learning model to identify tertiary lymphoid structures in histopathological H&E images as a potential clinical biomarker. J. ImmunoTher. Cancer 2022, 10, A1339. [Google Scholar] [CrossRef]
- Barmpoutis, P.; Di Capite, M.; Kayhanian, H.; Waddingham, W.; Alexander, D.C.; Jansen, M.; Kwong, F.N.K. Tertiary lymphoid structures (TLS) identification and density assessment on H&E-stained digital slides of lung cancer. PLoS ONE 2021, 16, e0256907. [Google Scholar] [CrossRef]
- Shariati, S.; Mehdipour, F.; Samadi, M.; Rasolmali, R.; Talei, A.R.; Ghaderi, A. The balance of regulatory and stimulatory B cell subsets in breast cancer draining lymph nodes correlates with tumor prognostic factors. Life Sci. 2020, 257, 118117. [Google Scholar] [CrossRef]
- Wennhold, K.; Weber, T.M.; Klein-Gonzalez, N.; Thelen, M.; Garcia-Marquez, M.; Chakupurakal, G.; Fiedler, A.; Schlosser, H.A.; Fischer, R.; Theurich, S.; et al. CD40-activated B cells induce anti-tumor immunity in vivo. Oncotarget 2017, 8, 27740–27753. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, J.R.; Pero, S.C.; Voss, W.N.; Shukla, G.S.; Sun, Y.; Schaetzle, S.; Lee, C.H.; Horton, A.P.; Harlow, S.; Gollihar, J.; et al. Identification of tumor-reactive B cells and systemic IgG in breast cancer based on clonal frequency in the sentinel lymph node. Cancer Immunol. Immunother. 2018, 67, 729–738. [Google Scholar] [CrossRef]
- Kleshchevnikov, V.; Shmatko, A.; Dann, E.; Aivazidis, A.; King, H.W.; Li, T.; Elmentaite, R.; Lomakin, A.; Kedlian, V.; Gayoso, A.; et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat. Biotechnol. 2022, 40, 661–671. [Google Scholar] [CrossRef]
- Andersson, A.; Larsson, L.; Stenbeck, L.; Salmen, F.; Ehinger, A.; Wu, S.Z.; Al-Eryani, G.; Roden, D.; Swarbrick, A.; Borg, A.; et al. Spatial deconvolution of HER2-positive breast cancer delineates tumor-associated cell type interactions. Nat. Commun. 2021, 12, 6012. [Google Scholar] [CrossRef]
- Tripodo, C.; Zanardi, F.; Iannelli, F.; Mazzara, S.; Vegliante, M.; Morello, G.; Di Napoli, A.; Mangogna, A.; Facchetti, F.; Sangaletti, S.; et al. A Spatially Resolved Dark- versus Light-Zone Microenvironment Signature Subdivides Germinal Center-Related Aggressive B Cell Lymphomas. iScience 2020, 23, 101562. [Google Scholar] [CrossRef]
- Fernandez-Martinez, A.; Tanioka, M.; Fan, C.; Parker, J.S.; Hoadley, K.A.; Krop, I.; Partridge, A.; Carey, L.; Perou, C.M. 174O—Predictive and prognostic value of B-cell gene-expression signatures and B-cell receptor (BCR) repertoire in HER2+ breast cancer: A correlative analysis of the CALGB 40601 clinical trial (Alliance). Ann. Oncol. 2019, 30, v55. [Google Scholar] [CrossRef]
- De Angelis, C.; Nagi, C.; Hoyt, C.C.; Liu, L.; Roman, K.; Wang, C.; Zheng, Y.; Veeraraghavan, J.; Sethunath, V.; Nuciforo, P.; et al. Evaluation of the Predictive Role of Tumor Immune Infiltrate in Patients with HER2-Positive Breast Cancer Treated with Neoadjuvant Anti-HER2 Therapy without Chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 738–745. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Quintana, A.; Milisenda, J.C.; Casal-Dominguez, M.; Muñoz-Braceras, S.; Derfoul, A.; Torres-Ruiz, J.; Pak, K.; Del Orso, S.; Naz, F.; et al. Transcriptomic profiling reveals distinct subsets of immune checkpoint inhibitor-induced myositis. Ann. Rheum. Dis. 2023. [Google Scholar] [CrossRef]
- Ghosh, N.; Chan, K.K.; Jivanelli, B.; Bass, A.R. Autoantibodies in Patients With Immune-Related Adverse Events From Checkpoint Inhibitors: A Systematic Literature Review. J. Clin. Rheumatol. 2022, 28, e498–e505. [Google Scholar] [CrossRef]
- Das, R.; Bar, N.; Ferreira, M.; Newman, A.M.; Zhang, L.; Bailur, J.K.; Bacchiocchi, A.; Kluger, H.; Wei, W.; Halaban, R.; et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Investig. 2018, 128, 715–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammen, A.L.; Rajan, A.; Pak, K.; Lehky, T.; Casciola-Rosen, L.; Donahue, R.N.; Lepone, L.M.; Zekeridou, A.; Pittock, S.J.; Hassan, R.; et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann. Rheum. Dis. 2019, 78, 150–152. [Google Scholar] [CrossRef] [PubMed]
- De Martin, E.; Michot, J.-M.; Papouin, B.; Champiat, S.; Mateus, C.; Lambotte, O.; Roche, B.; Antonini, T.M.; Coilly, A.; Laghouati, S.; et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J. Hepatol. 2018, 68, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Foster, R.E.; Horgan, K.; Mounsey, K.; Nixon, H.; Smalle, N.; Hughes, T.A.; Carter, C.R. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Tashireva, L.A.; Popova, N.O.; Kalinchuk, A.Y.; Goldberg, V.E.; Kovalenko, E.I.; Artamonova, E.V.; Manikhas, A.G.; Ponomarenko, D.M.; Levchenko, N.V.; Rossokha, E.I.; et al. B Lymphocytes Are a Predictive Marker of Eribulin Response and Overall Survival in Locally Advanced or Metastatic Breast Cancer: A Multicenter, Two-Cohort, Non-Randomized, Open-Label, Retrospective Study. Front. Oncol. 2022, 12, 909505. [Google Scholar] [CrossRef]
- Song, I.H.; Heo, S.H.; Bang, W.S.; Park, H.S.; Park, I.A.; Kim, Y.A.; Park, S.Y.; Roh, J.; Gong, G.; Lee, H.J. Predictive Value of Tertiary Lymphoid Structures Assessed by High Endothelial Venule Counts in the Neoadjuvant Setting of Triple-Negative Breast Cancer. Cancer Res. Treat. 2017, 49, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, A.; Horimoto, Y.; Onagi, H.; Ikarashi, D.; Nakayama, T.; Nakatsura, T.; Shimizu, H.; Kojima, K.; Yao, T.; Matsumoto, T.; et al. Plasma cell infiltration and treatment effect in breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res. 2021, 23, 99. [Google Scholar] [CrossRef]
- Brown, J.R.; Wimberly, H.; Lannin, D.R.; Nixon, C.; Rimm, D.L.; Bossuyt, V. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin. Cancer Res. 2014, 20, 5995–6005. [Google Scholar] [CrossRef] [Green Version]
- Miligy, I.; Mohan, P.; Gaber, A.; Aleskandarany, M.A.; Nolan, C.C.; Diez-Rodriguez, M.; Mukherjee, A.; Chapman, C.; Ellis, I.O.; Green, A.R.; et al. Prognostic significance of tumour infiltrating B lymphocytes in breast ductal carcinoma in situ. Histopathology 2017, 71, 258–268. [Google Scholar] [CrossRef]
- Xu, Y.; Lan, S.; Zheng, Q. Prognostic significance of infiltrating immune cell subtypes in invasive ductal carcinoma of the breast. Tumori 2018, 104, 196–201. [Google Scholar] [CrossRef]
- Lundberg, A.; Li, B.; Li, R. B cell-related gene signature and cancer immunotherapy response. Br. J. Cancer 2022, 126, 899–906. [Google Scholar] [CrossRef]
- Barone, F.; Nayar, S.; Campos, J.; Cloake, T.; Withers, D.R.; Toellner, K.M.; Zhang, Y.; Fouser, L.; Fisher, B.; Bowman, S.; et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc. Natl. Acad. Sci. USA 2015, 112, 11024–11029. [Google Scholar] [CrossRef] [Green Version]
- Sautes-Fridman, C.; Lawand, M.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Dieu-Nosjean, M.C. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front. Immunol. 2016, 7, 407. [Google Scholar] [CrossRef] [Green Version]
- Good-Jacobson, K.L.; Szumilas, C.G.; Chen, L.; Sharpe, A.H.; Tomayko, M.M.; Shlomchik, M.J. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 2010, 11, 535–542. [Google Scholar] [CrossRef]
- Banna, G.L.; Cantale, O.; Bersanelli, M.; Del Re, M.; Friedlaender, A.; Cortellini, A.; Addeo, A. Are anti-PD1 and anti-PD-L1 alike? The non-small-cell lung cancer paradigm. Oncol. Rev. 2020, 14, 490. [Google Scholar] [CrossRef]
- Musolino, A.; Gradishar, W.J.; Rugo, H.S.; Nordstrom, J.L.; Rock, E.P.; Arnaldez, F.; Pegram, M.D. Role of Fcgamma receptors in HER2-targeted breast cancer therapy. J. Immunother. Cancer 2022, 10, e003171. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, D.; Cai, L.; Yao, H.; Yan, M.; Wang, X.; Shen, W.; Du, Y.; Pang, H.; Lai, X.; et al. First-in-human HER2-targeted Bispecific Antibody KN026 for the Treatment of Patients with HER2-positive Metastatic Breast Cancer: Results from a Phase I Study. Clin. Cancer Res. 2022, 28, 618–628. [Google Scholar] [CrossRef]
- Sharma, M.; Carvajal, R.D.; Hanna, G.J.; Li, B.T.; Moore, K.N.; Pegram, M.D.; Rasco, D.W.; Spira, A.I.; Alonso, M.; Fang, L.; et al. Preliminary results from a phase 1/2 study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. J. Clin. Oncol. 2021, 39, 2549. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yu, K.D. Breast Cancer Vaccines: Disappointing or Promising? Front. Immunol. 2022, 13, 828386. [Google Scholar] [CrossRef]
| Clinical Trial | # Patients and Cancer Type | Treatment | Prognostic Value | Predictive Value | Reference |
|---|---|---|---|---|---|
| BIG 02-98 | 136 HER2+ and 113 TNBC | Adjuvant CT (not specified) HER2+ patients also received trastuzumab | B cell levels correlated with longer disease-free and overall survival | Not reported | Garaud et al. [16] |
| CALGB 40601 | 256 HER2+ | Neoadjuvant paclitaxel and trastuzumab +/− lapatinib | Patients with high IGHG counts had a greater 5-year disease-free survival benefit | High IgG-signature (especially the presence of IGHG) showed a significantly higher pCR rate when treated with both anti-HER2 treatments | Fernandez-Martinez et al. [96] |
| TBCRC006 | 59 HR+/HR- HER2+ | Lapatinib plus trastuzumab HR+ also received endocrine therapy | Not reported | High stromal and intratumoral CD20+ cells were independently associated with a higher pCR rate | De Angelis et al. [97] |
| Cohort study | 30 TNBC | Eribulin | PD-1-neg/PD-1-pos B cell ratio >3 was an independent prognostic factor for worse overall survival | PD-1-neg/PD-1-pos B cell ratio >3 was an independent predictor of shorter response to eribulin (short-term and long-term responders had comparable numbers of B cells levels at their tumours) | Tashireva et al. [108] |
| Cohort study | 368 all breast cancer subtypes (first cohort) 683 all breast cancer subtypes (second cohort) 103 HER2+ cohort | Neoadjuvant CT (doxorubicin + cyclophosphamide followed by paclitaxel, or cyclophosphamide + docetaxel) HER2+ patients also received trastuzumab | B cells induced anti-tumour T cell immunity by upregulating ICOSL and CR2, and downregulating IL-10 after neoadjuvant CT, which is associated with improved prognosis, especially in TNBC | CD55 expression was significantly lower in patients who responded to chemotherapy, which is thought to inhibit the complement and ICOSL | Lu et al. [44] |
| Cohort study | 108 TNBC | Neoadjuvant CT (adriamycin + cyclophosphamide followed by docetaxel) | TLS presence was not associated with overall survival. CXCL13 gene expression correlated with overall survival | HEV density (MECA79+), TLS, B cells, and CXCL13 correlated with each other and with pCR | Song et al. [109] |
| Cohort study | 146 all breast cancer subtypes 71 HR- (validation cohort) | Neoadjuvant CT (cyclophosphamide + epirubicin + fluorouracil (CEF) followed by paclitaxel or docetaxel. Some patients only received CEF, paclitaxel, or docetaxel) | In HR- tumours, high plasma cell infiltration was associated with significantly longer disease-free survival | Tumours with high plasma cells and B cells were associated with pCR There was a higher expression of both PD-1 and PD-L1 on B cells from patients with pCR | Sakaguchi et al. [110] |
| Cohort study | 95 all breast cancer subtypes | Neoadjuvant CT (a combination of different regiments of: adriamycin, (nab)paclitaxel, docetaxel, carboplatin, vinorelbine, and etoposide) +/− bevacizumab (aVEGF) HER2+ patients also received trastuzumab | Not reported | Automate quantitative analysis of CD20 in patient’s tumour independently predicted pCR Patients with tumours displaying high CD20 expression had 5.5 times more pCR | Brown et al. [111] |
| Cohort study | 114 TNBC | Not reported | Stromal CD20 and CD38 correlated with disease-free and overall survival. Intratumoral CD38 and CD138, and stromal CD138 did not correlate with disease-free survival | Not reported | Kuroda et al. [15] |
| Cohort study | 80 DCIS cases (36 pure DCIS and 44 mixed with invasive cancer) | Not reported | Patients with pure DCIS showed a higher number of B cells and lymphoid aggregates than patients with DCIS associated with invasive cancers. Pure DCIS associated with higher numbers of B lymphocytes had shorter recurrence-free interval, but this association was not significant with CD138+ plasma cell count | Not reported | Miligy et al. [112] |
| Cohort study | 102 all breast cancer subtypes | Not reported | Patients with CD20 cells at the primary lesion had better disease-free survival and overall survival | Not reported | Xu et al. [113] |
| Cohort study | 766 TNBC (discovery cohort) 1247 Lung, 1247 colorectal, and 325 melanoma (validation cohort) | Nivolumab (aPD-1) Ipilimumab (aCTLA-4) | Patients with B cells at primary carcinoma had better prognosis in overall survival only when a B cell signature of 9 cytokines (CXCR6, IL18RAP, LCK, IL2RG, CXCL13, PSMB10, TNFRSF14, BATF, TNFRSF4) was low (discovery cohort) | Low levels of the 9 cytokines B cell signature are predictive of response to immunotherapy and have an impact on overall survival (validation cohort) | Lundberg et al. [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Quintana, A.; Alberts, E.; Hung, M.S.; Boulat, V.; Ripoll, M.M.; Grigoriadis, A. B Cells in Breast Cancer Pathology. Cancers 2023, 15, 1517. https://doi.org/10.3390/cancers15051517
Li M, Quintana A, Alberts E, Hung MS, Boulat V, Ripoll MM, Grigoriadis A. B Cells in Breast Cancer Pathology. Cancers. 2023; 15(5):1517. https://doi.org/10.3390/cancers15051517
Chicago/Turabian StyleLi, Mengyuan, Angela Quintana, Elena Alberts, Miu Shing Hung, Victoire Boulat, Mercè Martí Ripoll, and Anita Grigoriadis. 2023. "B Cells in Breast Cancer Pathology" Cancers 15, no. 5: 1517. https://doi.org/10.3390/cancers15051517
APA StyleLi, M., Quintana, A., Alberts, E., Hung, M. S., Boulat, V., Ripoll, M. M., & Grigoriadis, A. (2023). B Cells in Breast Cancer Pathology. Cancers, 15(5), 1517. https://doi.org/10.3390/cancers15051517







