The Transcription Factor Twist1 Has a Significant Role in Mycosis Fungoides (MF) Cell Biology: An RNA Sequencing Study of 40 MF Cases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Material
2.2. Immunohistochemical Staining, Analysis, and Correlation with Disease Presentation and Outcome
2.3. Microdissection and RNA Extraction
2.4. The TWIST1 Promoter Methylation Analysis
2.5. RNA Sequencing Data Analysis
2.6. Read Counts and Principal Component Analysis
2.7. Differential Expression Analyses
2.8. Ingenuity Pathway Analysis
2.9. Hub Gene Analysis
3. Results
3.1. Patients
3.2. Immunohistochemistry of Twist1 and Zeb1, Correlation with Histomorphology, Disease Presentation and Outcome
3.3. The Analysis of TWIST1 Promoter Methylation
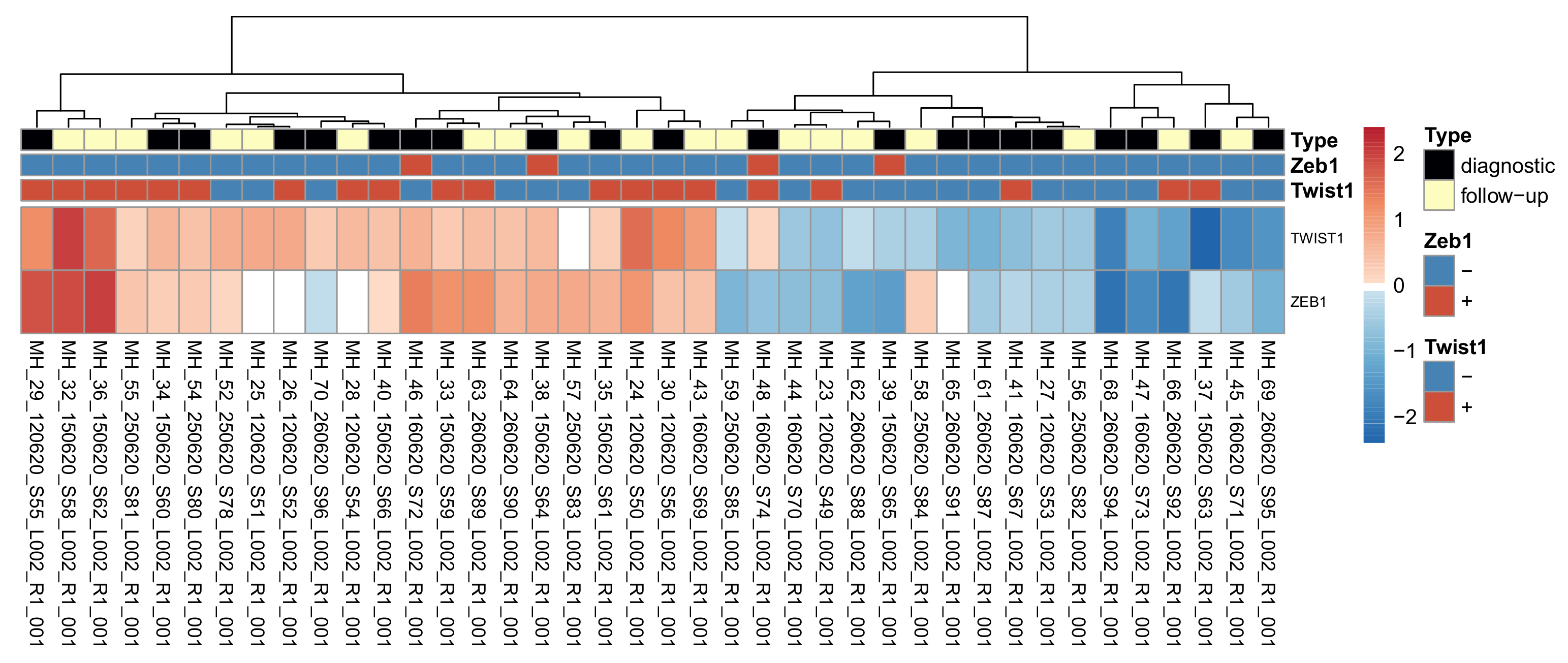
3.4. The Association between Twist1 and Zeb1 Protein Levels and RNA Levels
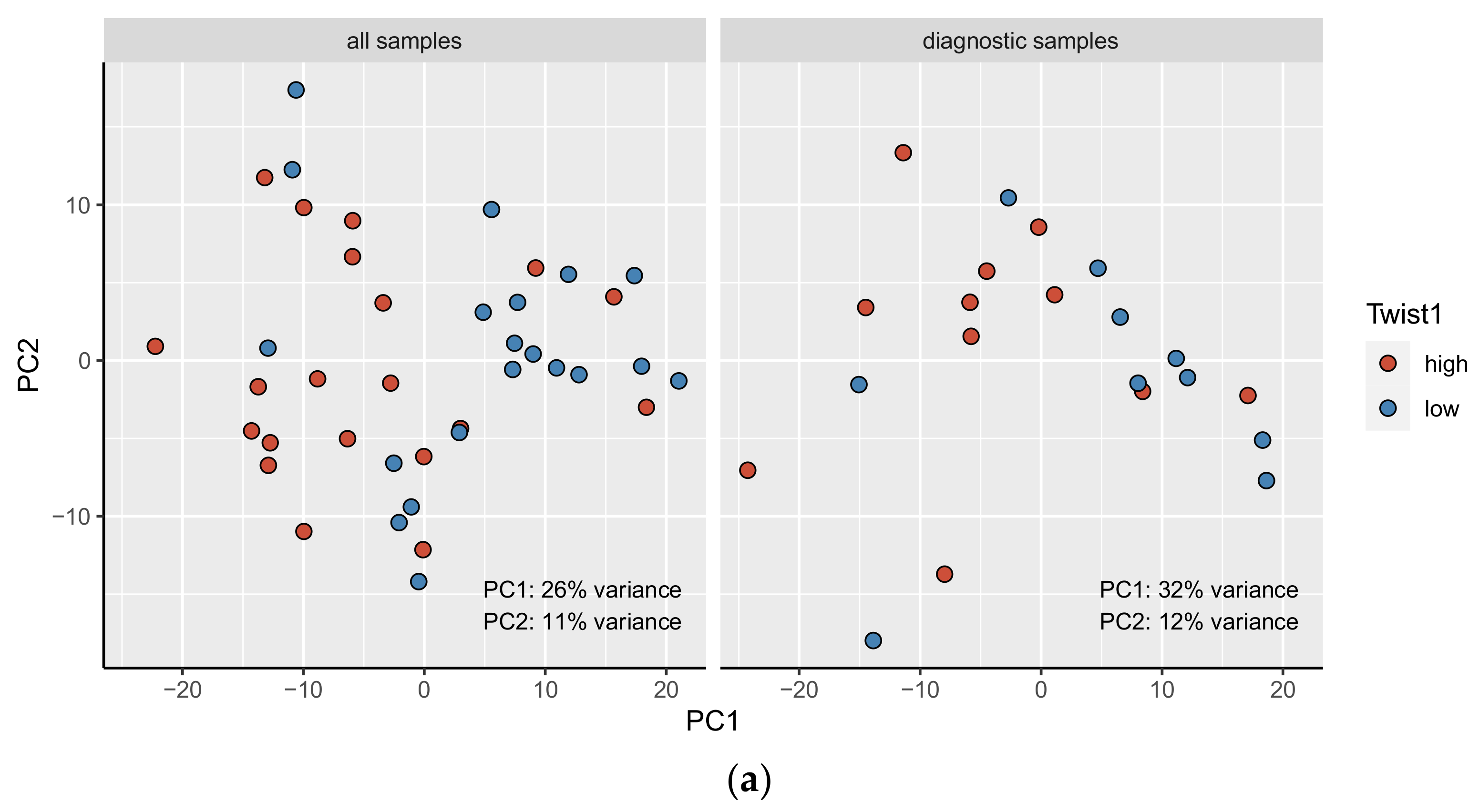
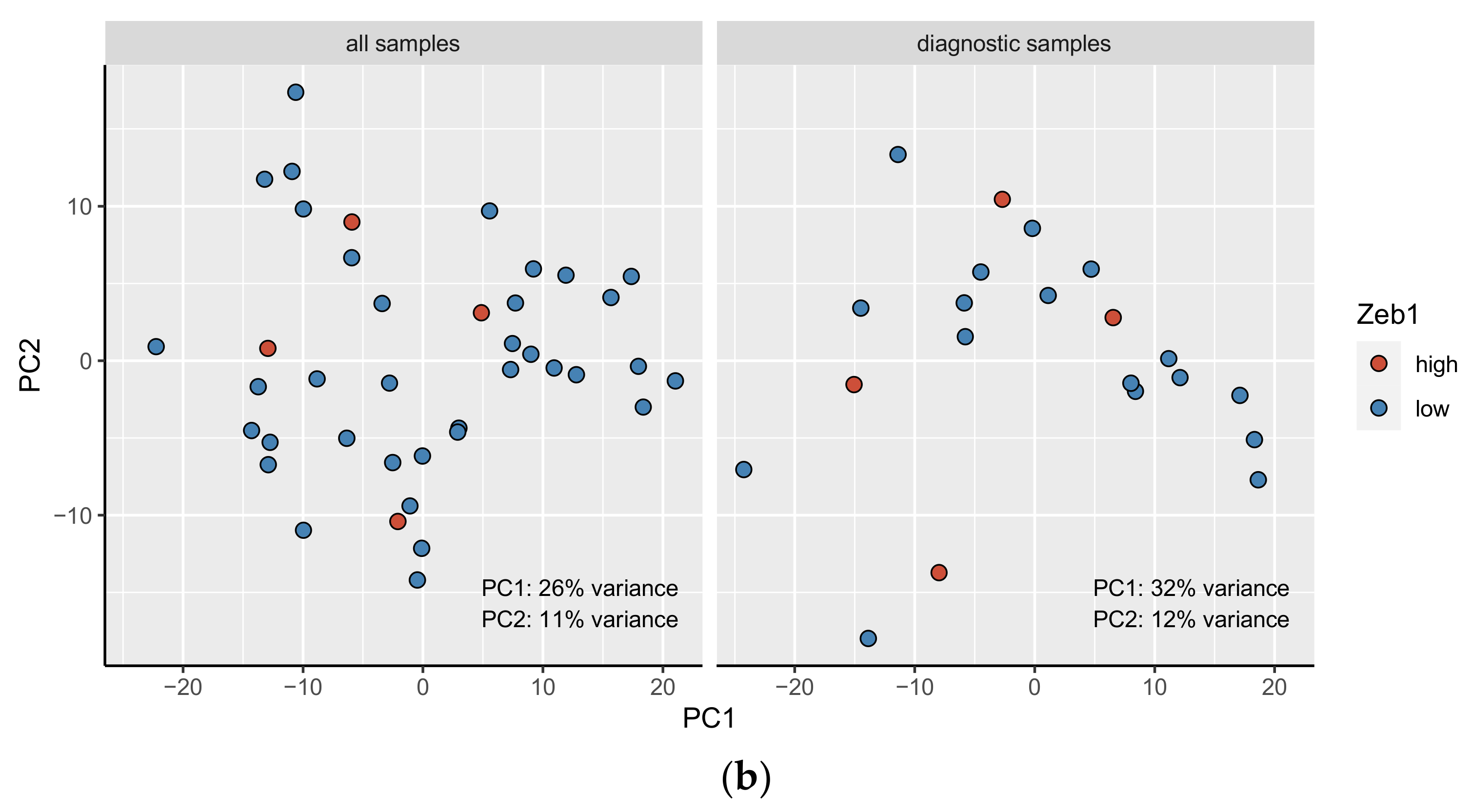
3.5. The Principal Component Analysis (PCA)
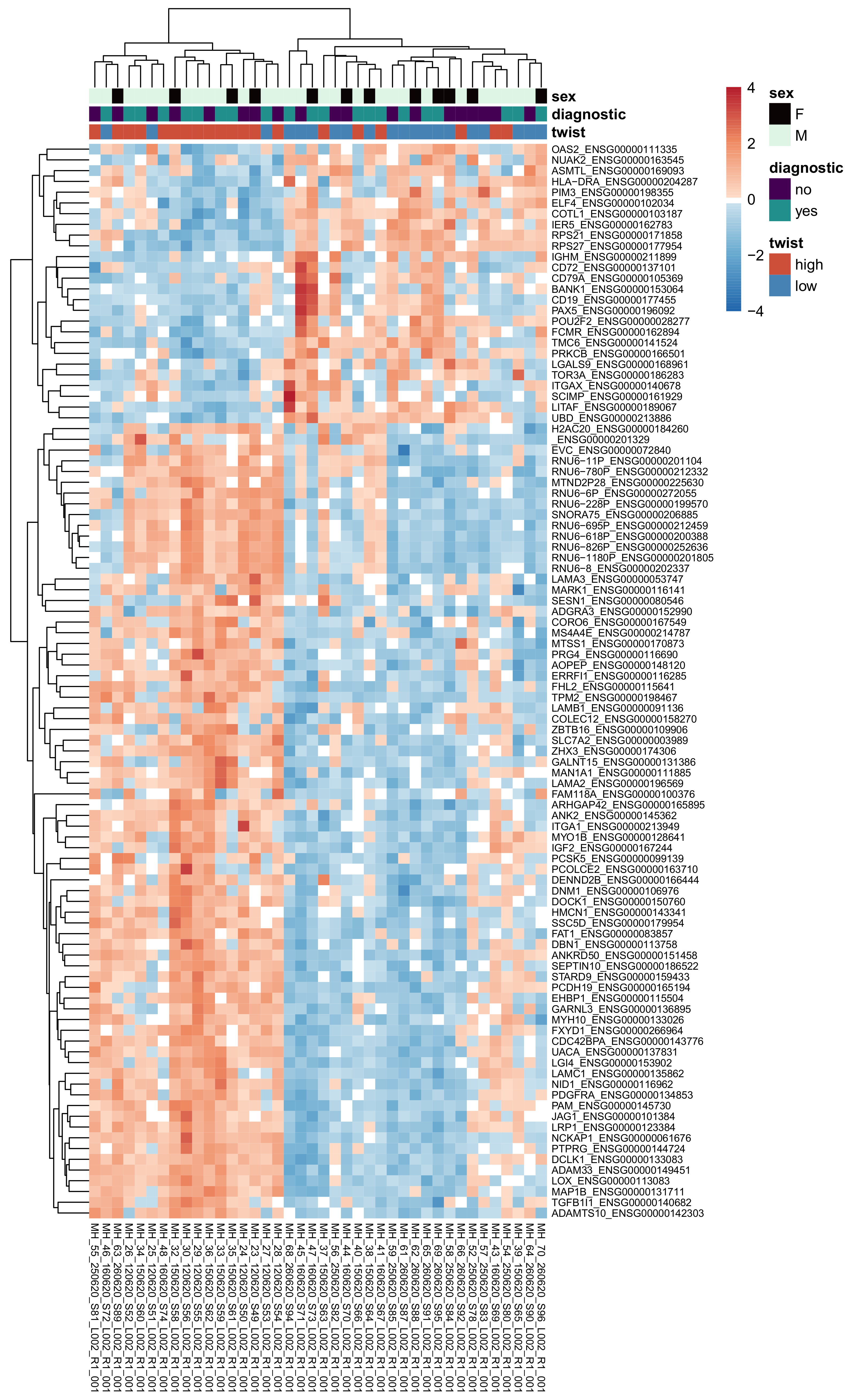
3.6. High vs. Low Twist1 and Zeb1—Differential Expression Analysis
3.7. High vs. Low Twist1—Ingenuity Pathway Analysis
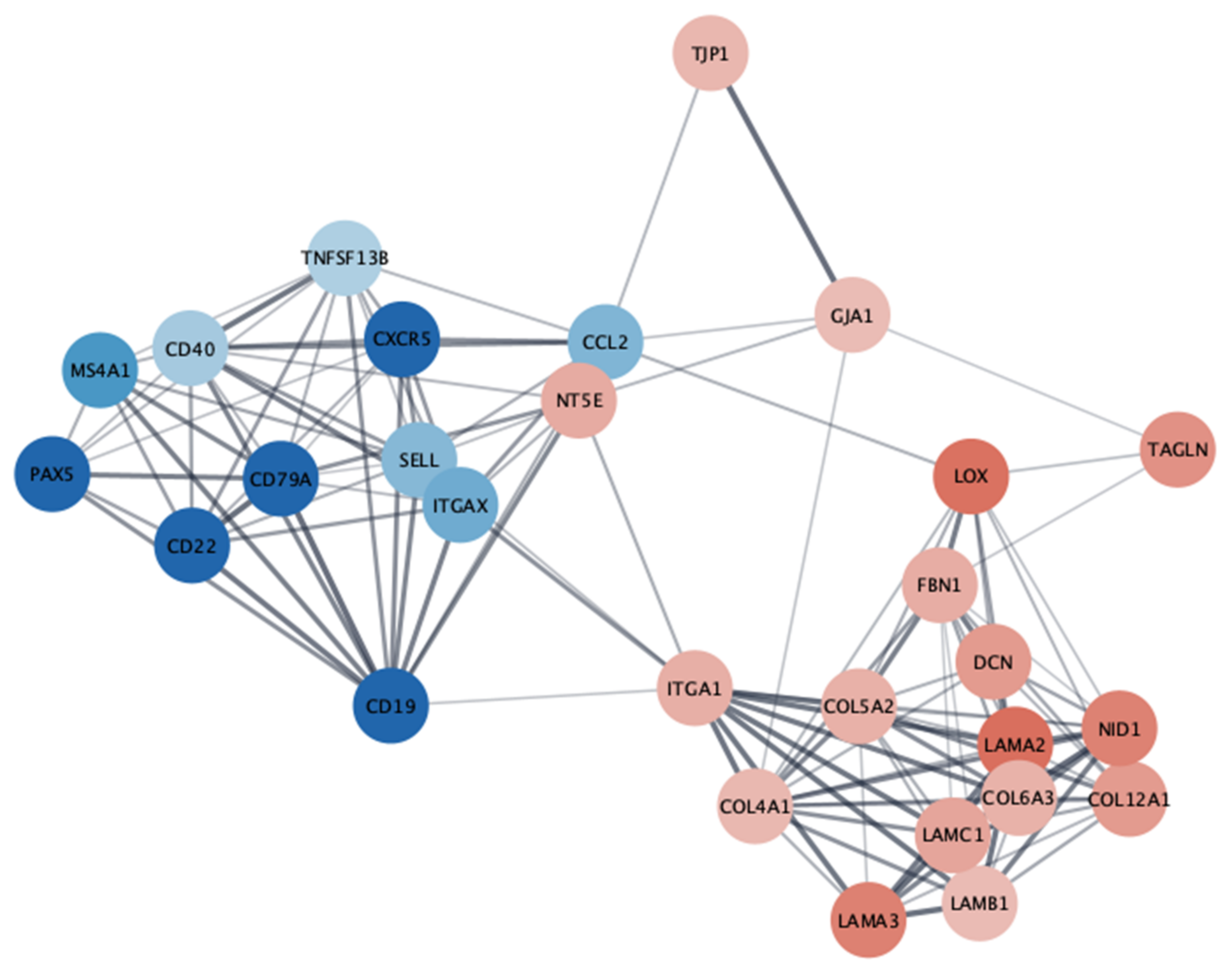
3.8. Hub Gene Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| AML | Acute myeloid leukemia |
| bHLH | Basic helix-loop-helix |
| CCL2 | C-C motif chemokine ligand2 |
| CD | Cluster of differentiation |
| CDK5 | Cyclin-dependent kinase 5 |
| CK2 | Casein kinase 2 |
| COL5A2 | Collagen type V alpha 2 chain |
| CTCL | Primary cutaneous T-cell lymphoma |
| CXCR5 | C-X-C chemokine receptor type 5 |
| DCN | Decorin |
| DE | Differential expression |
| EGFR | Epidermal growth factor receptor |
| eIF4 | Eukaryotic translation initiation factor 4E |
| EMT | Epithelia-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| FBLN5 | Fibulin 5 |
| FBN1 | Fibrillin 1 |
| FCER1G | Fc epsilon receptor Ig |
| FFPE | Formalin-fixed, paraffin-embedded |
| FZD | Frizzled |
| GJA1 | Gap junction alpha-1 protein |
| GP6 | Glycoprotein VI |
| HGF | Hepatocyte growth factor |
| HIF-1 | Hypoxia inducible factor-1 |
| HLA-A | Major histocompatibility complex, class I, A |
| HLA-DRA | Major histocompatibility complex, class II, DR Alpha |
| HMGA2 | High-mobility group AT-hook 2 |
| IGHM | Immunoglobulin heavy constant |
| IHC | Immunohistochemistry |
| ILK | Integrin-linked kinase |
| IL-7 | Interleukin 7 |
| ITGA1 | Integrin alpha 1 |
| ITGAX | Integrin subunit alpha X |
| LAMA | Laminin subunit alpha 1 |
| LAMB | Laminin subunit beeta |
| LAMC | Laminin subunit gamma |
| LCM | Laser capture microdissection |
| LDH | Lactate dehydrogenase |
| LGALS9 | Galectin 9 |
| LITAF | Lipopolysaccharide induced TNF factor |
| LOX | Lysyl oxidase |
| LYZ | Lysozyme |
| MAPK | Mitogen activated protein kinase |
| MF | Mycosis fungoides |
| MSX2 | Muscle segment homeobox 2 |
| MS4A1 | Membrane Spanning 4-Domains A1 |
| NDUFA4 | NDUFA4 Mitochondrial Complex Associated |
| NF-kB | Nuclear factor-κB |
| NK cell | Natural killer cell |
| NT5E | 5′-Nucleotidase Ecto |
| OAS2 | 2′-5′-oligoadenylate synthetase |
| PAX5 | Paired box 5 |
| PCA | Principal component analysis |
| Pet | Polyethylene terephthalate |
| PI3K | Phosphoinositide 3-kinase |
| p70S6K | Ribosomal protein S6 kinase beta 1 |
| RCC | Clear cell renal cell carcinomas |
| RTK | Receptor tyrosine kinase |
| RPGR | Retinitis pigmentosa GTPase regulator |
| SCC | Squamous carcinogenesis |
| SELL | Selectin L |
| Smad | Mothers against decapentaplegic homolog 2 |
| SS | Sézary syndroma |
| STAT3 | Signal transducer and activator of transcription 3 |
| SRC-1 | Steroid receptor coactivator |
| TAGLN | Transgelin |
| TF | Transcription factor |
| TGFß | Tumor growth factor beta |
| Th | T helper |
| TIM-3+ | T-cell immunoglobulin and mucin-domain containing-3 |
| TJP1 | Tight junction protein 1 |
| TNFR | Tumor necrosis factor receptor |
| TNFSF13B | TNF superfamily member 13b |
| TTNST | Time to next systemic treatment |
| TREM | Triggering receptor expressed on myeloid cells 1 |
| TSEB | Total skin radiation therapy |
| TTST | Time to systemic therapy |
| TWIST1 | TWIST1 gene |
| Twist1 | Twist1 protein |
| Twist1+ | High Twist1 expression |
| Twist1- | Low Twist1 expression |
| Zeb1+ | High Zeb1 expression |
| Zeb1- | Low Zeb1 expression |
| ZBTB16 | Zinc finger and BTB domain containing 16 |
| B-TRCP | Beta-transducing repeat containing protein |
References
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 Update of the WHO-EORTC Classification for Primary Cutaneous Lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Kempf, W.; Mitteldorf, C. Cutaneous T-cell Lymphomas—An Update 2021. Hematol. Oncol. 2021, 39, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.H. Signaling Networks Guiding Epithelial–Mesenchymal Transitions during Embryogenesis and Cancer Progression. Cancer Sci. 2007, 98, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Weinberg, R.A. Epithelial-Mesenchymal Transition: At the Crossroads of Development and Tumor Metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef]
- Kahlert, U.D.; Joseph, J.V.; Kruyt, F.A.E.; Kruyt, F.A.E. EMT-and MET-Related Processes in Nonepithelial Tumors: Importance for Disease Progression, Prognosis, and Therapeutic Opportunities. Mol. Oncol. 2017, 11, 860–877. [Google Scholar] [CrossRef]
- Pei, H.; Li, Y.; Liu, M.; Chen, Y. Targeting Twist Expression with Small Molecules. Medchemcomm 2017, 8, 268–275. [Google Scholar] [CrossRef]
- Merindol, N.; Riquet, A.; Szablewski, V.; Eliaou, J.F.; Puisieux, A.; Bonnefoy, N. The Emerging Role of Twist Proteins in Hematopoietic Cells and Hematological Malignancies. Blood Cancer J. 2014, 4, e206. [Google Scholar] [CrossRef]
- Vandewalle, C.; van Roy, F.; Berx, G. The Role of the ZEB Family of Transcription Factors in Development and Disease. Cell. Mol. Life Sci. 2009, 66, 773–787. [Google Scholar] [CrossRef]
- Van Doorn, R.; Dijkman, R.; Vermeer, M.H.; Out-Luiting, J.J.; van der Raaij-Helmer, E.M.H.; Willemze, R.; Tensen, C.P. Aberrant Expression of the Tyrosine Kinase Receptor EphA4 and the Transcription Factor Twist in Sézary Syndrome Identified by Gene Expression Analysis. Cancer Res. 2004, 64, 5578–5586. [Google Scholar] [CrossRef]
- Goswami, M.; Duvic, M.; Dougherty, A.; Ni, X. Increased Twist Expression in Advanced Stage of Mycosis Fungoides and Sézary Syndrome. J. Cutan. Pathol. 2012, 39, 500. [Google Scholar] [CrossRef] [PubMed]
- Häyrinen, M.J.; Uotila, P.M.; Sahi, H.; Haapasaari, K.-M.; Teppo, H.-R.; Soini, Y.; Lapela, M.; Vasala, K.; Turpeenniemi-Hujanen, T.; Ranki, A.; et al. Twist and Zeb1 Expression Identify Mycosis Fungoides Patients with Low Risk of Disease Progression. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e95–e98. [Google Scholar] [CrossRef]
- Lemma, S.; Karihtala, P.; Haapasaari, K.M.; Jantunen, E.; Soini, Y.; Bloigu, R.; Pasanen, A.K.; Turpeenniemi-Hujanen, T.; Kuittinen, O. Biological Roles and Prognostic Values of the Epithelial–Mesenchymal Transition-Mediating Transcription Factors Twist, ZEB1 and Slug in Diffuse Large B-Cell Lymphoma. Histopathology 2013, 62, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kolde, R. Pheatmap: Pretty Heatmaps; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal Analysis Approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. CytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Rendón-Serna, N.; Correa-Londoño, L.A.; Velásquez-Lopera, M.M.; Bermudez-Muñoz, M. Cell Signaling in Cutaneous T-Cell Lymphoma Microenvironment: Promising Targets for Molecular-Specific Treatment. Int. J. Dermatol. 2021, 60, 1462–1480. [Google Scholar] [CrossRef] [PubMed]
- Dobos, G.; de Cevins, C.; Ly Ka So, S.; Jean-Louis, F.; Mathieu, S.; Ram-Wolff, C.; Resche-Rigon, M.; Bensussan, A.; Bagot, M.; Michel, L. The Value of Five Blood Markers in Differentiating Mycosis Fungoides and Sézary Syndrome: A Validation Cohort. Br. J. Dermatol. 2021, 185, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chang, S.; Seminario-Vidal, L.; de Mingo Pulido, A.; Tordesillas, L.; Song, X.; Reed, R.A.; Harkins, A.; Whiddon, S.; Nguyen, J.V.; et al. Genomic and Single-Cell Landscape Reveals Novel Drivers and Therapeutic Vulnerabilities of Transformed Cutaneous T-Cell Lymphoma. Cancer Discov. 2022, 12, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Rahman, M.A.; Chen, Z.G.; Shin, D.M. Multiple Biological Functions of Twist1 in Various Cancers. Oncotarget 2017, 8, 20380. [Google Scholar] [CrossRef]
- Galván, J.A.; Helbling, M.; Koelzer, V.H.; Tschan, M.P.; Berger, M.D.; Hädrich, M.; Schnüriger, B.; Karamitopoulou, E.; Dawson, H.; Inderbitzin, D.; et al. TWIST1 and TWIST2 Promoter Methylation and Protein Expression in Tumor Stroma Influence the Epithelial-Mesenchymal Transition-like Tumor Budding Phenotype in Colorectal Cancer. Oncotarget 2015, 6, 874–885. [Google Scholar] [CrossRef]
- Zhong, J.; Ogura, K.; Wang, Z.; Inuzuka, H. Degradation of the Transcription Factor Twist, an Oncoprotein That Promotes Cancer Metastasis. Discov. Med. 2013, 15, 7. [Google Scholar]
- Zhang, Y.; Yu, C. Prognostic Characterization of OAS1/OAS2/OAS3/OASL in Breast Cancer. BMC Cancer 2020, 20, 575. [Google Scholar] [CrossRef]
- Gao, L.; Ren, R.; Shen, J.; Hou, J.; Ning, J.; Feng, Y.; Wang, M.; Wu, L.; Sun, Y.; Wang, H.; et al. Values of OAS Gene Family in the Expression Signature, Immune Cell Infiltration and Prognosis of Human Bladder Cancer. BMC Cancer 2022, 22, 1016. [Google Scholar] [CrossRef]
- Kim, J.C.; Ha, Y.J.; Tak, K.H.; Roh, S.A.; Kwon, Y.H.; Kim, C.W.; Yoon, Y.S.; Lee, J.L.; Park, Y.; Kim, S.K.; et al. Opposite Functions of GSN and OAS2 on Colorectal Cancer Metastasis, Mediating Perineural and Lymphovascular Invasion, Respectively. PLoS ONE 2018, 13, e0202856. [Google Scholar] [CrossRef]
- Zhou, H.; Jia, X.; Yang, F.; Shi, P. Long Noncoding RNA SATB1-AS1 Contributes to the Chemotherapy Resistance through the MicroRNA-580/ 2′-5′-Oligoadenylate Synthetase 2 Axis in Acute Myeloid Leukemia. Bioengineered 2021, 12, 6403. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Miyagaki, T.; Kamijo, H.; Oka, T.; Shishido-Takahashi, N.; Suga, H.; Sugaya, M.; Sato, S. Possible Therapeutic Applicability of Galectin-9 in Cutaneous T-Cell Lymphoma. J. Dermatol. Sci. 2019, 96, 134–142. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, M.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 Interacts with PD-1 and TIM-3 to Regulate T Cell Death and Is a Target for Cancer Immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Liang, L.; Ao, S.; Zhang, J.; Chang, Z.; Wang, Z.; Zhou, Y.; Duan, X.; Deng, T. Fc Fragment of IgE Receptor Ig (FCER1G) Acts as a Key Gene Involved in Cancer Immune Infiltration and Tumour Microenvironment. Immunology 2022, 168, 302–319. [Google Scholar] [CrossRef]
- Andreu, P.; Johansson, M.; Affara, N.I.; Pucci, F.; Tan, T.; Junankar, S.; Korets, L.; Lam, J.; Tawfik, D.; DeNardo, D.G.; et al. FcRgamma Activation Regulates Inflammation-Associated Squamous Carcinogenesis. Cancer Cell 2010, 17, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhou, M.; Zhu, X. Deciphering Immune-Associated Genes to Predict Survival in Clear Cell Renal Cell Cancer. Biomed. Res. Int. 2019, 2019, 2506843. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, L.; Wang, Y.; Wang, G.; Zhu, Y.; Cao, R.; Qian, G.; Xie, C.; Liu, X.; Xiao, Y.; et al. Co-Expression Network Analysis Identified FCER1G in Association with Progression and Prognosis in Human Clear Cell Renal Cell Carcinoma. Int. J. Biol. Sci. 2017, 13, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, K.; Li, X.; Liu, J. Identification of Key Genes and Pathways in Myeloma Side Population Cells by Bioinformatics Analysis. Int. J. Med. Sci. 2020, 17, 2063–2076. [Google Scholar] [CrossRef]
- Fu, L.; Cheng, Z.; Dong, F.; Quan, L.; Cui, L.; Liu, Y.; Zeng, T.; Huang, W.; Chen, J.; Pang, Y.; et al. Enhanced Expression of FCER1G Predicts Positive Prognosis in Multiple Myeloma. J. Cancer 2020, 11, 1182–1194. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, J.; Yu, X.; Jiang, X.; Shi, Y.; Weng, Y.; Kuai, Y.; Lei, L.; Ren, G.; Feng, X.; et al. LITAF Is a Potential Tumor Suppressor in Pancreatic Cancer. Oncotarget 2018, 9, 3131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Z.; Tsuji, T.; Gong, J.; Xie, J.; Chen, C.; Li, W.; Amar, S.; Luo, Z. LITAF and TNFSF15, Two Downstream Targets of AMPK, Exert Inhibitory Effects on Tumor Growth. Oncogene 2011, 30, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xing, H.; Chen, Y.; Wang, L.; Wang, D.; Rao, Q.; Tang, K.; Tian, Z.; He, K.; Wang, M.; et al. PIG7, Transactivated by AML1, Promotes Apoptosis and Differentiation of Leukemia Cells with AML1-ETO Fusion Gene. Leukemia 2012, 26, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.R.; Phelan, J.J.; Michielsen, A.J.; Maguire, A.A.; Dunne, C.; Martin, P.; Noonan, S.; Tosetto, M.; Geraghty, R.; Fennelly, D.; et al. Characterising the Prognostic Potential of HLA-DR during Colorectal Cancer Development. Cancer Immunol. Immunother. 2020, 69, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.E.; Braun, M.; Syring, I.; Klümper, N.; Schmidt, D.; Perner, S.; Hauser, S.; Müller, S.C.; Ellinger, J. NDUFA4 Expression in Clear Cell Renal Cell Carcinoma Is Predictive for Cancer-Specific Survival. Am. J. Cancer Res. 2015, 5, 2816. [Google Scholar] [PubMed]
- Kopp, H.G.; Placke, T.; Salih, H.R. Platelet-Derived Transforming Growth Factor-β down-Regulates NKG2D Thereby Inhibiting Natural Killer Cell Antitumor Reactivity. Cancer Res. 2009, 69, 7775–7783. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Lee, T.Y.; Hsu, J.B.K.; Huang, H.D.; Yang, W.C.V.; Chang, T.H. Computational Analysis for Identification of the Extracellular Matrix Molecules Involved in Endometrial Cancer Progression. PLoS ONE 2020, 15, e0231594. [Google Scholar] [CrossRef]
- Shankar, J.; Messenberg, A.; Chan, J.; Underhill, T.M.; Foster, L.J.; Nabi, I.R. Pseudopodial Actin Dynamics Control Epithelial-Mesenchymal Transition in Metastatic Cancer Cells. Cancer Res. 2010, 70, 3780–3790. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between Epithelial and Mesenchymal States: Acquisition of Malignant and Stem Cell Traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef]
- Datta, A.; Deng, S.; Gopal, V.; Yap, K.C.H.; Halim, C.E.; Lye, M.L.; Ong, M.S.; Tan, T.Z.; Sethi, G.; Hooi, S.C.; et al. Cytoskeletal Dynamics in Epithelial-Mesenchymal Transition: Insights into Therapeutic Targets for Cancer Metastasis. Cancers 2021, 13, 1882. [Google Scholar] [CrossRef]
- Mikheeva, S.A.; Mikheev, A.M.; Petit, A.; Beyer, R.; Oxford, R.G.; Khorasani, L.; Maxwell, J.P.; Glackin, C.A.; Wakimoto, H.; González-Herrero, I.; et al. TWIST1 Promotes Invasion through Mesenchymal Change in Human Glioblastoma. Mol. Cancer 2010, 9, 1–18. [Google Scholar] [CrossRef]
- Hardie, W.D.; Hagood, J.S.; Dave, V.; Perl, A.K.T.; Whitsett, J.A.; Korfhagen, T.R.; Glasser, S. Signaling Pathways in the Epithelial Origins of Pulmonary Fibrosis. Cell Cycle 2010, 9, 2769. [Google Scholar] [CrossRef]
- Zheng, C.-C.; Hu, H.-F.; Hong, P.; Zhang, Q.-H.; Xu, W.W.; He, Q.-Y.; Li, B. Significance of Integrin-Linked Kinase (ILK) in Tumorigenesis and Its Potential Implication as a Biomarker and Therapeutic Target for Human Cancer. Am. J. Cancer Res. 2019, 9, 186. [Google Scholar] [PubMed]
- Shen, H.; Ma, J.L.; Zhang, Y.; Deng, G.L.; Qu, Y.L.; Wu, X.L.; He, J.X.; Zhang, S.; Zeng, S. Integrin-Linked Kinase Overexpression Promotes Epithelial-Mesenchymal Transition via Nuclear Factor-ΚB Signaling in Colorectal Cancer Cells. World J. Gastroenterol. 2016, 22, 3969. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Z.; He, X.; Jiang, Y.; Pan, R.; Chen, S.; Chen, Y.; Han, Y.; Yu, H.; Zhang, T. GJA1 Reverses Arsenic-Induced EMT via Modulating MAPK/ERK Signaling Pathway. Toxicol. Appl. Pharmacol. 2022, 450, 116138. [Google Scholar] [CrossRef] [PubMed]
- Beksaç, B.; Gleason, L.; Baik, S.; Ringe, J.M.; Porcu, P.; Nikbakht, N. Dermal Fibroblasts Promote Cancer Cell Proliferation and Exhibit Fibronectin Overexpression in Early Mycosis Fungoides. J. Dermatol. Sci. 2022, 106, 53–60. [Google Scholar] [CrossRef]
- Jaspars, L.H.; Beljaards, R.C.; Bonnet, P.; Willemze, R.; Meijer, C.J.L.M. Distinctive Adhesion Pathways Are Involved in Epitheliotropic Processes at Different Sites. J. Pathol. 1996, 178, 385–392. [Google Scholar] [CrossRef]
- Jullié, M.L.; Carlotti, M.; Vivot, A.; Beylot-Barry, M.; Ortonne, N.; Frouin, E.; Carlotti, A.; de Muret, A.; Balme, B.; Franck, F.; et al. CD20 Antigen May Be Expressed by Reactive or Lymphomatous Cells of Transformed Mycosis Fungoides: Diagnostic and Prognostic Impact. Am. J. Surg. Pathol. 2013, 37, 1845–1854. [Google Scholar] [CrossRef]
- Nielsen, P.R.; Eriksen, J.O.; Sørensen, M.D.; Wehkamp, U.; Lindahl, L.M.; Bzorek, M.; Iversen, L.; Woetman, A.; Ødum, N.; Litman, T.; et al. Role of B-Cells in Mycosis Fungoides. Acta Derm. Venereol. 2021, 101, adv00413. [Google Scholar] [CrossRef]
- Stolearenco, V.; Namini, M.R.J.; Hasselager, S.S.; Gluud, M.; Buus, T.B.; Willerslev-Olsen, A.; Ødum, N.; Krejsgaard, T. Cellular Interactions and Inflammation in the Pathogenesis of Cutaneous T-Cell Lymphoma. Front. Cell Dev. Biol. 2020, 8, 851. [Google Scholar] [CrossRef]
- Hahtola, S.; Tuomela, S.; Elo, L.; Häkkinen, T.; Karenko, L.; Nedoszytko, B.; Heikkilä, H.; Saarialho-Kere, U.; Roszkiewicz, J.; Aittokallio, T.; et al. Th1 Response and Cytotoxicity Genes Are Down-Regulated in Cutaneous T-Cell Lymphoma. Clin. Cancer Res. 2006, 12, 4812–4821. [Google Scholar] [CrossRef]
- Beavis, P.A.; Stagg, J.; Darcy, P.K.; Smyth, M.J. CD73: A Potent Suppressor of Antitumor Immune Responses. Trends Immunol. 2012, 33, 231–237. [Google Scholar] [CrossRef]
- Bagdonaite, I.; Wandall, H.H.; Litvinov, I.V.; Nastasi, C.; Becker, J.C.; Dabelsteen, S.; Geisler, C.; Bonefeld, C.M.; Zhang, Q.; Wasik, M.A.; et al. Ectopic Expression of a Novel CD22 Splice-Variant Regulates Survival and Proliferation in Malignant T Cells from Cutaneous T Cell Lymphoma (CTCL) Patients. Oncotarget 2015, 6, 14374–14384. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK Signalling Pathway and Tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Zhou, J.; Fu, J.; He, T.; Qin, J.; Wang, L.; Liao, L.; Xu, J. Phosphorylation of Serine 68 of Twist1 by MAPKs Stabilizes Twist1 Protein and Promotes Breast Cancer Cell Invasiveness. Cancer Res. 2011, 71, 3980. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.B.; Abel, E.V.; Mayberry, M.M.; Basile, K.J.; Berger, A.C.; Aplin, A.E. TWIST1 Is an ERK1/2 Effector That Promotes Invasion and Regulates MMP-1 Expression in Human Melanoma Cells. Cancer Res. 2012, 72, 6382. [Google Scholar] [CrossRef] [PubMed]
- Matejka, M.; Konrad, K. Epidermal Langerhans Cells in Mycosis Fungoides and Sézary Syndrome. Wien. Klin. Wochenschr. 1983, 95, 847–852. [Google Scholar] [PubMed]
- Xu, W.; Yang, Z.; Lu, N. A New Role for the PI3K/Akt Signaling Pathway in the Epithelial-Mesenchymal Transition. Cell Adh. Migr. 2015, 9, 317. [Google Scholar] [CrossRef]


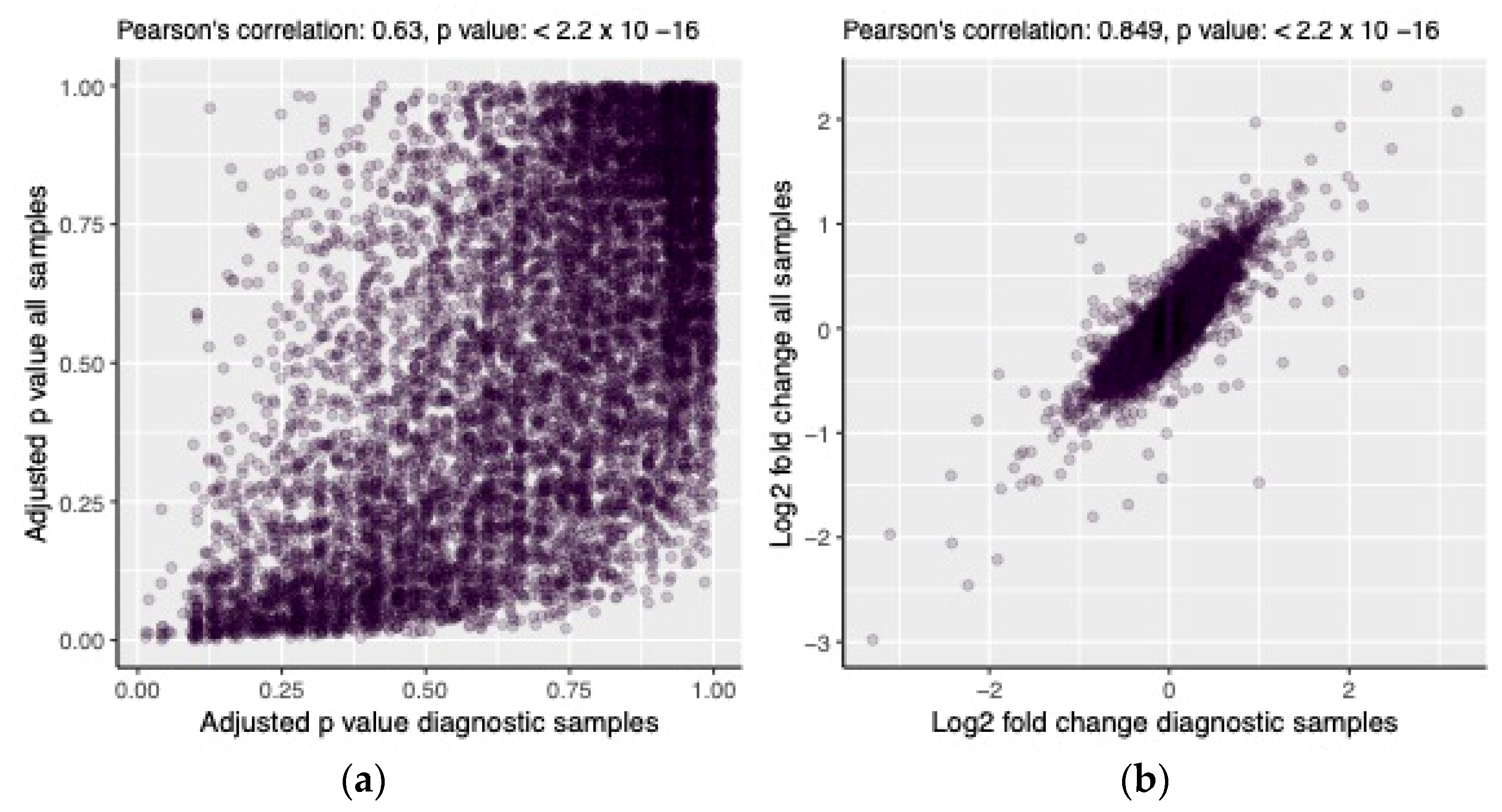
| Diagnostic Samples, n (%) | Follow-up Samples, n (%) | All Samples, n (%) | |
|---|---|---|---|
| Number of cases | 21 | 19 | 40 |
| Male | 16/21 (76%) | 12/19 (63%) | 28/40 (70%) |
| Age 60 years or older | 16/21 (76%) | 11/19 (58%) | 27/40 (68%) (median 63 years, range 19–86) |
| Stage I–IIA | 16/21 (76%) | 13/19 (68%) | 29/40 (73%) |
| Stage IIB–IV | 5/21 (24%) | 6/19 (32%) | 11/40 (28%) |
| Elevated LDH | 7/21 (33%) | 7/19 (37%) | 14/40 (35%) |
| Presenting lesions | |||
| Solitary | 1/21 (5%) | 0/19 (0%) | 1/40 (3%) |
| Multiple | 16/21 (76%) | 15/19 (79%) | 31/40 (78%) |
| Erythrodermic | 4/21 (19%) | 4/19 (21%) | 8/40 (20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Häyrinen, M.J.; Kiiskilä, J.; Ranki, A.; Väkevä, L.; Barton, H.J.; Kuusisto, M.E.L.; Porvari, K.; Kuitunen, H.; Haapasaari, K.-M.; Teppo, H.-R.; et al. The Transcription Factor Twist1 Has a Significant Role in Mycosis Fungoides (MF) Cell Biology: An RNA Sequencing Study of 40 MF Cases. Cancers 2023, 15, 1527. https://doi.org/10.3390/cancers15051527
Häyrinen MJ, Kiiskilä J, Ranki A, Väkevä L, Barton HJ, Kuusisto MEL, Porvari K, Kuitunen H, Haapasaari K-M, Teppo H-R, et al. The Transcription Factor Twist1 Has a Significant Role in Mycosis Fungoides (MF) Cell Biology: An RNA Sequencing Study of 40 MF Cases. Cancers. 2023; 15(5):1527. https://doi.org/10.3390/cancers15051527
Chicago/Turabian StyleHäyrinen, Marjaana J., Jenni Kiiskilä, Annamari Ranki, Liisa Väkevä, Henry J. Barton, Milla E. L. Kuusisto, Katja Porvari, Hanne Kuitunen, Kirsi-Maria Haapasaari, Hanna-Riikka Teppo, and et al. 2023. "The Transcription Factor Twist1 Has a Significant Role in Mycosis Fungoides (MF) Cell Biology: An RNA Sequencing Study of 40 MF Cases" Cancers 15, no. 5: 1527. https://doi.org/10.3390/cancers15051527
APA StyleHäyrinen, M. J., Kiiskilä, J., Ranki, A., Väkevä, L., Barton, H. J., Kuusisto, M. E. L., Porvari, K., Kuitunen, H., Haapasaari, K.-M., Teppo, H.-R., & Kuittinen, O. (2023). The Transcription Factor Twist1 Has a Significant Role in Mycosis Fungoides (MF) Cell Biology: An RNA Sequencing Study of 40 MF Cases. Cancers, 15(5), 1527. https://doi.org/10.3390/cancers15051527





