Impact of the 21-Gene Assay in Patients with High-Clinical Risk ER-Positive and HER2-Negative Early Breast Cancer: Results of the KARMA Dx Study
Abstract
:Simple Summary
Abstract
1. Introduction
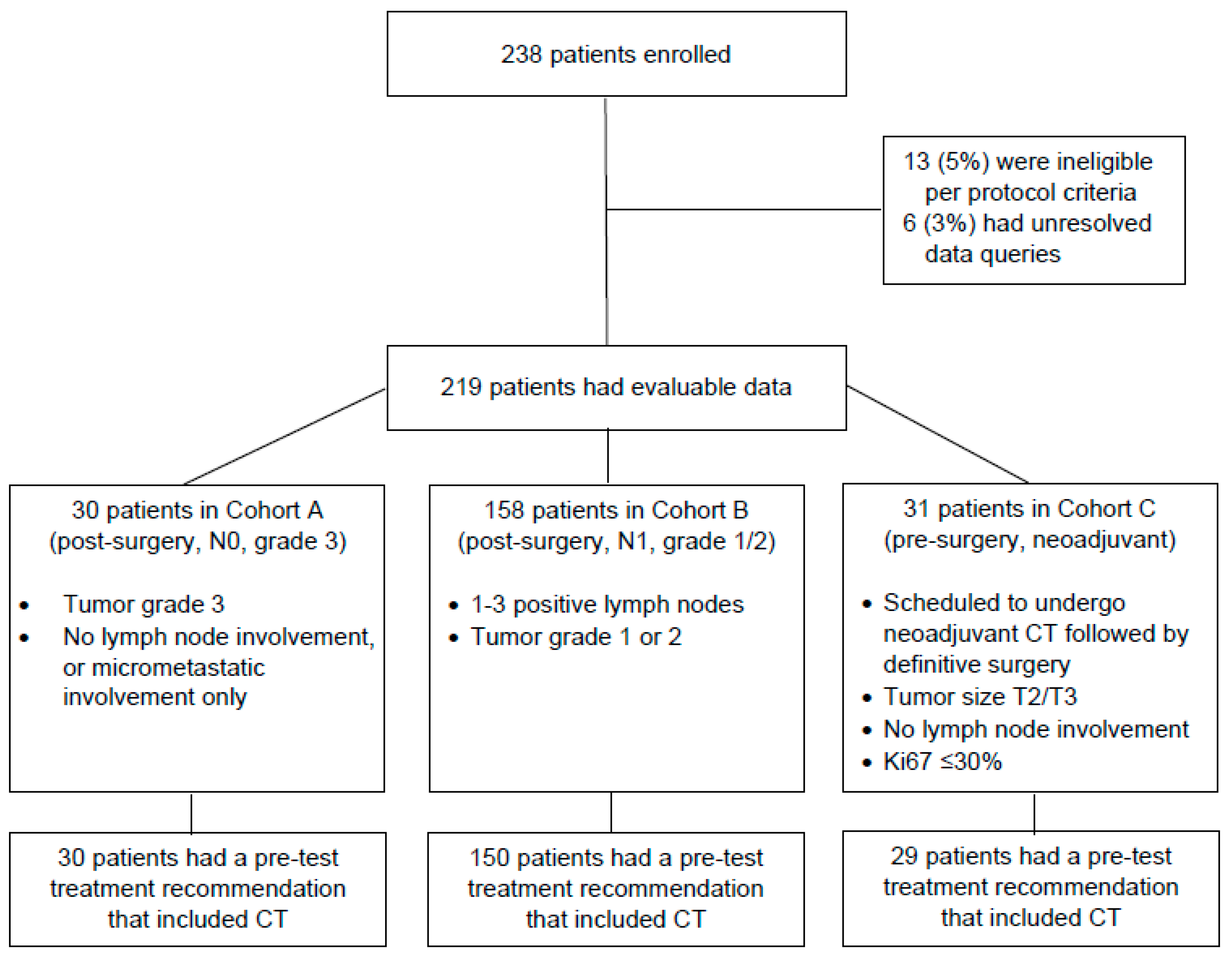
2. Methods
2.1. Study Design
2.2. Study Participants
2.3. Ethical Considerations
2.4. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Change in Treatment Recommendations after 21-Gene Testing
3.3. Physician Confidence Level with 21-Gene Test Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E.; Dees, E.; Perez, E.A.; Olson, J.A.; et al. Prospective validation of a 21-gene expression assay in breast cancer. N. Engl. J. Med. 2015, 373, 2005–2014. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Albain, K.S.; Saphner, T.J.; Badve, S.S.; Wagner, L.I.; Kaklamani, V.G.; Keane, M.M.; Gomez, H.L.; et al. Clinical outcomes in early breast cancer with a high 21-gene Recurrence Score of 26 to 100 assigned to adjuvant chemotherapy plus endocrine therapy: A secondary analysis of the TAILORx randomized clinical trial. JAMA Oncol. 2020, 6, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hortobagyi, G.N.; Connolly, J.L.; D’Orsi, C.J.; Edge, S.B.; Mitendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Giuliano, A. Breast. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S., Greene, F., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer Publishing: New York, NY, USA, 2017; pp. 589–628. [Google Scholar]
- Andre, F.; Ismaila, N.; Henry, N.L.; Somerfield, M.R.; Bast, R.C.; Barlow, W.; Collyar, D.E.; Hammond, M.E.; Kuderer, N.M.; Liu, M.C.; et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO Clinical Practice Guideline update-Integration of results from TAILORx. J. Clin. Oncol. 2019, 37, 1956–1964. [Google Scholar] [CrossRef] [Green Version]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (V4.2021); National Comprehensive Cancer Network: Fort Washington, PA, USA, 2021. [Google Scholar]
- Sparano, J.A.; Gray, R.J.; Ravdin, P.M.; Makower, D.F.; Pritchard, K.; Albain, K.; Hayes, D.F.; Geyer, C.E.; Dees, E.C.; Goetz, M.P.; et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N. Engl. J. Med. 2019, 380, 2395–2405. [Google Scholar] [CrossRef]
- Dowsett, M.; Cuzick, J.; Wale, C.; Forbes, J.; Mallon, E.; Salter, J.; Quinn, E.; Dunbier, A.; Baum, M.; Buzdar, A.; et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J. Clin. Oncol. 2010, 28, 1829–1834. [Google Scholar] [CrossRef] [Green Version]
- Albain, K.S.; Barlow, W.E.; Shak, S.; Hortobagyi, G.N.; Livingston, R.B.; Yeh, I.; Ravdin, P.; Bugarini, R.; Baehner, F.L.; Davidson, N.E.; et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010, 11, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Kalinsky, K.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldtein, L.J.; Chia, S.K.; et al. 21-gene Assay to inform chemotherapy benefit in node-positive Breast Cancer. N. Engl. J. Med. 2021, 385, 2336–2347. [Google Scholar] [CrossRef]
- Zelnak, A.B.; Murali, S.; Styblo, T.M.; Carlson, G.W.; Gabram, S.G.; Rizzo, M.; Torres, M.; Newell, M.A.; Liu, M.; O’Regan, R. Phase II trial evaluating the use of 21-gene Recurrence Score to select preoperative therapy in hormone receptor-positive breast cancer. J. Clin. Oncol. 2013, 31, 562. [Google Scholar] [CrossRef]
- Pivot, X.; Mansi, L.; Chaigneau, L.; Montcuquet, P.; Thiery-Vuillemin, A.; Bazan, F.; Dobi, E.; Sautiere, J.L.; Rigenbach, F.; Algors, M.P.; et al. In the era of genomics, should tumor size be reconsidered as a criterion for neoadjuvant chemotherapy? Oncologist 2015, 20, 344–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yardley, D.A.; Peacock, N.W.; Shastry, M.; Burris, H.A.; Bechhold, R.G.; Hendricks, C.B.; Yoshizawa, C.N.; Sing, A.; Hainsworth, J. A phase II trial of ixabepilone and cyclophosphamide as neoadjuvant therapy for patients with HER2-negative breast cancer: Correlation of pathologic complete response with the 21-gene Recurrence Score. Breast Cancer Res. Treat. 2015, 154, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Bear, H.D.; Wan, W.; Robidoux, A.; Rubin, P.; Limentani, S.; White, R.L.; Granfortuna, J.; Hopkins, J.O.; Oldham, D.; Rodriguez, A.; et al. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: A multicenter trial. J. Surg. Oncol. 2017, 115, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Kantor, O.; Barrera, E.; Kopkash, K.; Pesce, C.; Barrera, E.; Winchester, D.; Yao, K. Are we overtreating hormone receptor positive breast cancer with neoadjuvant chemotherapy? Role of Oncotype DX for hormone receptor positive patients undergoing neoadjuvant chemotherapy. Ann. Surg. Oncol. 2019, 26, 3232–3239. [Google Scholar] [CrossRef]
- Thekkekara, R.J.; Bharadwaj, S.; Yadav, U.; Baranwal, A.; Peace, D.; Rogowski, W.; Sekosan, M.; La, T.; Marcus, E.A.; Ferrer, K.; et al. Predicting response to neoadjuvant chemotherapy in nonmetastatic hormone receptor-positive breast cancer using 21-gene Breast Recurrence Score test. J. Clin. Oncol. 2019, 37, e12093. [Google Scholar] [CrossRef]
- Morales Murillo, S.; Gasol Cudos, A.; Veas Rodriguez, J.; Canosa Morales, C.; Olivé, J.M.; Vilardell, F.; Sanchez, D.R.; Iglesias, E.; Salud, A. Selection of neoadjuvant treatment based on the 21-gene test results in luminal breast cancer. Breast 2021, 56, 35–41. [Google Scholar] [CrossRef]
- Ibarrondo, O.; Alvarez-Lopez, I.; Freundlich, F.; Arrospide, A.; Galve-Calvo, E.; Gutierrez-Toribio, M.; Plazaola, A.; Mar, J. Probabilistic cost-utility analysis and expected value of perfect information for the Oncotype multigenic test: A discrete event simulation model. Gac. Sanit. 2020, 34, 61–68. [Google Scholar] [CrossRef]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef] [Green Version]
- Albanell, J.; Gonzalez, A.; Ruiz-Borrego, M.; Alba, E.; García-Saenz, J.A.; Corominas, J.M.; Burgues, O.; Furio, V.; Rojo, A.; Palacios, J.; et al. Prospective transGEICAM study of the impact of the 21-gene Recurrence Score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann. Oncol. 2012, 23, 625–631. [Google Scholar] [CrossRef]
- Curtit, E.; Vannetzel, J.M.; Darmon, J.C.; Roche, S.; Bourgeois, H.; Dewas, S.; Catala, S.; Mereb, E.; Furtos Fanget, C.; Genet, D.; et al. Results of PONDx, a prospective multicenter study of the Oncotype DX((R)) breast cancer assay: Real-life utilization and decision impact in French clinical practice. Breast 2019, 44, 39–45. [Google Scholar] [CrossRef]
- Cognetti, F.; Masetti, R.; Fabi, A.; Bianchi, G.; Santini, D.; Rognone, A.; Catania, G.; Angelucci, D.; Naso, G.; Giuliano, M.; et al. PONDx: Real-life utilization and decision impact of the 21-gene assay on clinical practice in Italy. NPJ Breast Cancer 2021, 7, 47. [Google Scholar] [CrossRef]
- Albanell, J.; Svedman, C.; Gligorov, J.; Holt, S.D.H.; Bertelli, G.; Blohmer, J.U.; Rouzier, R.; Lluch, A.; Eiermann, W. Pooled analysis of prospective European studies assessing the impact of using the 21-gene Recurrence Score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer. Eur. J. Cancer 2016, 66, 104–113. [Google Scholar] [PubMed] [Green Version]
- Gluz, O.; Nitz, U.A.; Christgen, M.; Kates, R.E.; Shak, S.; Clemens, M.; Kraemer, S.; Aktas, B.; Kuemmel, S.; Reimer, T.; et al. West German Study Group phase III PlanB trial: First prospective outcome data for the 21-Gene Recurrence Score assay and concordance of prognostic markers by central and local pathology assessment. J. Clin. Oncol. 2016, 34, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Iorgulescu, J.B.; Freedman, R.A.; Lester, S.C.; Mittendorf, E.; Brock, J. 21-Gene Recurrence Score adds significant value for grade 3 breast cancers: Results from a national cohort. JCO Precis. Oncol. 2019, 3. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Shak, S.; Sledge, G.W.; Winer, E.; Albain, K.; Mamounas, E.; Jakubowski, D.; Petkov, V.; Wolmark, N. Breast cancer-specific mortality in patients with node-negative and node-positive breast cancer guided by the 21-gene assay: A SEER-genomic population-based study. In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 4–8 December 2018. [Google Scholar]
- Nitz, U.; Gluz, O.; Christgen, M.; Kates, R.E.; Clemens, M.; Malter, W.; Nuding, B.; Aktas, B.; Kuemmel, S.; Reimer, T.; et al. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: Five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res. Treat. 2017, 165, 573–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.; Miller, D.P.; Shak, S.; Petkov, V.I. Breast cancer specific survival in patients with node-positive hormone receptor positive invasive breast cancer and Oncotype DX Recurrence Score results in the SEER database. Breast Can. Res. Treat. 2017, 163, 303–310. [Google Scholar] [CrossRef]
- Stemmer, S.M.; Steiner, M.; Rizel, S.; Geffen, D.B.; Nisenbaum, B.; Peretz, T.; Soussan-Gutman, L.; Bareket-Samish, A.; Isaacs, K.; Rosengarten, O.; et al. Clinical outcomes in ER+ HER2-, node-positive breast cancer patients who were treated according to the Recurrence Score results: Evidence from a large prospectively designed registry. NPJ Breast Cancer 2017, 3, 32. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. Tamoxifen Citrate, Letrozole, Anastrozole, or Exemestane with or without Chemotherapy in Treating Patients with Invasive RxPONDER Breast Cancer; National Cancer Institute: Bethesda, MD, USA, 2011. [Google Scholar]
- Iwata, H.; Masuda, N.; Yamamoto, Y.; Fujisawa, T.; Toyama, T.; Kashiwaba, M.; Ohtani, S.; Taira, N.; Sakai, T.; Hasegawa, Y.; et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: The TransNEOS study. Breast Cancer Res. Treat. 2019, 173, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Paik, S.; Tang, G.; Shak, S.; Kim, C.; Baker, J.; Kim, W.; Cronin, M.; Baehner, F.L.; Watson, D.; Bryant, J.; et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006, 24, 3726–3734. [Google Scholar] [CrossRef]
- Cobleigh, M.A.; Tabesh, B.; Bitterman, P.; Baker, J.; Cronin, M.; Liu, M.L.; Borchik, R.; Mosquera, J.M.; Walker, M.G.; Shak, S. Tumor gene expression and prognosis in breast cancer patients with 10 or more positive lymph nodes. Clin. Cancer Res. 2005, 11, 8623–8631. [Google Scholar] [CrossRef] [Green Version]
- Esteban, J.; Baker, J.; Liu, M.L.; MGLlamas, M.G.; Walker, M.G.; Mena, R. Tumor gene expression and prognosis in breast cancer: Multi-gene RT-PCR assay of paraffin-embedded tissue. Proc. Am. Soc. Clin. Oncol. 2003. Available online: https://www.scienceopen.com/document?vid=f5fe5404-4d43-4ac6-acb0-b04ebc37d14d (accessed on 17 December 2022).




| All Patients (n = 219) | Cohort A N0 (n = 30) | Cohort B N+ (n = 158) | Cohort C Neoadjuvant (n = 31) | ||
|---|---|---|---|---|---|
| Age, years | Median (IQR) | 55 (47–66) | 59 (51–67) | 55 (47–66) | 49 (44–59) |
| ≤50 | 78 (36%) | 7 (23%) | 54 (34%) | 17 (55%) | |
| >50 | 141 (64%) | 23 (77%) | 104 (66%) | 14 (45%) | |
| Nodal status | N0/N1mi | 59 (27%) | 30 (100%) | 0 | 29 (94%) |
| N+ | 160 (73%) | 0 | 158 (100%) | 2 (6%) | |
| Tumor grade | I | 56 (26%) | 0 | 47 (30%) | 9 (29%) |
| II | 127 (58%) | 0 | 111 (70%) | 16 (52%) | |
| III | 31 (14%) | 30 (100%) | 0 | 1 (3%) | |
| Unknown | 5 (2%) | 0 | 0 | 5 (16%) | |
| Tumor classification | T1a | 2 (1%) | 0 | 2 (1%) | 0 |
| T1b | 20 (9%) | 4 (13%) | 16 (10%) | 0 | |
| T1c | 111 (51%) | 17 (57%) | 88 (56%) | 6 (19%) | |
| T2 | 79 (36%) | 8 (27%) | 50 (32%) | 21 (68%) | |
| T3 | 7 (3%) | 1 (3%) | 2 (1%) | 4 (13%) | |
| Recurrence Score | Median (IQR) | 17 (12–22) | 22 (17–40) | 17 (12–20) | 16 (13–22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llombart-Cussac, A.; Anton-Torres, A.; Rojas, B.; Andrés, R.; Martinez, N.; Rodríguez, C.A.; Marin, S.; Puértolas, T.; González, A.F.; Fernández-Murga, M.L.; et al. Impact of the 21-Gene Assay in Patients with High-Clinical Risk ER-Positive and HER2-Negative Early Breast Cancer: Results of the KARMA Dx Study. Cancers 2023, 15, 1529. https://doi.org/10.3390/cancers15051529
Llombart-Cussac A, Anton-Torres A, Rojas B, Andrés R, Martinez N, Rodríguez CA, Marin S, Puértolas T, González AF, Fernández-Murga ML, et al. Impact of the 21-Gene Assay in Patients with High-Clinical Risk ER-Positive and HER2-Negative Early Breast Cancer: Results of the KARMA Dx Study. Cancers. 2023; 15(5):1529. https://doi.org/10.3390/cancers15051529
Chicago/Turabian StyleLlombart-Cussac, Antonio, Antonio Anton-Torres, Beatriz Rojas, Raquel Andrés, Noelia Martinez, César A. Rodríguez, Sara Marin, Teresa Puértolas, Alejandro Falcón González, María Leonor Fernández-Murga, and et al. 2023. "Impact of the 21-Gene Assay in Patients with High-Clinical Risk ER-Positive and HER2-Negative Early Breast Cancer: Results of the KARMA Dx Study" Cancers 15, no. 5: 1529. https://doi.org/10.3390/cancers15051529






