Myelotoxicity of Temozolomide Treatment in Patients with Glioblastoma Is It Time for a More Mechanistic Approach?
1. Introduction
2. Myelotoxicity Is Not a Rare Event and Leads to Significant Treatment Alterations
3. A More Systematic Approach towards Myeloxicity
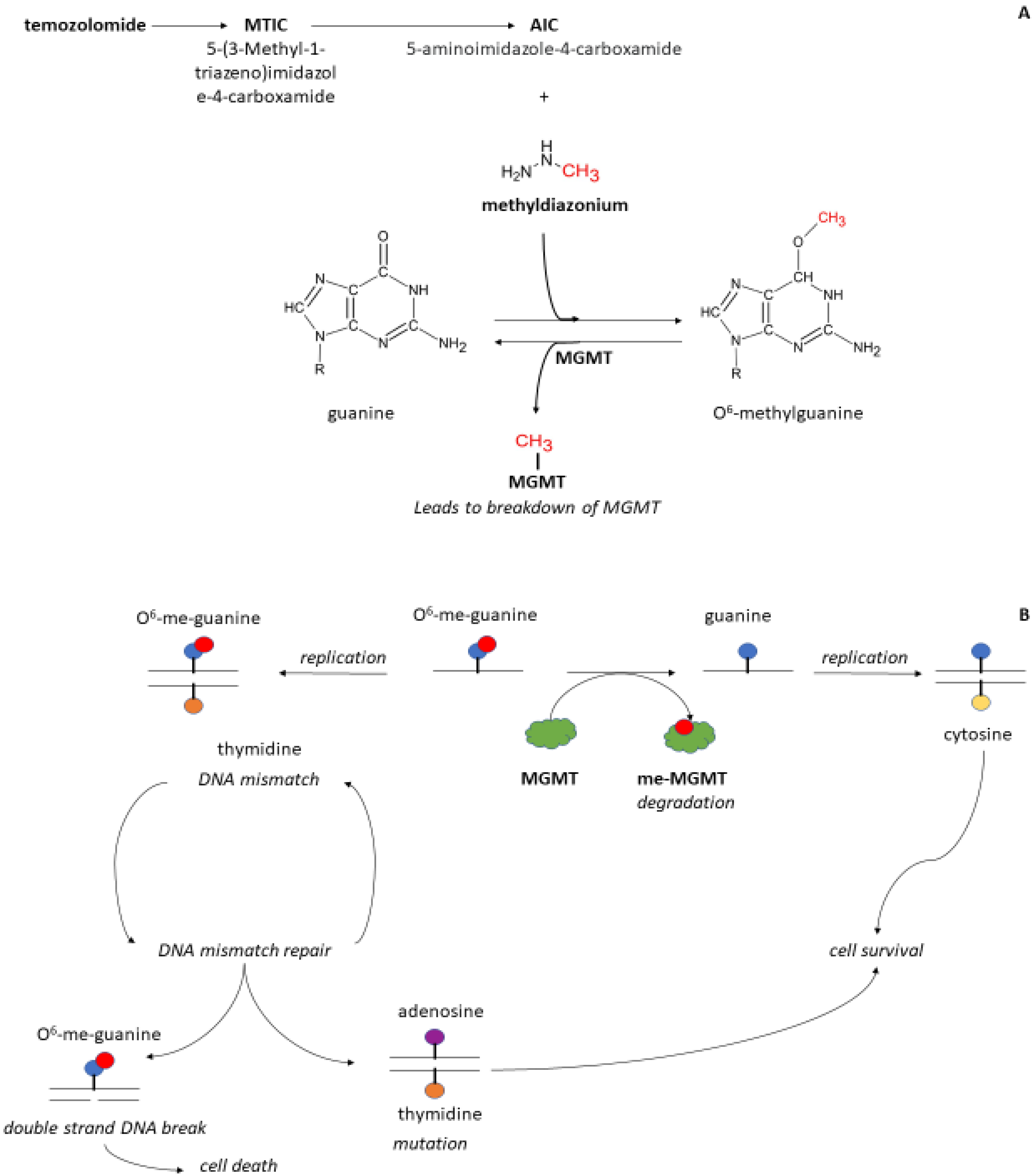
4. Should MGMT Be Included in the PK-PD Model for Myeloxicity?
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23 (Suppl. S2), iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, S.; Baker, L.; Walmsley, V.; Hingorani, M. Temozolomide-Related Idiosyncratic and Other Uncommon Toxicities: A Systematic Review. Anticancer. Drugs 2012, 23, 1099–1106. [Google Scholar] [CrossRef]
- Le Rhun, E.; Oppong, F.B.; Vanlancker, M.; Stupp, R.; Nabors, B.; Chinot, O.; Wick, W.; Preusser, M.; Gorlia, T.; Weller, M. Prognostic Significance of Therapy-Induced Myelosuppression in Newly Diagnosed Glioblastoma. Neuro-Oncology 2022, 24, 1533–1545. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5; US Department of Health and Human Services, National Institutes of Health, National Cancer Institute: Bethesda, MD, USA, 2017.
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.-K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide Combined with Standard Treatment for Patients with Newly Diagnosed Glioblastoma with Methylated MGMT Promoter (CENTRIC EORTC 26071-22072 Study): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Nabors, L.B.; Fink, K.L.; Mikkelsen, T.; Grujicic, D.; Tarnawski, R.; Nam, D.H.; Mazurkiewicz, M.; Salacz, M.; Ashby, L.; Zagonel, V.; et al. Two Cilengitide Regimens in Combination with Standard Treatment for Patients with Newly Diagnosed Glioblastoma and Unmethylated MGMT Gene Promoter: Results of the Open-Label, Controlled, Randomized Phase II CORE Study. Neuro-Oncology 2015, 17, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Wick, W.; Gorlia, T.; Bady, P.; Platten, M.; van den Bent, M.J.; Taphoorn, M.J.B.; Steuve, J.; Brandes, A.A.; Hamou, M.-F.; Wick, A.; et al. Phase II Study of Radiotherapy and Temsirolimus versus Radiochemotherapy with Temozolomide in Patients with Newly Diagnosed Glioblastoma without MGMT Promoter Hypermethylation (EORTC 26082). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 4797–4806. [Google Scholar] [CrossRef] [Green Version]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy-Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [Green Version]
- Skaga, E.; Skretteberg, M.A.; Johannesen, T.B.; Brandal, P.; Vik-Mo, E.O.; Helseth, E.; Langmoen, I.A. Real-World Validity of Randomized Controlled Phase III Trials in Newly Diagnosed Glioblastoma: To Whom Do the Results of the Trials Apply? Neuro-Oncol. Adv. 2021, 3, vdab008. [Google Scholar] [CrossRef]
- Armstrong, T.S.; Cao, Y.; Scheurer, M.E.; Vera-Bolaños, E.; Manning, R.; Okcu, M.F.; Bondy, M.; Zhou, R.; Gilbert, M.R. Risk Analysis of Severe Myelotoxicity with Temozolomide: The Effects of Clinical and Genetic Factors. Neuro-Oncology 2009, 11, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, S.H.; Park, M.-J.; Lee, M.M.; Kim, T.M.; Lee, S.-H.; Cho, S.Y.; Kim, Y.-H.; Kim, Y.J.; Park, C.-K.; Kim, C.-Y. Toxicity Profile of Temozolomide in the Treatment of 300 Malignant Glioma Patients in Korea. J. Korean Med. Sci. 2014, 29, 980–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, D.E.; Grossman, S.A.; Zeltzman, M.; Parisi, M.A.; Kleinberg, L. The Impact of Thrombocytopenia from Temozolomide and Radiation in Newly Diagnosed Adults with High-Grade Gliomas. Neuro-Oncology 2007, 9, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Niewald, M.; Berdel, C.; Fleckenstein, J.; Licht, N.; Ketter, R.; Rübe, C. Toxicity after Radiochemotherapy for Glioblastoma Using Temozolomide—A Retrospective Evaluation. Radiat. Oncol. Lond. Engl. 2011, 6, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, T.; Mohanty, S.; Moiyadi, A.; Jalali, R. Factors Predicting Temozolomide Induced Clinically Significant Acute Hematologic Toxicity in Patients with High-Grade Gliomas: A Clinical Audit. Clin. Neurol. Neurosurg. 2013, 115, 1814–1819. [Google Scholar] [CrossRef]
- Garcia, C.R.; Myint, Z.W.; Jayswal, R.; Wang, C.; Morgan, R.M.; Butts, A.R.; Weiss, H.L.; Villano, J.L. Hematological Adverse Events in the Management of Glioblastoma. J. Neurooncol. 2022, 156, 153–161. [Google Scholar] [CrossRef]
- Elting, L.S.; Rubenstein, E.B.; Martin, C.G.; Kurtin, D.; Rodriguez, S.; Laiho, E.; Kanesan, K.; Cantor, S.B.; Benjamin, R.S. Incidence, Cost, and Outcomes of Bleeding and Chemotherapy Dose Modification among Solid Tumor Patients with Chemotherapy-Induced Thrombocytopenia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 1137–1146. [Google Scholar] [CrossRef]
- Lombardi, G.; Rumiato, E.; Bertorelle, R.; Saggioro, D.; Farina, P.; Della Puppa, A.; Zustovich, F.; Berti, F.; Sacchetto, V.; Marcato, R.; et al. Clinical and Genetic Factors Associated With Severe Hematological Toxicity in Glioblastoma Patients During Radiation Plus Temozolomide Treatment: A Prospective Study. Am. J. Clin. Oncol. 2015, 38, 514–519. [Google Scholar] [CrossRef]
- Robins, H.I.; Eickhoff, J.; Gilbert, M.R.; Armstrong, T.S.; Shi, W.; De Groot, J.F.; Schultz, C.J.; Hunter, G.K.; Valeinis, E.; Roach, M.; et al. The Association between BMI and BSA-Temozolomide-Induced Myelosuppression Toxicities: A Correlative Analysis of NRG Oncology RTOG 0525. Neuro-Oncol. Pract. 2019, 6, 473–478. [Google Scholar] [CrossRef]
- de Vries Schultink, A.H.M.; Suleiman, A.A.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R. Pharmacodynamic Modeling of Adverse Effects of Anti-Cancer Drug Treatment. Eur. J. Clin. Pharmacol. 2016, 72, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Temozolomide in Malignant Glioma Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jen, J.F.; Cutler, D.L.; Pai, S.M.; Batra, V.K.; Affrime, M.B.; Zambas, D.N.; Heft, S.; Hajian, G. Population Pharmacokinetics of Temozolomide in Cancer Patients. Pharm. Res. 2000, 17, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L.E.; Henningsson, A.; Maas, H.; Nguyen, L.; Karlsson, M.O. Model of Chemotherapy-Induced Myelosuppression With Parameter Consistency Across Drugs. J. Clin. Oncol. 2002, 20, 4713–4721. [Google Scholar] [CrossRef] [PubMed]
- Carl Panetta, J.; Kirstein, M.N.; Gajjar, A.J.; Nair, G.; Fouladi, M.; Stewart, C.F. A Mechanistic Mathematical Model of Temozolomide Myelosuppression in Children with High-Grade Gliomas. Math. Biosci. 2003, 186, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Panetta, J.C.; Kirstein, M.N.; Gajjar, A.; Nair, G.; Fouladi, M.; Heideman, R.L.; Wilkinson, M.; Stewart, C.F. Population Pharmacokinetics of Temozolomide and Metabolites in Infants and Children with Primary Central Nervous System Tumors. Cancer Chemother. Pharmacol. 2003, 52, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Mehrotra, S.; Gopalakrishnan, M.; Gojo, I.; Karp, J.E.; Greer, J.M.; Chen, A.; Piekarz, R.; Kiesel, B.F.; Gobburu, J.; et al. Population Pharmacokinetics and Exposure-Response Assessment of Veliparib Co-Administered with Temozolomide in Patients with Myeloid Leukemias. Cancer Chemother. Pharmacol. 2019, 83, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, C.A.; Bohlke, K.; Delaney, M.; Hume, H.; Magdalinski, A.J.; McCullough, J.J.; Omel, J.L.; Rainey, J.M.; Rebulla, P.; Rowley, S.D.; et al. Platelet Transfusion for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 283–299. [Google Scholar] [CrossRef]
- Smith, T.J.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.L.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Hegi, M.E.; Gilbert, M.R.; Chakravarti, A. Chemoradiotherapy in Malignant Glioma: Standard of Care and Future Directions. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4127–4136. [Google Scholar] [CrossRef]
- Gerson, S.L.; Miller, K.; Berger, N.A. O6 Alkylguanine-DNA Alkyltransferase Activity in Human Myeloid Cells. J. Clin. Investig. 1985, 76, 2106–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeek, B.; Southgate, T.D.; Gilham, D.E.; Margison, G.P. O6-Methylguanine-DNA Methyltransferase Inactivation and Chemotherapy. Br. Med. Bull. 2008, 85, 17–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, K.; Eichhorn-Grombacher, U.; Schlink, K.; Nitzsche, S.; Oesch, F.; Kaina, B. Long-Time Expression of DNA Repair Enzymes MGMT and APE in Human Peripheral Blood Mononuclear Cells. Arch. Toxicol. 2001, 75, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Fang, Q.; Loktionova, N.A. Human Variants of O6-Alkylguanine-DNA Alkyltransferase. DNA Repair 2007, 6, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugni, J.M.; Han, J.; Tsai, M.; Hunter, D.J.; Samson, L.D. Genetic Association and Functional Studies of Major Polymorphic Variants of MGMT. DNA Repair 2007, 6, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Egyházi, S.; Ueno, T.; Lindholm, C.; Kreklau, E.L.; Stierner, U.; Ringborg, U.; Hansson, J. O6-Methylguanine-DNA-Methyltransferase Expression and Gene Polymorphisms in Relation to Chemotherapeutic Response in Metastatic Melanoma. Br. J. Cancer 2003, 89, 1517–1523. [Google Scholar] [CrossRef] [Green Version]
- D’Atri, S.; Graziani, G.; Lacal, P.M.; Nisticò, V.; Gilberti, S.; Faraoni, I.; Watson, A.J.; Bonmassar, E.; Margison, G.P. Attenuation of O(6)-Methylguanine-DNA Methyltransferase Activity and MRNA Levels by Cisplatin and Temozolomide in Jurkat Cells. J. Pharmacol. Exp. Ther. 2000, 294, 664–671. [Google Scholar]
- Sabharwal, A.; Waters, R.; Danson, S.; Clamp, A.; Lorigan, P.; Thatcher, N.; Margison, G.P.; Middleton, M.R. Predicting the Myelotoxicity of Chemotherapy: The Use of Pretreatment O6-Methylguanine-DNA Methyltransferase Determination in Peripheral Blood Mononuclear Cells. Melanoma Res. 2011, 21, 502–508. [Google Scholar] [CrossRef]
- Sylvester, R.K.; Steen, P.; Tate, J.M.; Mehta, M.; Petrich, R.J.; Berg, A.; Kolesar, J. Temozolomide-Induced Severe Myelosuppression: Analysis of Clinically Associated Polymorphisms in Two Patients. Anticancer. Drugs 2011, 22, 104–110. [Google Scholar] [CrossRef]
- Chakravarti, A.; Erkkinen, M.G.; Nestler, U.; Stupp, R.; Mehta, M.; Aldape, K.; Gilbert, M.R.; Black, P.M.; Loeffler, J.S. Temozolomide-Mediated Radiation Enhancement in Glioblastoma: A Report on Underlying Mechanisms. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 4738–4746. [Google Scholar] [CrossRef] [Green Version]
- Scheurer, M.E.; Zhou, R.; Gilbert, M.R.; Bondy, M.L.; Sulman, E.P.; Yuan, Y.; Liu, Y.; Vera, E.; Wendland, M.M.; Youssef, E.F.; et al. Germline Polymorphisms in MGMT Associated with Temozolomide-Related Myelotoxicity Risk in Patients with Glioblastoma Treated on NRG Oncology/RTOG 0825. Neuro-Oncol. Adv. 2022, 4, vdac152. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, M.M.; Broen, M.P.G.; Swart, E.L.; Bartelink, I.H.; Kouwenhoven, M.C.M. Myelotoxicity of Temozolomide Treatment in Patients with Glioblastoma Is It Time for a More Mechanistic Approach? Cancers 2023, 15, 1561. https://doi.org/10.3390/cancers15051561
Said MM, Broen MPG, Swart EL, Bartelink IH, Kouwenhoven MCM. Myelotoxicity of Temozolomide Treatment in Patients with Glioblastoma Is It Time for a More Mechanistic Approach? Cancers. 2023; 15(5):1561. https://doi.org/10.3390/cancers15051561
Chicago/Turabian StyleSaid, Medhat M., Martinus P. G. Broen, Eleonora L. Swart, Imke H. Bartelink, and Mathilde C. M. Kouwenhoven. 2023. "Myelotoxicity of Temozolomide Treatment in Patients with Glioblastoma Is It Time for a More Mechanistic Approach?" Cancers 15, no. 5: 1561. https://doi.org/10.3390/cancers15051561
APA StyleSaid, M. M., Broen, M. P. G., Swart, E. L., Bartelink, I. H., & Kouwenhoven, M. C. M. (2023). Myelotoxicity of Temozolomide Treatment in Patients with Glioblastoma Is It Time for a More Mechanistic Approach? Cancers, 15(5), 1561. https://doi.org/10.3390/cancers15051561




