ImmunoPET Imaging Identifies the Optimal Timepoint for Combination Therapy in Xenograft Models of Triple-Negative Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Evaluation of gpNMB Expression in the Presence of Dasatinib In Vitro
2.3. Lysate Preparation and Western Blotting of gpNMB Expression
2.4. Xenograft Models
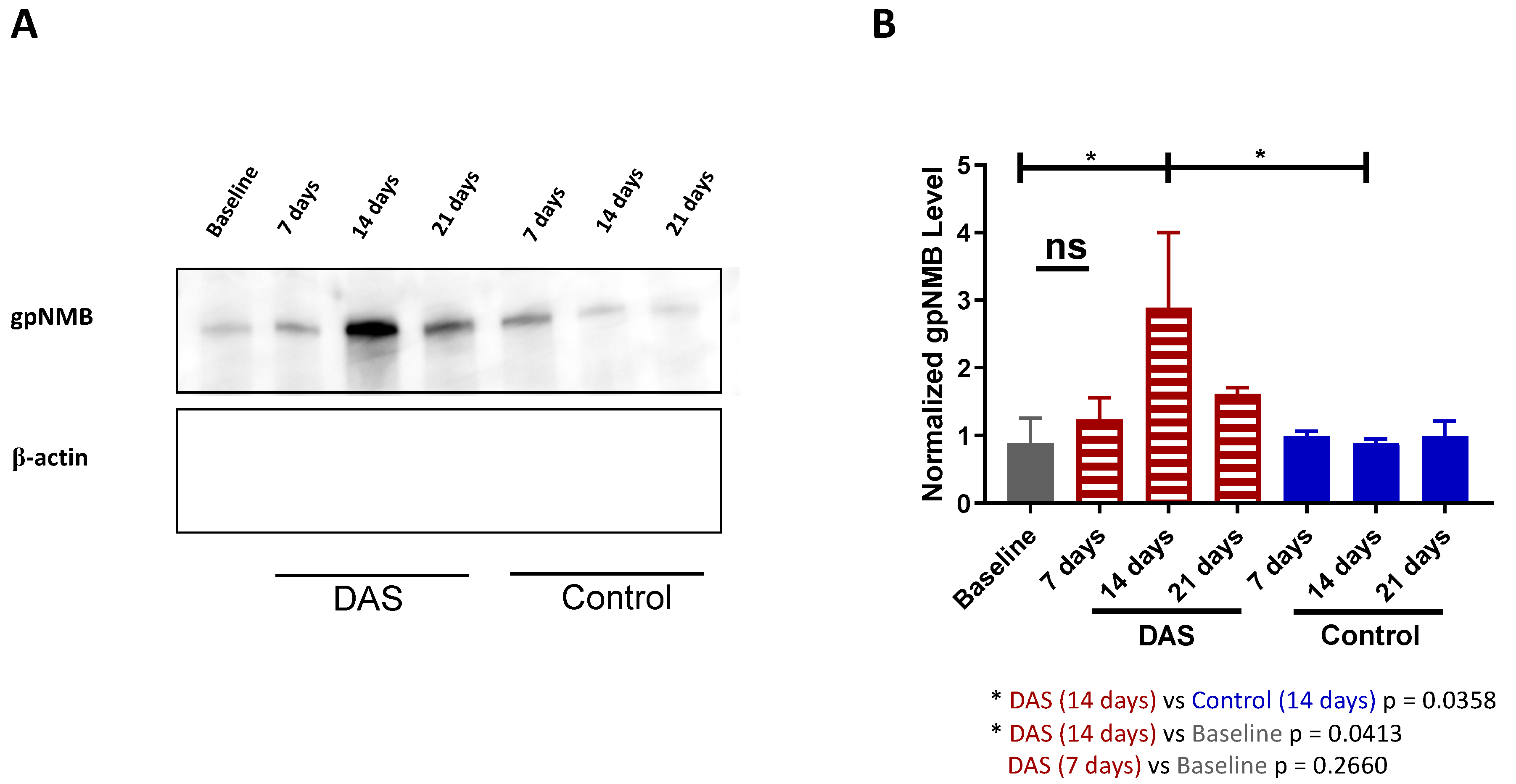
2.5. Determining the Timepoint of gpNMB Upregulation after Dasatinib Treatment in MDA-MB-468 Xenograft Models by Western Blot Analysis of Tumor Lysates
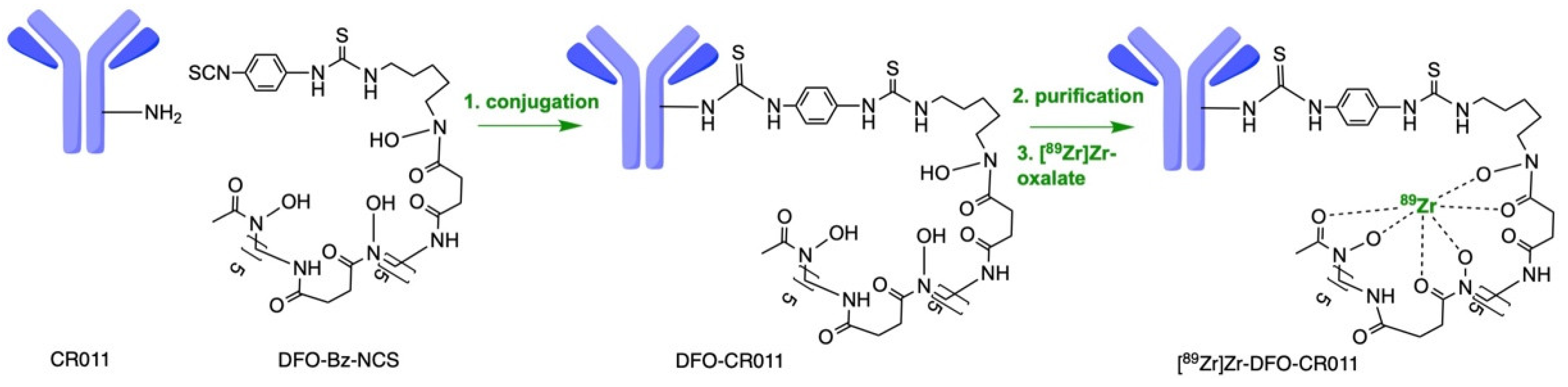
2.6. Conjugation and Radiolabeling of CR011
2.7. PET Imaging Acquisition
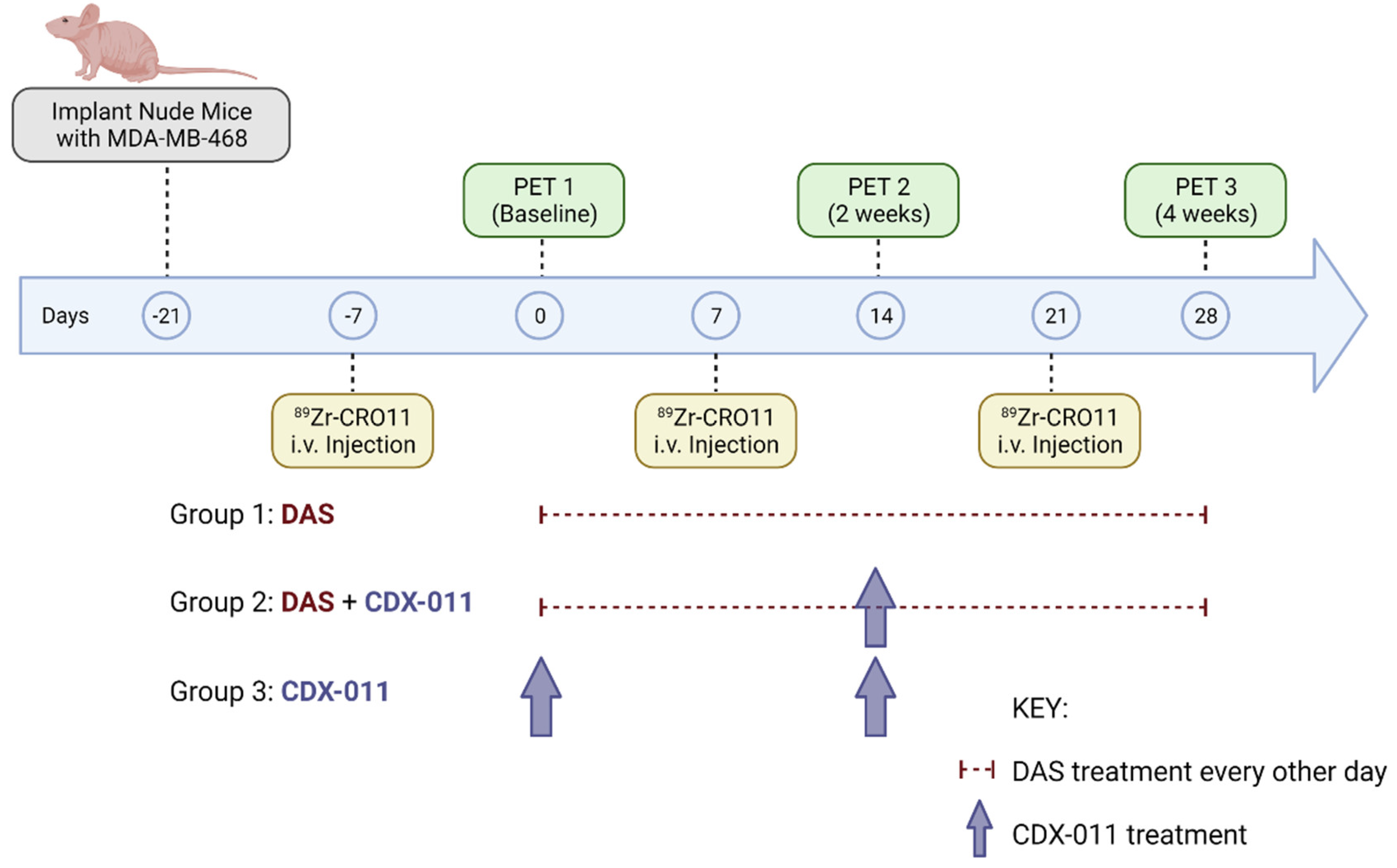
2.8. PET Imaging and Treatment Paradigm of gpNMB-Positive MDA-MB-468 Xenograft Models
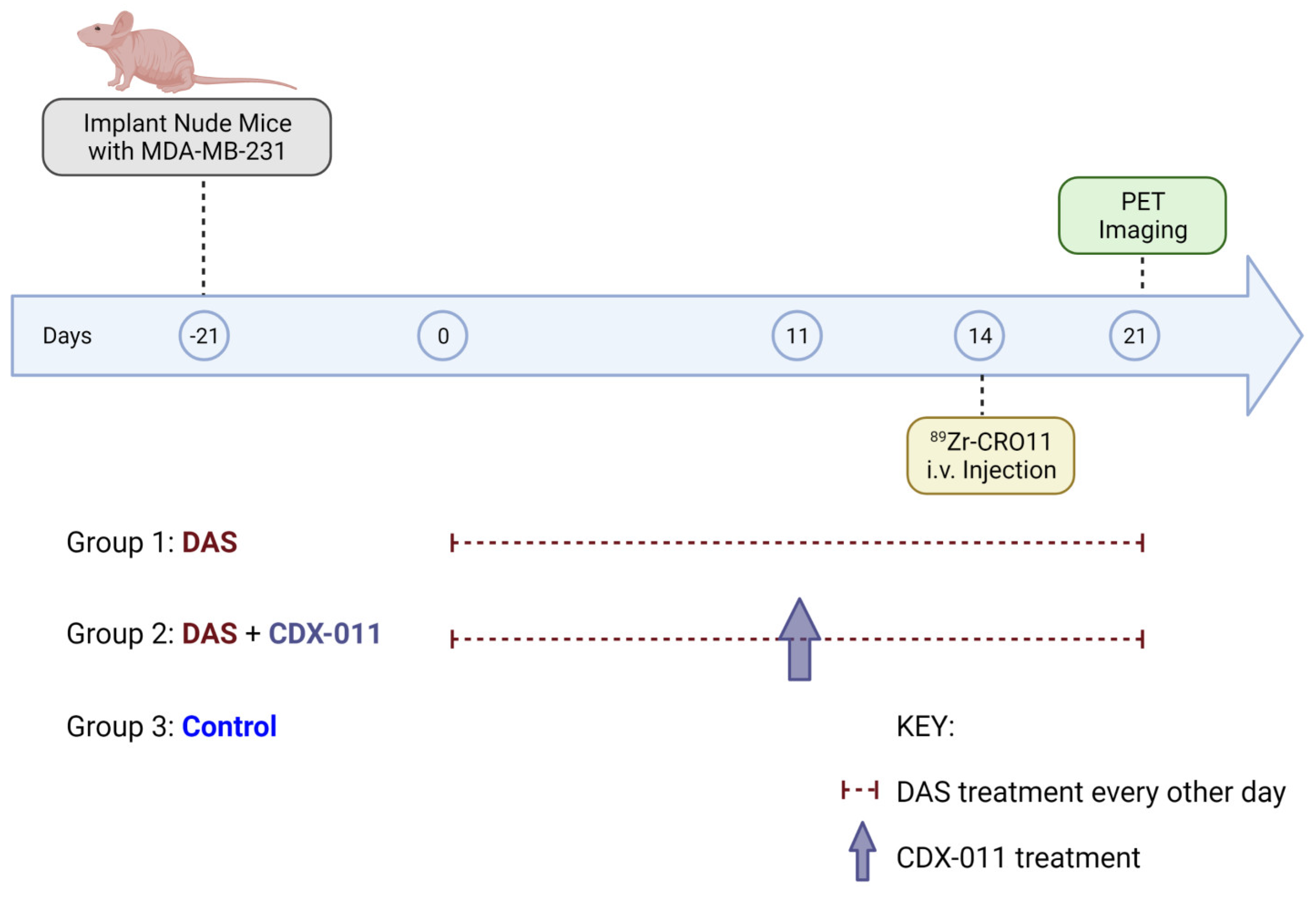
2.9. PET Imaging and Treatment Paradigm of gpNMB-Negative MDA-MB-231 Xenograft Models
2.10. Statistical Evaluation
3. Results
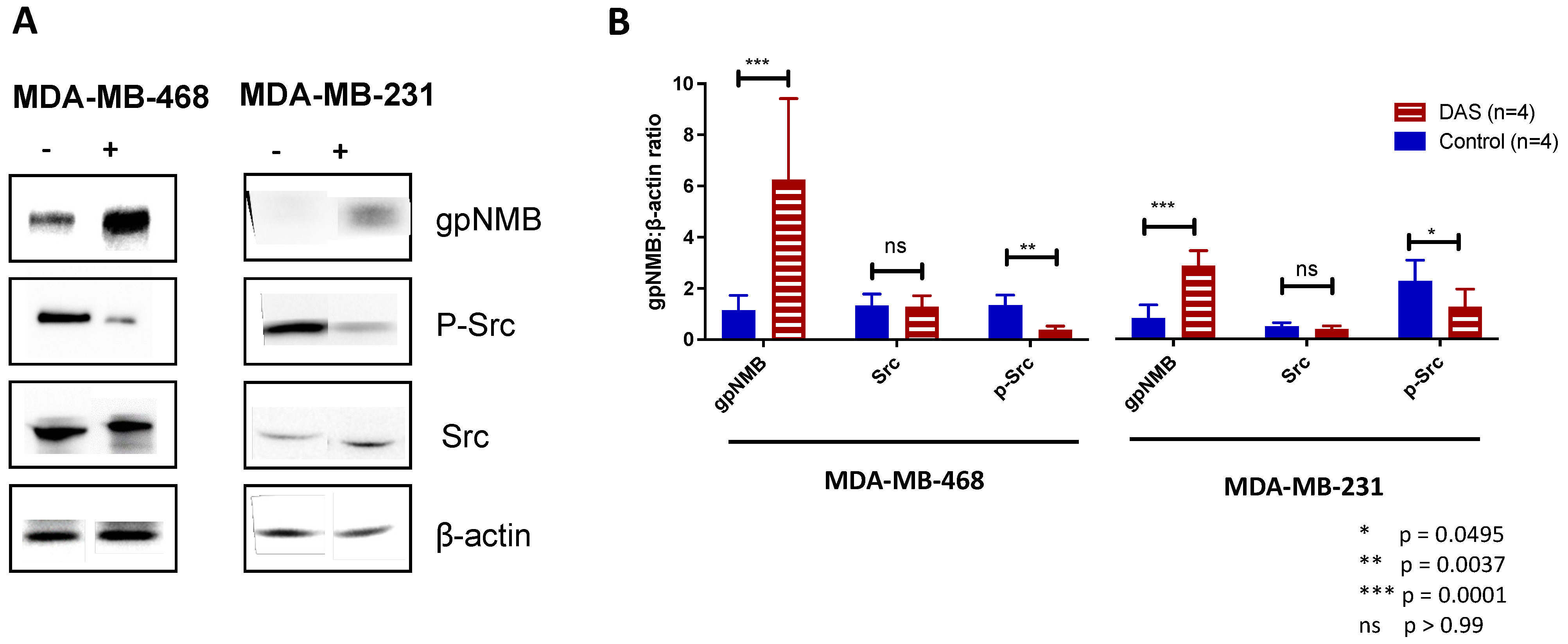
3.1. Dasatinib Treatment Upregulates gpNMB Expression in MDA-MB-468 Cells In Vitro
3.2. gpNMB Upregulation Was Determined to Be 14 Days after Dasatinib Treatment In Vivo
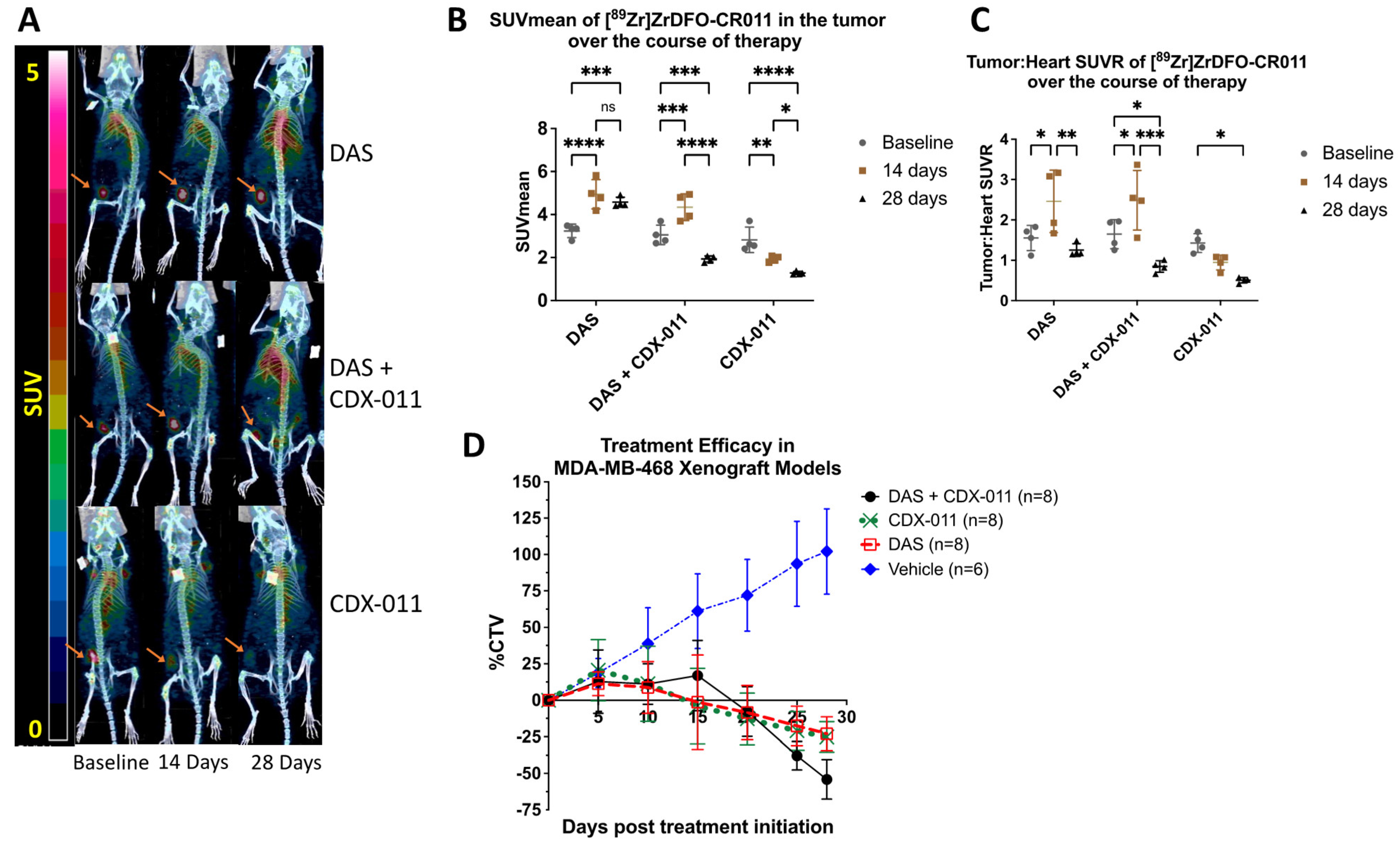
3.3. PET Imaging with [89Zr]Zr-DFO-CR011 Demonstrated Increased Tracer Uptake in MDA-MB-468 Tumors In Vivo at 14 Days after Dasatinib Treatment
3.4. Combination Treatment with CDX-011 and Dasatinib Was More Effective in Tumor Shrinkage Than Monotherapy in MDA-MB-468 Xenograft Models at Endpoint
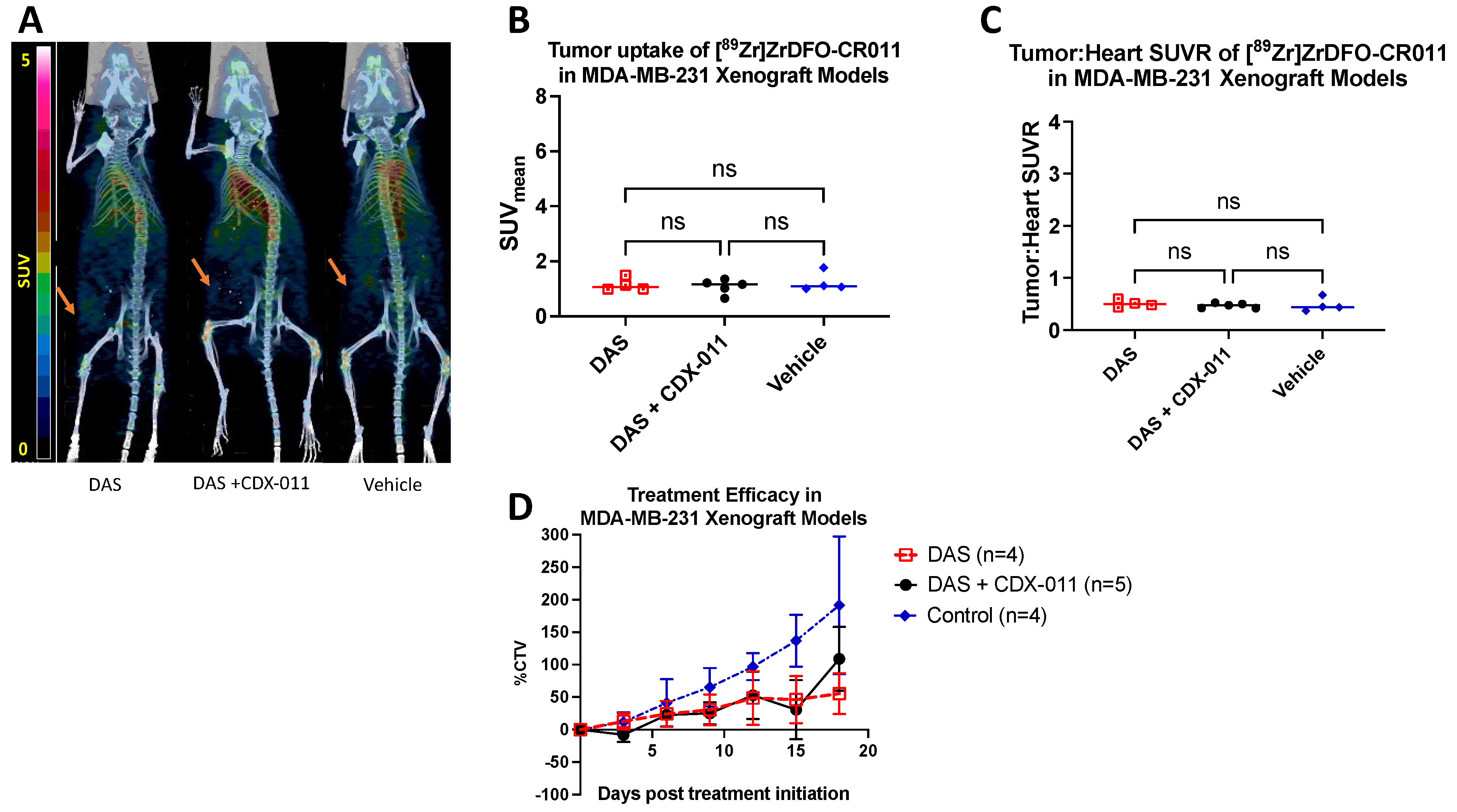
3.5. Dasatinib Did Not Affect the Tumor Uptake of [89Zr]ZrDFO-CR011 in gpNMB-Negative MDA-MB-231 Xenograft Models
4. Discussion
4.1. Monitoring gpNMB Expression via Immunopet with [89Zr]Zr-DFO-CR011
4.2. Rational Design for Combination Therapy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Jadid, H.; Denson, A.C.; Gray, J.E. Targeti ng Trop-2 in solid tumors: Future prospects. Oncotargets Ther. 2019, 12, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wei, R.; Lin, Y.; Kwok, H.F. Clinical and Recent Patents Applications of PD-1/PD-L1 Targeting Immunotherapy in Cancer Treatment-Current Progress, Strategy, and Future Perspective. Front. Immunol. 2020, 11, 1508. [Google Scholar] [CrossRef]
- Tzikas, A.-K.; Nemes, S.; Linderholm, B.K. A comparison between young and old patients with triple-negative breast cancer: Biology, survival and metastatic patterns. Breast Cancer Res. Treat. 2020, 182, 643–654. [Google Scholar] [CrossRef]
- Chen, C.; Okita, Y.; Watanabe, Y.; Abe, F.; Fikry, M.A.; Ichikawa, Y.; Suzuki, H.; Shibuya, A.; Kato, M. Glycoprotein nmb Is Exposed on the Surface of Dormant Breast Cancer Cells and Induces Stem Cell-like Properties. Cancer Res. 2018, 78, 6424–6435. [Google Scholar] [CrossRef]
- Taya, M.; Hammes, S.R. Glycoprotein Non-Metastatic Melanoma Protein B (GPNMB) and Cancer: A Novel Potential Therapeutic Target. Steroids 2018, 133, 102–107. [Google Scholar] [CrossRef]
- Kolb, E.A.; Gorlick, R.; Billups, C.A.; Hawthorne, T.; Kurmasheva, R.T.; Houghton, P.J.; Smith, M.A. Initial testing (stage 1) of glembatumumab vedotin (CDX-011) by the pediatric preclinical testing program. Pediatr. Blood Cancer 2014, 61, 1816–1821. [Google Scholar] [CrossRef]
- Rose, A.A.; Annis, M.G.; Frederick, D.T.; Biondini, M.; Dong, Z.; Kwong, L.; Chin, L.; Keler, T.; Hawthorne, T.; Watson, I.R.; et al. MAPK Pathway Inhibitors Sensitize BRAF-Mutant Melanoma to an Antibody-Drug Conjugate Targeting GPNMB. Clin. Cancer Res. 2016, 22, 6088–6098. [Google Scholar] [CrossRef]
- Rose, A.A.N.; Biondini, M.; Curiel, R.; Siegel, P.M. Targeting GPNMB with glembatumumab vedotin: Current developments and future opportunities for the treatment of cancer. Pharmacol. Ther. 2017, 179, 127–141. [Google Scholar] [CrossRef]
- Qian, X.; Mills, E.; Torgov, M.; LaRochelle, W.J.; Jeffers, M. Pharmacologically enhanced expression of GPNMB increases the sensitivity of melanoma cells to the CR011-vcMMAE antibody-drug conjugate. Mol. Oncol. 2008, 2, 81–93. [Google Scholar] [CrossRef]
- Wolska-Washer, A.; Robak, T. Safety and Tolerability of Antibody-Drug Conjugates in Cancer. Drug Saf. 2019, 42, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; Bossenmaier, B.; Kollmorgen, G.; Niederfellner, G. Acquired Resistance to Antibody-Drug Conjugates. Cancers 2019, 11, 394. [Google Scholar] [CrossRef]
- Riganti, C.; Contino, M. New Strategies to Overcome Resistance to Chemotherapy and Immune System in Cancer. Int. J. Mol. Sci. 2019, 20, 4783. [Google Scholar] [CrossRef]
- Biondini, M.; Kiepas, A.; El-Houjeiri, L.; Annis, M.G.; Hsu, B.E.; Fortier, A.M.; Morin, G.; Martina, J.A.; Sirois, I.; Aguilar-Mahecha, A.; et al. HSP90 inhibitors induce GPNMB cell-surface expression by modulating lysosomal positioning and sensitize breast cancer cells to glembatumumab vedotin. Oncogene 2022, 41, 1701–1717. [Google Scholar] [CrossRef]
- Maric, G.; Rose, A.A.; Annis, M.G.; Siegel, P.M. Glycoprotein non-metastatic b (GPNMB): A metastatic mediator and emerging therapeutic target in cancer. Oncotargets Ther. 2013, 6, 839–852. [Google Scholar]
- Kurebayashi, J.; Kanomata, N.; Moriya, T.; Kozuka, Y.; Watanabe, M.; Sonoo, H. Preferential antitumor effect of the Src inhibitor dasatinib associated with a decreased proportion of aldehyde dehydrogenase 1-positive cells in breast cancer cells of the basal B subtype. BMC Cancer 2010, 10, 568. [Google Scholar] [CrossRef] [PubMed]
- Bahman, F.; Pittalà, V.; Haider, M.; Greish, K. Enhanced anticancer activity of nanoformulation of dasatinib against triple-negative breast cancer. J. Pers. Med. 2021, 11, 559. [Google Scholar] [CrossRef]
- García-Ferrer, M.; Wojnicz, A.; Mejía, G.; Koller, D.; Zubiaur, P.; Abad-Santos, F. Utility of therapeutic drug monitoring of imatinib, nilotinib, and dasatinib in chronic myeloid leukemia: A systematic review and meta-analysis. Clin. Ther. 2019, 41, 2558–2570.e7. [Google Scholar] [CrossRef] [PubMed]
- Ocana, A.; Gil-Martin, M.; Antolín, S.; Atienza, M.; Montaño, Á.; Ribelles, N.; Urruticoechea, A.; Falcón, A.; Pernas, S.; Orlando, J. Efficacy and safety of dasatinib with trastuzumab and paclitaxel in first line HER2-positive metastatic breast cancer: Results from the phase II GEICAM/2010-04 study. Breast Cancer Res. Treat. 2019, 174, 693–701. [Google Scholar] [CrossRef]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.Y.; Cai, W. ImmunoPET: Concept, Design, and Applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef]
- McKnight, B.N.; Kim, S.; Boerner, J.L.; Viola, N.T. Cetuximab PET delineated changes in cellular distribution of EGFR upon dasatinib treatment in triple negative breast cancer. Breast Cancer Res. 2020, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cavaliere, A.; Gallezot, J.D.; Keler, T.; Michelhaugh, S.K.; Belitzky, E.; Liu, M.; Mulnix, T.; Maher, S.E.; Bothwell, A.L.M.; et al. [89Zr]ZrDFO-CR011 PET Correlates with Response to Glycoprotein Nonmetastatic Melanoma B-targeted Therapy in Triple-negative Breast Cancer. Mol. Cancer Ther. 2022, 21, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Nostra, B.V.; Lee, S.; Laforest, R.; Vitale, L.; Nie, X.; Hyrc, K.; Keler, T.; Hawthorne, T.; Hoog, J.; Li, S.; et al. Preclinical PET imaging of glycoprotein non-metastatic melanoma B in triple negative breast cancer: Feasibility of an antibody-based companion diagnostic agent. Oncotarget 2017, 8, 104303–104314. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, J.; Yu, Y.; Aboukameel, A.; Kanwar, S.S.; Das, J.K.; Du, J.; Patel, B.B.; Sarkar, F.H.; Rishi, A.K.; Mohammad, R.M.; et al. ErbB-inhibitory protein: A modified ectodomain of epidermal growth factor receptor synergizes with dasatinib to inhibit growth of breast cancer cells. Mol. Cancer Ther. 2010, 9, 1503–1514. [Google Scholar] [CrossRef]
- Ullman-Cullere, M.H.; Foltz, C.J. Body condition scoring: A rapid and accurate method for assessing health status in mice. Lab. Anim. Sci. 1999, 49, 319–323. [Google Scholar]
- Qian, X.L.; Zhang, J.; Li, P.Z.; Lang, R.G.; Li, W.D.; Sun, H.; Liu, F.F.; Guo, X.J.; Gu, F.; Fu, L. Dasatinib inhibits c-src phosphorylation and prevents the proliferation of Triple-Negative Breast Cancer (TNBC) cells which overexpress Syndecan-Binding Protein (SDCBP). PLoS ONE 2017, 12, e0171169. [Google Scholar] [CrossRef]
- Chen, B.; Xu, X.; Luo, J.; Wang, H.; Zhou, S. Rapamycin Enhances the Anti-Cancer Effect of Dasatinib by Suppressing Src/PI3K/mTOR Pathway in NSCLC Cells. PLoS ONE 2015, 10, e0129663. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, X.; Jia, Y.; Chi, F.; Qin, K.; Pei, J.; Zhang, C.; Mu, X.; Zhang, H.; Dong, X.; et al. Dasatinib inhibits proliferation of liver cancer cells, but activation of Akt/mTOR compromises dasatinib as a cancer drug. Acta Biochim. Biophys. Sin. 2021, 53, 823–836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Belitzky, E.; Blaha, O.; Cavaliere, A.; Katz, S.R.; Aboian, M.; Melegari, L.; Rajabimoghadam, K.; Kurpiewski, S.; Zhu, X.; et al. ImmunoPET Imaging Identifies the Optimal Timepoint for Combination Therapy in Xenograft Models of Triple-Negative Breast Cancer. Cancers 2023, 15, 1589. https://doi.org/10.3390/cancers15051589
Li Z, Belitzky E, Blaha O, Cavaliere A, Katz SR, Aboian M, Melegari L, Rajabimoghadam K, Kurpiewski S, Zhu X, et al. ImmunoPET Imaging Identifies the Optimal Timepoint for Combination Therapy in Xenograft Models of Triple-Negative Breast Cancer. Cancers. 2023; 15(5):1589. https://doi.org/10.3390/cancers15051589
Chicago/Turabian StyleLi, Ziqi, Erika Belitzky, Ondrej Blaha, Alessandra Cavaliere, Samantha R. Katz, Mariam Aboian, Lindy Melegari, Khashayar Rajabimoghadam, Stephen Kurpiewski, Xiaohua Zhu, and et al. 2023. "ImmunoPET Imaging Identifies the Optimal Timepoint for Combination Therapy in Xenograft Models of Triple-Negative Breast Cancer" Cancers 15, no. 5: 1589. https://doi.org/10.3390/cancers15051589
APA StyleLi, Z., Belitzky, E., Blaha, O., Cavaliere, A., Katz, S. R., Aboian, M., Melegari, L., Rajabimoghadam, K., Kurpiewski, S., Zhu, X., & Marquez-Nostra, B. (2023). ImmunoPET Imaging Identifies the Optimal Timepoint for Combination Therapy in Xenograft Models of Triple-Negative Breast Cancer. Cancers, 15(5), 1589. https://doi.org/10.3390/cancers15051589





