Role of the Molecular Tumor Board for the Personalized Treatment of Patients with Metastatic Breast Cancer: A Focus on the State of the Art in Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. New Strategies for Precision Oncology
3. Variant Classification Scales
4. New Clinical Trial Designs
5. Molecular Screening Studies
6. MTBs: Current Status and Future Prospects
7. MTBs: The State of the Art in Italy
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Di Pilla, A.; Cozzolino, M.R.; Mannocci, A.; Carini, E.; Spina, F.; Castrini, F.; Grieco, A.; Messina, R.; Damiani, G.; Specchia, M.L. The Impact of Tumor Boards on Breast Cancer Care: Evidence from a Systematic Literature Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14990. [Google Scholar] [CrossRef] [PubMed]
- Gori, S.; Miglietta, F.; Modena, A. Mammella. I numeri del cancro in Italia; Intermedia Editore: Brescia, Italy, 2021; pp. 64–66. Available online: https://www.aiom.it/wp-content/uploads/2021/11/2021_NDC.pdf (accessed on 11 February 2023).
- Gori, S.; Miglietta, F.; Biganzoli, L.; Calabrese, M.; Cortesi, L.; Conte, B.; Criscitiello, C.; Del Mastro, L.; Dieci, M.V.; Folli, S.; et al. Linee guida “Neoplasie della mammella”; Associazione Italiana di Oncologia Medica: Milano, Italy, 2021; pp. 27–30. Available online: https://www.aiom.it/wp-content/uploads/2021/11/2021_LG_AIOM_Neoplasie_Mammella_11112021.pdf.pdf (accessed on 11 February 2023).
- Biganzoli, L.; Cardoso, F.; Beishon, M.; Cameron, D.; Cataliotti, L.; Coles, C.E.; Delgado Bolton, R.C.; Die Trill, M.; Erdem, S.; Fjell, M.; et al. The requirements of a specialist breast centre. Breast 2020, 51, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; De Michele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agenese, D.; Allison, K.H.; Andreson, B.; Burstein, H.J.; Chew, H.; et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer. Version 2.2023. 2023. Available online: www.nccn.org/patients (accessed on 11 February 2023).
- Luchini, C.; Lawlor, R.T.; Milella, M.; Aldo Scarpa, A. Molecular Tumor Boards in Clinical Practice. Trends Cancer. 2020, 6, 738–744. [Google Scholar] [CrossRef]
- Frost, H.; Graham, D.M.; Carter, L.; O’Regan, P.; Landers, D.; Freitas, A. Patient attrition in Molecular Tumour Boards: A systematic review. Br. J. Cancer 2022, 127, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.L.; Huang, B.; Weiss, H.L.; Hull, P.; Westgate, P.M.; Miller, R.W.; Arnold, S.M.; Kolesar, J.M. Clinical Outcomes of Molecular Tumor Boards: A Systematic Review. JCO Precis. Oncol. 2021, 5, PO.20.00495. [Google Scholar] [CrossRef]
- Martini, N.; Piccinni, C. The tools for the governance of mutational oncology and agnostic drugs: From place in therapy to place in pathway. Recenti Prog Med. 2021, 112, 805–806. [Google Scholar]
- Sultova, E.; Westphalen, C.B.; Jung, A.; Kumbrink, J.; Kirchner, T.; Mayr, D.; Rudelius, M.; Ormanns, S.; Heinemann, V.; Metzeler, K.H.; et al. Implementation of Precision Oncology for Patients with Metastatic Breast Cancer in an Interdisciplinary MTB Setting. Diagnostics 2021, 11, 733. [Google Scholar] [CrossRef]
- Russo, A.; Incorvaia, L.; Beretta, G.; Chiari, R.; Cinieri, S.; Ferrara, R.; Galvano, A.; Gori, S.; Guadagni, F.; Marchetti, P.; et al. Raccomandazioni AIOM “Tumor Board Molecolare”; Associazione Italiana di Oncologia Medica: Milano, Italy, 2020; pp. 1–20. Available online: https://www.aiom.it/raccomandazioni-2020-tumor-board-molecolare/ (accessed on 11 February 2023).
- Schwartzberg, L.; Kim, E.S.; Liu, D.; Schrag, D. Precision Oncology: Who, How, What, When, and When Not? Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 160–169. [Google Scholar] [CrossRef]
- Crimini, E.; Repetto, M.; Tarantino, P.; Ascione, L.; Antonarelli, G.; Guerini Rocco, E.; Barberis, M.; Mazzarella, L.; Curigliano, G. Challenges and Obstacles in Applying Therapeutical Indications Formulated in Molecular Tumor Boards. Cancers 2022, 14, 3193. [Google Scholar] [CrossRef]
- Pinto, C.; Biffoni, M.; Popoli, P.; Marchetti, A.; Marchetti, P.; Martini, N.; Normanno, N. Molecular tests and target therapies in oncology: Recommendations from the Italian workshop. Future Oncol. 2021, 17, 3529–3539. [Google Scholar] [CrossRef]
- Andre, F.; Mardis, E.; Salm, M.; Soria, J.C.; Siu, L.L.; Swanton, C. Prioritizing targets for precision cancer medicine. Ann Oncol. 2014, 25, 2295–2303. [Google Scholar] [CrossRef]
- Van Allen, E.M.; Wagle, N.; Stojanov, P.; Perrin, D.L.; Cibulskis, K.; Marlow, S.; Jane-Valbuena, J.; Friedrich, D.C.; Kryukov, G.; Carter, S.L.; et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat. Med. 2014, 20, 682–688. [Google Scholar] [CrossRef]
- Dienstmann, R.; Sock Jang, I.; Bot, B.; Friend, S.; Guinney, J. Database of genomic biomarkers for cancer drugs and clinical targetability in solid tumors. Cancer Discov. 2015, 5, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Sukhai, M.A.; Craddock, K.J.; Thomas, M.; Hansen, A.R.; Zhang, T.; Siu, L.; Bedard, P.; Stockley, T.L.; Kamel-Reid, S. A classification system for clinical relevance of somatic variants identified in molecular profiling of cancer. Genet. Med. 2016, 18, 128–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarty, D.; Gao, J.; Phillips, S.M.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 2017, PO.17.00011. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condorelli, R.; Mosele, F.; Verret, B.; Bachelot, T.; Bedard, P.L.; Cortes, J.; Hyman, D.M.; Juric, D.; Krop, I.; Bieche, I.; et al. Genomic alterations in breast cancer: Level of evidence for actionability according to ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 2019, 30, 365–373. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Botticelli, A.; Scagnoli, S.; Conte, P.; Cremolini, C.; Ascierto, P.A.; Cappuzzo, F.; Aglietta, M.; Mazzuca, F.; Capoluongo, E.; Blandino, G.; et al. Mutational landscape of breast cancer patients in ROME trial: Preliminary results. Cancer Res. 2023, 83, P6-10-09. [Google Scholar] [CrossRef]
- Trotta, F.; Traversa, G. La Ricerca di Precisione tra Ombrelli e Cestini. 2016. Available online: https://forward.recentiprogressi.it/wp-content/uploads/2016/08/recprogrmed_2016_suppl1_trotta_traversa.pdf (accessed on 11 February 2023).
- Lewis, C.; Harvey, R.D. Precision Oncology Comes of Age: Tumor-Agnostic Approaches. J. Adv. Pract. Oncol. 2020, 11, 221–225. [Google Scholar]
- Massard, C.; Michiels, S.; Ferté, C.; Le Deley, M.C.; Lacroix, L.; Hollebecque, A.; Verlingue, L.; Ileana, E.; Rosellini, S.; Ammari, S.; et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017, 7, 586–595. [Google Scholar] [CrossRef] [Green Version]
- André, F.; Bachelot, T.; Commo, F.; Campone, M.; Arnedos, M.; Dieras, V.; Lacroix-Triki, M.; Lacroix, L.; Cohen, P.; Gentien, D.; et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: A multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 2014, 15, 267–274. [Google Scholar] [CrossRef]
- Pezo, R.C.; Chen, T.W.; Berman, H.K.; Mulligan, A.M.; Razak, A.A.; Siu, L.L.; Cescon, D.W.; Amir, E.; Elser, C.; Warr, D.G.; et al. Impact of multi-gene mutational profiling on clinical trial outcomes in metastatic breast cancer. Breast Cancer Res. Treat. 2018, 168, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernas, S.; Villagrasa, P.; Vivancos, A.; Scaltriti, M.; Rodón, J.; Burgués, O.; Nuciforo, P.; Canes, J.; Paré, L.; Dueñas, M.; et al. First Nationwide Molecular Screening Program in Spain for Patients with Advanced Breast Cancer: Results from the AGATA SOLTI-1301 Study. Front. Oncol. 2021, 11, 744112. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.A.; Schwaederlé, M.; Scur, M.D.; Boles, S.G.; Helsten, T.; Subramanian, R.; Schwab, R.B.; Kurzrock, R. Breast Cancer Experience of the Molecular Tumor Board at the University of California, San Diego Moores Cancer Center. J. Oncol. Pract. 2015, 11, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Hartkopf, A.; Koch, A.; Klaumünzer, M.; Schulze, M.; Grischke, E.M.; Taran, F.A.; Brucker, S.; Battke, F.; Biskup, S. Sequencing for an interdisciplinary molecular tumor board in patients with advanced breast cancer: Experiences from a case series. Oncotarget 2020, 11, 3279–3285. [Google Scholar] [CrossRef]
- Aftimos, P.; Oliveira, M.; Irrthum, A.; Fumagalli, D.; Sotiriou, C.; Nili Gal-Yam, E.; Robson, M.E.; Ndozeng, J.; Di Leo, A.; Ciruelos, E.M.; et al. Genomic and Transcriptomic Analyses of Breast Cancer Primaries and Matched Metastases in AURORA, the Breast International Group (BIG) Molecular Screening Initiative. Cancer Discov. 2021, 11, 2796–2811. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Delord, J.P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Trédan, O.; Massiani, M.A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- André, F.; Gonçalves, A.; Filleron, T.; Dalenc, F.; Lusque, A.; Campone, M.; Sablin, M.P.; Bonnefoi, H.; Bieche, I.; Lacroix, L.; et al. Clinical utility of molecular tumor profiling: Results from the randomized trial SAFIR02-BREAST. Cancer Res. 2022, 82, GS1-10. [Google Scholar] [CrossRef]
- Hlevnjak, M.; Schulze, M.; Elgaafary, S.; Fremd, C.; Michel, L.; Beck, K.; Pfütze, K.; Richter, D.; Wolf, S.; Horak, P.; et al. CATCH: A Prospective Precision Oncology Trial in Metastatic Breast Cancer. JCO Precis. Oncol. 2021, 5, PO.20.00248. [Google Scholar] [CrossRef]
- Bruzas, S.; Kuemmel, S.; Harrach, H.; Breit, E.; Ataseven, B.; Traut, A.; Rüland, A.; Kostara, A.; Chiari, O.; Dittmer-Grabowski, C.; et al. Next-Generation Sequencing-Directed Therapy in Patients with Metastatic Breast Cancer in Routine Clinical Practice. Cancers 2021, 13, 4564. [Google Scholar] [CrossRef] [PubMed]
- Fukada, I.; Mori, S.; Hayashi, N.; Hosonaga, M.; Yamazaki, M.; Wang, X.; Kawai, S.; Inagaki, L.; Ozaki, Y.; Kobayashi, K.; et al. Assessment of a cancer genomic profile test for patients with metastatic breast cancer. Sci. Rep. 2022, 12, 4813. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Kingston, B.; Kilburn, L.S.; Kernaghan, S.; Wardley, A.M.; Macpherson, I.R.; Baird, R.D.; Roylance, R.; Stephens, P.; Oikonomidou, O.; et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): A multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020, 21, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- van Geelen, C.T.; Savas, P.; Ling Teo, Z.; Luen, S.J.; Weng, C.F.; Ko, Y.A.; Kuykhoven, K.S.; Caramia, F.; Salgado, R.; Francis, P.A.; et al. Clinical implications of prospective genomic profiling of metastatic breast cancer patients. Breast Cancer Res. 2020, 22, 91. [Google Scholar] [CrossRef]
- The Rome Trial from Histology to Target: The Road to Personalize Target Therapy and Immunotherapy (ROME). Available online: https://clinicaltrials.gov/ct2/show/NCT04591431 (accessed on 11 February 2023).
- Kurnit, K.C.; Dumbrava, E.E.I.; Litzenburger, B.; Khotskaya, Y.B.; Johnson, A.M.; Yap, T.A.; Rodon, J.; Zeng, J.; Shufean, M.A.; Bailey, A.M.; et al. Precision Oncology Decision Support: Current Approaches and Strategies for the Future. Clin. Cancer Res. 2018, 24, 2719–2731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angel, M.O.; Pupareli, C.; Soule, T.; Tsou, F.; Leiva, M.; Losco, F.; Esteso, F.; O Connor, J.M.; Luca, R.; Petracci, F.; et al. Implementation of a molecular tumour board in LATAM: The impact on treatment decisions for patients evaluated at Instituto Alexander Fleming, Argentina. Ecancermedicalscience 2021, 15, 1312. [Google Scholar] [CrossRef]
- Kato, S.; Kim, K.H.; Lim, H.J.; Boichard, A.; Nikanjam, M.; Weihe, E.; Kuo, D.J.; Eskander, R.N.; Goodman, A.; Galanina, N.; et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 2020, 11, 4965. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Guo, H.; Srivastava, P.; James, T.; Birch, W.; Siu, L.L.; Tew, W.P.; Tolaney, S.M. Utilization of tumor genomics in clinical practice: An international survey among ASCO members. Future Oncol. 2019, 15, 2463–2470. [Google Scholar] [CrossRef]
- Hamamoto, R.; Koyama, T.; Kouno, N.; Yasuda, T.; Yui, S.; Sudo, K.; Hirata, M.; Sunami, K.; Kubo, T.; Takasawa, K.; et al. Introducing AI to the molecular tumor board: One direction toward the establishment of precision medicine using large-scale cancer clinical and biological information. Exp. Hematol. Oncol. 2022, 11, 82. [Google Scholar] [CrossRef]
- Tamborero, D.; Dienstmann, R.; Rachid, M.H.; Boekel, J.; Lopez-Fernandez, A.; Jonsson, M.; Razzak, A.; Braña, I.; De Petris, L.; Yachnin, J.; et al. The Molecular Tumor Board Portal supports clinical deci-sions and automated reporting for precision oncology. Nat. Cancer 2022, 2, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, L.; Russo, A.; Cinieri, S. The molecular tumor board: A tool for the governance of precision oncology in the real world. Tumori 2022, 108, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Gramolini, E. Oncologia di Precisione, sì ai Molecular Tumor Board. Panorama della Sanità. 2021. Available online: https://www.panoramasanita.it/2021/12/17/oncologia-di-precisione-si-ai-molecular-tumor-board/ (accessed on 11 February 2023).
- Bollettino Delle Giunte E Delle Commissioni Parlamentari. 2021. Available online: http://documenti.camera.it/leg18/resoconti/commissioni/bollettini/pdf/2021/12/14/leg.18.bol0713.data20211214.pdf (accessed on 11 February 2023).
- Marchetti, A.; Barbareschi, M.; Barberis, M.; Buglioni, S.; Buttitta, F.; Fassan, M.; Fontanini, G.; Marchiò, C.; Papotti, M.; Pruneri, G.; et al. Real-World Data on NGS Diagnos-tics: A survey from the Italian Society of Pathology (SIAPeC) NGS Network. Pathologica 2021, 113, 262–271. [Google Scholar] [CrossRef]
- ISO 15189:2022; Medical Laboratories—Requirements for Quality and Competence. ISO Standards: Geneva, Switzerland, 2022. Available online: https://www.iso.org/standard/76677.html (accessed on 3 March 2023).
- Wolff, L.; Kiesewetter, B. Applicability of ESMO-MCBS and ESCAT for Molecular Tumor Boards. memo 2022, 15, 190–195. [Google Scholar] [CrossRef]
- Curigliano, G.; Ciliberto, G.; Criscitiello, C.; Lazzari, C.; Lorusso, D.; Montemurro, F.; Nanni, O.; Tommasi, S. Capitolo 7: Accesso ai Farmaci. Linee Guida per l’istituzione e la gestione dei Molecular Tumor Board negli Istituti di Alleanza Contro il Cancro. 2020. Available online: https://www.alleanzacontroilcancro.it/wp-content/uploads/2021/03/Linee-guida.pdf (accessed on 11 February 2023).
- Martini, N.; De Maria, R.; Amunni, G.; Apolone, G.; Beretta, G.; Bertetto, O.; Blasi, L.; Ciliberto, G.; Cinieri, S.; Conte, P.F.; et al. Documento di consenso sullo sviluppo e sull’organizzazione dell’oncologia mutazionale in Italia. Il Pensiero Scientifico Editore. Suppl. A Politiche Sanit. 2020, 21, 1–12. [Google Scholar]
- Fasola, G.; Barducci, M.C.; Pelizzari, G.; Grossi, F.; Pinto, C.; Daniele, B.; Giordano, M.; Ortega, C.; Silva, R.R.; Tozzi, V.D.; et al. Implementation of Precision Oncology in Clinical Practice: Results of a National Survey for Health Care Professionals. Oncologist 2023, oyad020. [Google Scholar] [CrossRef]
- Bardia, A.; Bidard, F.C.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. EMERALD phase 3 trial of elacestrant versus standard of care endo-crine therapy in patients with ER+/HER2- metastatic breast cancer: Updated results by duration of prior CDK4/6i in metastatic setting. Cancer Res. 2023, 83, GS3-01. [Google Scholar] [CrossRef]
- Turner, N.C.; Oliveira, M.; Howell, S.; Dalenc, F.; Cortes, J.; Gomez, H.; Hu, X.; Jhaveri, K.; Loibl, S.; Morales Murillo, S.; et al. Capivasertib and fulvestrant for patients with aromatase inhibitor-resistant hormone receptor-positive/human epi-dermal growth factor receptor 2-negative advanced breast cancer: Results from the Phase III CAPItello-291 trial. Cancer Res. 2023, 83, GS3-04. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated with ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Falcone, R.; Lombardi, P.; Filetti, M.; Fabi, A.; Altamura, V.; Scambia, G.; Daniele, G. Molecular Profile and Matched Targeted Therapy for Advanced Breast Cancer Patients. Curr. Oncol. 2023, 30, 2501–2509. [Google Scholar] [CrossRef]

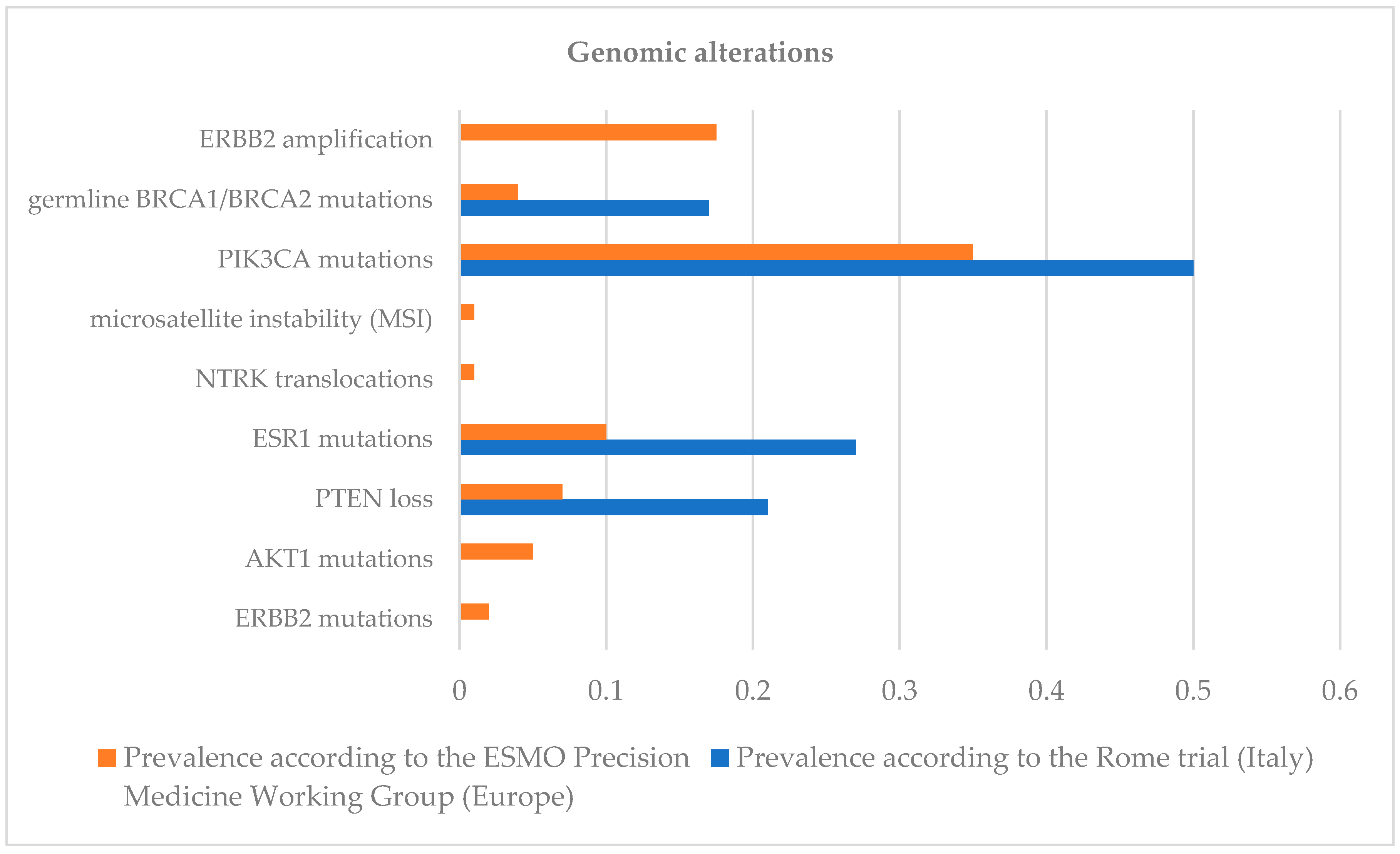
| Genomic Alteration | Level |
|---|---|
| ERBB2 amplification | I |
| Germline BRCA1/BRCA2 mutations | I |
| PIK3CA mutations | I |
| Microsatellite instability (MSI) | I |
| NTRK translocations | I |
| ESR1 mutations | II |
| PTEN loss | II |
| AKT1 mutations | II |
| ERBB2 mutations | II |
| Author/Study | Type of Metastatic Tumor (n, %) | Enrolled Patients (n) | Evaluable Patients (n) | Patients with Actionable Alterations (n, %) | Patients Receiving Targetable Therapies (n, %) | Results |
|---|---|---|---|---|---|---|
| Massard et al. [27]/ Moscato-01 | solid tumors (breast: 135, 14%) | 948 | 843 | 411, 49% | 199, 27% | PFS2/PFS1 > 1.3. Sixty-three out of 948 pts (7%) benefited in PFS. |
| Andrè et al. [28]/ Safir01/UNICANCER | breast (423, 100%) | 423 | 297 | 195, 66% | 55, 19% | Four out of 43 patients (9%) had an objective response. Nine (21%) had stable disease for more than 16 weeks. |
| Pezo et al. [29] | breast (483, 100%) | 483 | 440 | 203, 46% | 15% | No difference in median time on treatment between patients treated with matched therapies and those with unmatched therapies (3.6 vs. 3.8 months, p = 0.89). |
| Pernas et al. [30]/ SOLTI-1301 AGATA | breast (305, 100%) | 305 | 260 | 123, 47% | 13, 5% | Among 13 patients, 46.2% had PFS ≥ 6 months with combination therapy. |
| Parker et al. [31] | breast (43, 100%) | 43 | 40 | 17, 42% | 17, 42% | PFS was significantly worse for patients receiving a therapy not matched with the identified genomic alteration. |
| Walter et al. [32] | breast (52, 100%) | 52 | 52 | 45, 87% | 22, 42% | - |
| Aftimos et al. [33]/ AURORA | breast (381, 100%) | 381 | 88% | 51% | 7% | - |
| Le Tourneau et al. [34]/ SHIVA | solid tumor (breast: 59, 20%) | 741 | 741 | 293, 39.5% | 195, 26% | The use of targeted drugs did not statistically significantly improve PFS (HR = 0.88; p = 0.41). |
| Andrè et al. [35]/ SAFIR02-BREAST and SAFIR-PI3K | breast (1462, 100%) | 1462 | 238 | 115, 48% | - | Median PFS was 9.1 and 2.8 months in the targeted therapy and chemotherapy arms, respectively (HR = 0.41; p < 0.001). |
| Hlevnjak et al. [36]/ CATCH | breast (200, 100%) | 200 | 128 | 64, 50% | 53, 41% | Twenty-one out of 53 patients (40%) achieved stable disease (n = 13.25%) or partial response (n = 8.15%). Sixteen (30%) of those patients showed PFS improvement of at least 30% during MTB-recommended treatment compared to PFS of the previous line of treatment. |
| Bruzas et al. [37] | breast (95, 100%) | 95 | 83 | 63, 76% | 30, 36% | The ratio of PFS in NGS-based therapy to PFS in the last line of standard therapy before NGS was >1.3 of 13 (43.3%) patients, indicative of a clinical benefit to NGS-directed therapy. The one-year overall survival rates were 22.7% in the 65 patients assigned to standard therapy compared with 62.9% in the 30 patients who received combination therapy. |
| Fukada et al. [38] | 310 (breast: 37, 100%) | 37 | 35 | 30, 86% | 9, 26% | - |
| Turner et al. [39]/ plasmaMATCH | breast (1051, 100%) | 1051 | 1034 | - | Cohort A: 74, 7.2% Cohort B: 20, 1.9% Cohort C: 18, 1.7% Cohort D: 19, 1.8% | Five (25%) of 20 patients in cohort B and four (22%) of 18 patients in cohort C having a response. Six (8%) of 74 in cohort A and two (11%) of 19 patients in cohort D having a response. |
| van Geelen et al. [40] | breast (322, 100%) | 322 | 234 | 171, 73% | 74, 32% | Patients with a higher number of mutations had significantly worse overall survival. |
| Botticelli et al. [24]/ ROME | solid tumor (breast: 62, 6.3%) | 62 | 62 | 34, 55% | 28, 45% | Germline mutations have been identified in patients with no prior indication for germline testing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irelli, A.; Chiatamone Ranieri, S.; Di Giacomo, D.; Malatesta, S.; Patruno, L.V.; Tessitore, A.; Alesse, E.; Cannita, K. Role of the Molecular Tumor Board for the Personalized Treatment of Patients with Metastatic Breast Cancer: A Focus on the State of the Art in Italy. Cancers 2023, 15, 1727. https://doi.org/10.3390/cancers15061727
Irelli A, Chiatamone Ranieri S, Di Giacomo D, Malatesta S, Patruno LV, Tessitore A, Alesse E, Cannita K. Role of the Molecular Tumor Board for the Personalized Treatment of Patients with Metastatic Breast Cancer: A Focus on the State of the Art in Italy. Cancers. 2023; 15(6):1727. https://doi.org/10.3390/cancers15061727
Chicago/Turabian StyleIrelli, Azzurra, Sofia Chiatamone Ranieri, Daniela Di Giacomo, Sara Malatesta, Leonardo Valerio Patruno, Alessandra Tessitore, Edoardo Alesse, and Katia Cannita. 2023. "Role of the Molecular Tumor Board for the Personalized Treatment of Patients with Metastatic Breast Cancer: A Focus on the State of the Art in Italy" Cancers 15, no. 6: 1727. https://doi.org/10.3390/cancers15061727
APA StyleIrelli, A., Chiatamone Ranieri, S., Di Giacomo, D., Malatesta, S., Patruno, L. V., Tessitore, A., Alesse, E., & Cannita, K. (2023). Role of the Molecular Tumor Board for the Personalized Treatment of Patients with Metastatic Breast Cancer: A Focus on the State of the Art in Italy. Cancers, 15(6), 1727. https://doi.org/10.3390/cancers15061727






