Sandwich Culture Platforms to Investigate the Roles of Stiffness Gradients and Cell–Matrix Adhesions in Cancer Cell Migration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Alginate Stocks for Migration Assays
2.3. Cell Culture
2.4. Migration Assay Setup
2.5. Analysis of Cell Migration
3. Results
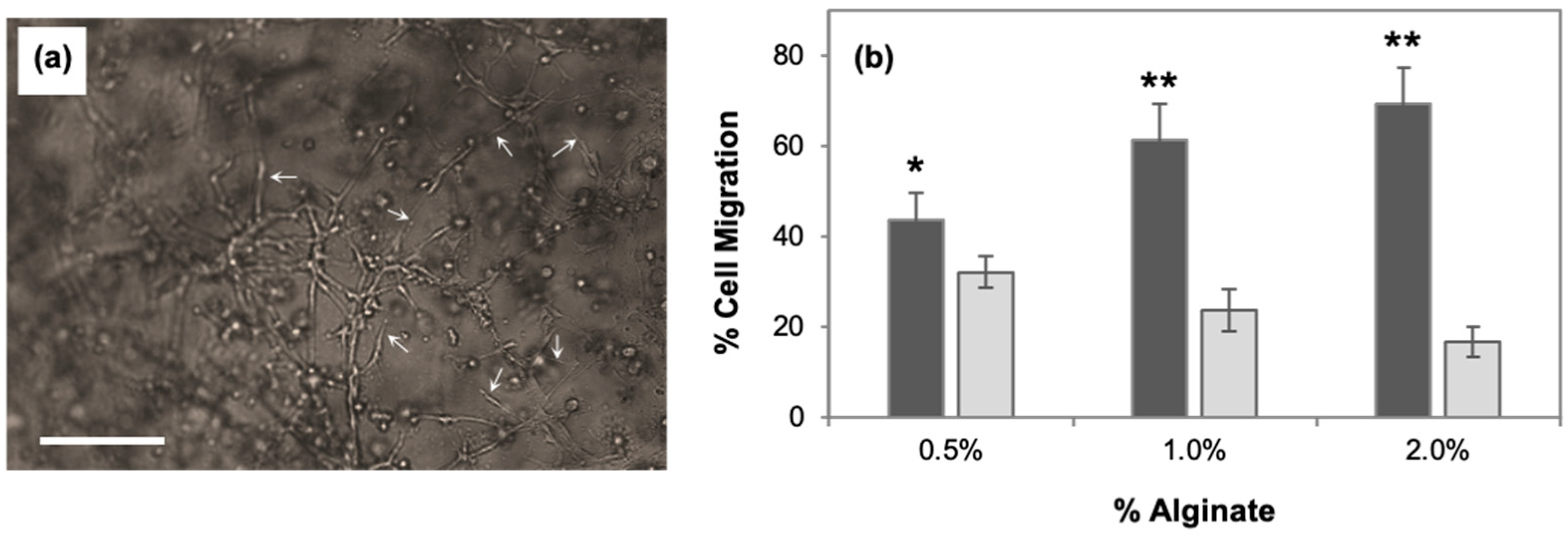
3.1. Alginate Concentration Affects 2.5D Cell Migration
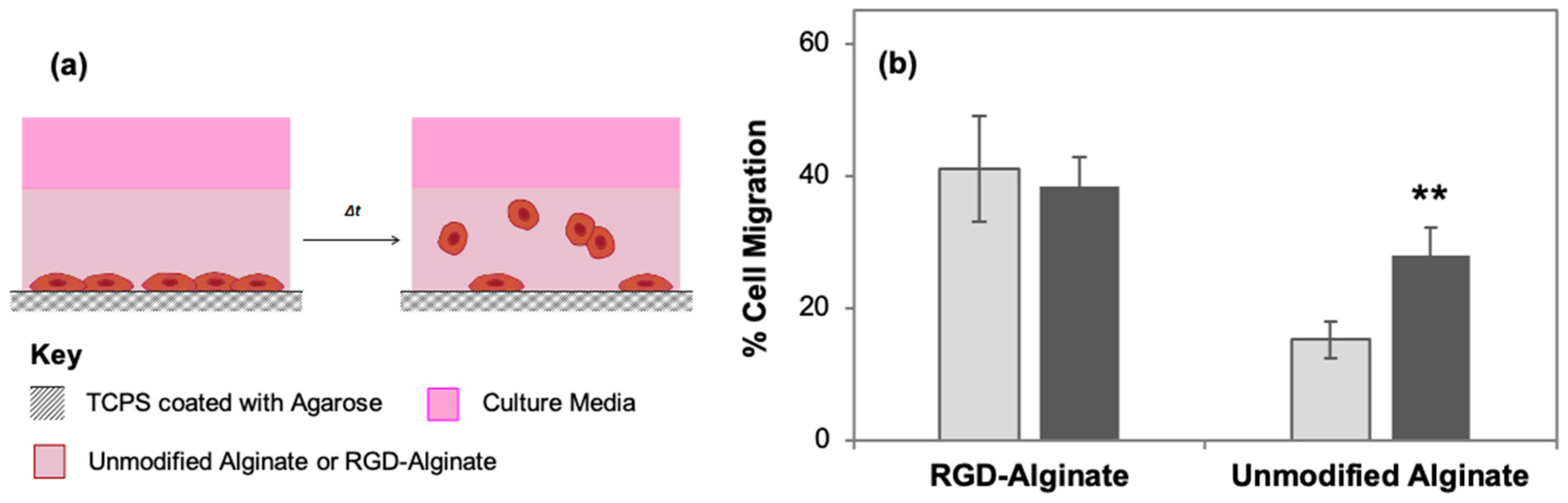
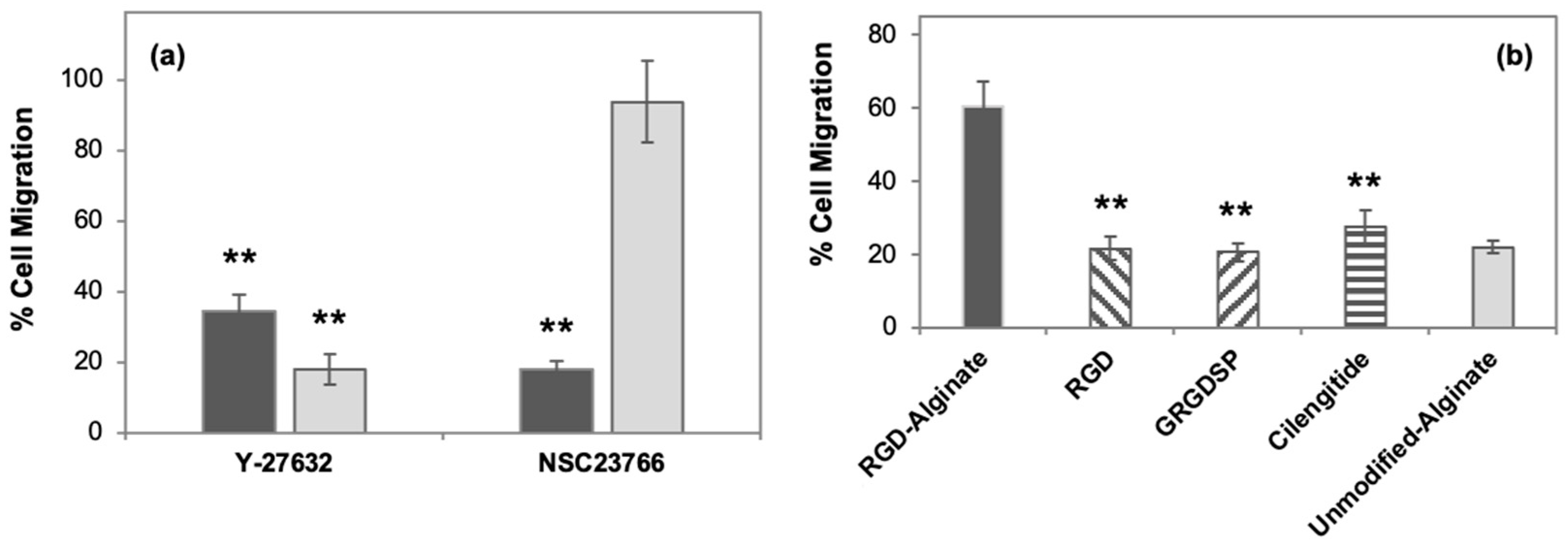
3.2. RGD Density Positively Influences Rate of 2.5D Cell Migration
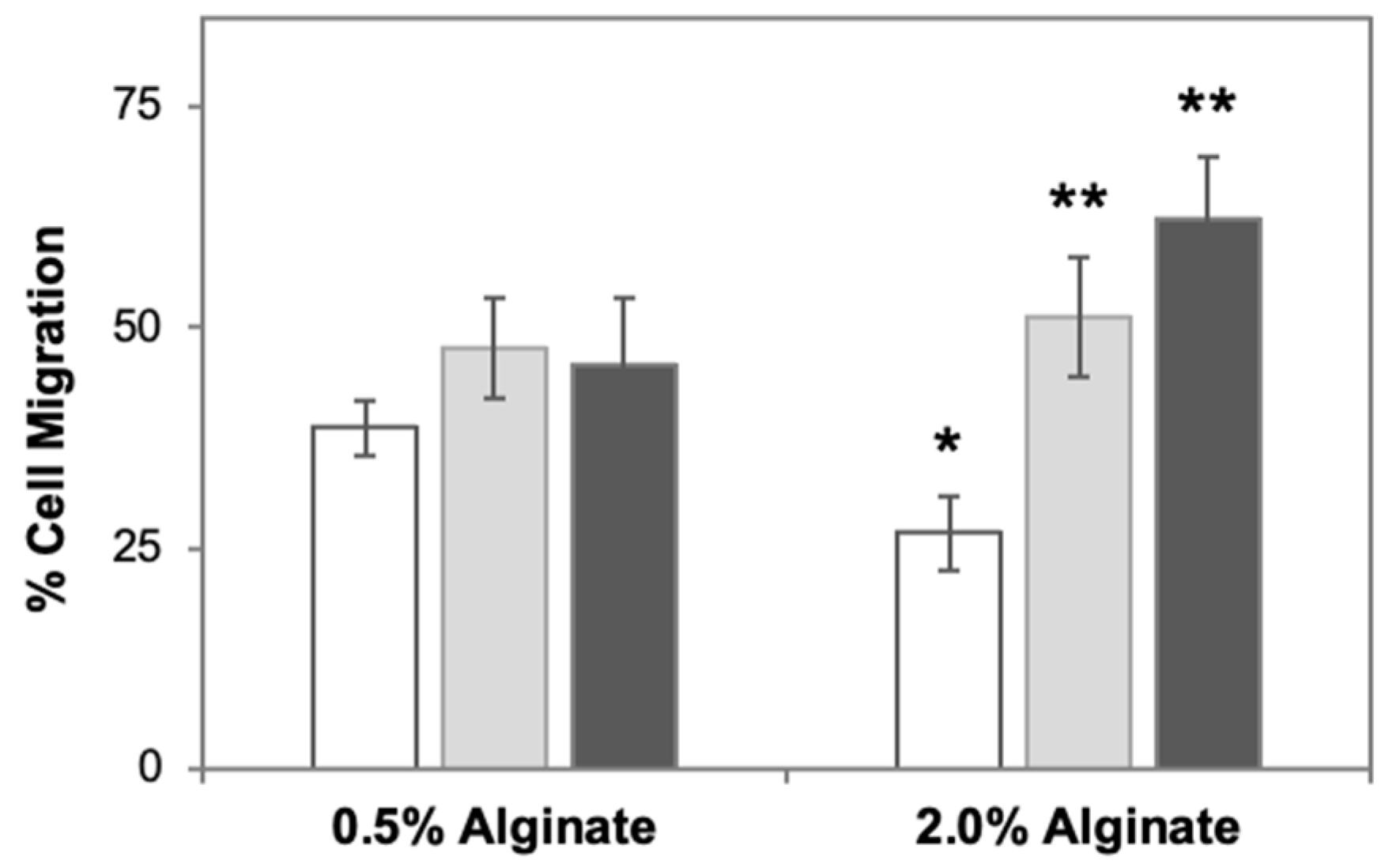
3.3. Reduced Role of Stiffness Gradients under Adhesion-Dependent Conditions
3.4. Inhibition of Rho GTPases and Cell Adhesions Influence 2.5D Cell Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauffenburger, D.A.; Horwitz, A.F. Cell Migration: A Physically Integrated Molecular Process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [CrossRef]
- Wu, J.; Mao, Z.; Tan, H.; Han, L.; Ren, T.; Gao, C. Gradient biomaterials and their influences on cell migration. Interface Focus 2012, 2, 337–355. [Google Scholar] [CrossRef]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. Mol. Mech. Mutagen. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.D.; Yamada, K.M. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp. Cell Res. 2016, 343, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 2019, 20, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Mellado, M.; Muñoz, L.M.; Cascio, G.; Lucas, P.; Pablos, J.L.; Frade, J.M.R. T cell migration in rheumatoid arthritis. Front. Immunol. 2015, 6, 384. [Google Scholar] [CrossRef]
- Nevius, E.; Gomes, A.C.; Pereira, J.P. Inflammatory Cell Migration in Rheumatoid Arthritis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 59–78. [Google Scholar] [CrossRef]
- Sliva, D. Signaling Pathways Responsible for Cancer Cell Invasion as Targets for Cancer Therapy. Curr. Cancer Drug Targets 2004, 4, 327–336. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef]
- Wu, J.-S.; Jiang, J.; Chen, B.-J.; Wang, K.; Tang, Y.-L.; Liang, X.-H. Plasticity of cancer cell invasion: Patterns and mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalipour, A.; Diaz, M.F.; Livingston, M.; Ewere, A.; Zhou, A.; Horton, P.D.; Olamigoke, L.T.; Lamar, J.M.; Hagan, J.P.; Lee, H.J.; et al. RhoA-ROCK competes with YAP to regulate amoeboid breast cancer cell migration in response to lymphatic-like flow. FASEB BioAdv. 2022, 4, 342–361. [Google Scholar] [CrossRef] [PubMed]
- Bachem, M.G.; Schünemann, M.; Ramadani, M.; Siech, M.; Beger, H.; Buck, A.; Zhou, S.; Schmid-Kotsas, A.; Adler, G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 2005, 128, 907–921. [Google Scholar] [CrossRef] [PubMed]
- DeClerck, Y.A. Desmoplasia: A Response or a Niche? Cancer Discov. 2012, 2, 772–774. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianopoulos, T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef]
- Parekh, A.; Weaver, A.M. Regulation of cancer invasiveness by the physical extracellular matrix environment. Cell Adhes. Migr. 2009, 3, 288–292. [Google Scholar] [CrossRef]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef]
- Artemenko, Y.; Axiotakis, L.; Borleis, J.; Iglesias, P.A.; Devreotes, P.N. Chemical and mechanical stimuli act on common signal transduction and cytoskeletal networks. Proc. Natl. Acad. Sci. USA 2016, 113, E7500–E7509. [Google Scholar] [CrossRef]
- Nam, K.-H.; Kim, P.; Wood, D.K.; Kwon, S.; Provenzano, P.P.; Kim, D.-H. Multiscale Cues Drive Collective Cell Migration. Sci. Rep. 2016, 6, 29749. [Google Scholar] [CrossRef]
- Mierke, C.T. Mechanical Cues Affect Migration and Invasion of Cells From Three Different Directions. Front. Cell Dev. Biol. 2020, 8, 583226. [Google Scholar] [CrossRef]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, K.; Hara, J.; Ito, C.; Asuri, P. Role of Three-Dimensional Matrix Stiffness in Regulating the Response of Human Neural Cells to Toxins. Cell. Mol. Bioeng. 2014, 7, 278–284. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Dadhwal, S.; Medina, C.; Steczina, S.; Chehreghanianzabi, Y.; Ashraf, A.; Asuri, P. Three-dimensional matrix stiffness and adhesive ligands affect cancer cell response to toxins. Biotechnol. Bioeng. 2016, 113, 443–452. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Petersen, O.W.; Rønnov-Jessen, L.; Howlett, A.R.; Bissell, M.J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064. [Google Scholar] [CrossRef]
- Zaman, M.H.; Trapani, L.M.; Sieminski, A.L.; MacKellar, D.; Gong, H.; Kamm, R.D.; Wells, A.; Lauffenburger, D.A.; Matsudaira, P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 10889–10894. [Google Scholar] [CrossRef]
- Ehrbar, M.; Sala, A.; Lienemann, P.; Ranga, A.; Mosiewicz, K.; Bittermann, A.; Rizzi, S.; Weber, F.; Lutolf, M. Elucidating the Role of Matrix Stiffness in 3D Cell Migration and Remodeling. Biophys. J. 2011, 100, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Pebworth, M.-P.; Cismas, S.A.; Asuri, P. A Novel 2.5D Culture Platform to Investigate the Role of Stiffness Gradients on Adhesion-Independent Cell Migration. PLoS ONE 2014, 9, e110453. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramaiyan, K.; Totey, S.; Bhat, V.; Totey, S.M.; Deb, K. Y-27632 enhances differentiation of blastocyst like cystic human embryoid bodies to endocrinologically active trophoblast cells on a biomimetic platform. J. Biomed. Sci. 2009, 16, 88. [Google Scholar] [CrossRef]
- Hou, H.; Chávez, A.E.; Wang, C.-C.; Yang, H.; Gu, H.; Siddoway, B.A.; Hall, B.J.; Castillo, P.E.; Xia, H. The Rac1 Inhibitor NSC23766 Suppresses CREB Signaling by Targeting NMDA Receptor Function. J. Neurosci. 2014, 34, 14006–14012. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.; Davison, I.; Helfrich, M.; Mason, W.; Horton, M. Integrin receptor-mediated mobilisation of intranuclear calcium in rat osteoclasts. J. Cell Sci. 1993, 105, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Ferrer, L.; Hauschild, J.; Fiedler, W.; Bokemeyer, C.; Nippgen, J.; Celik, I.; Schuch, G. Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of FAK/src/AKT pathway. J. Exp. Clin. Cancer Res. 2008, 27, 86. [Google Scholar] [CrossRef] [PubMed]
- Amann, K.; Haas, C.S.; Schüssler, J.; Daniel, C.; Hartner, A.; Schöcklmann, H.O. Beneficial effects of integrin v 3-blocking RGD peptides in early but not late phase of experimental glomerulonephritis. Nephrol. Dial. Transplant. 2012, 27, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Khavari, A.; Nydén, M.; Weitz, D.A.; Ehrlicher, A.J. Composite alginate gels for tunable cellular microenvironment mechanics. Sci. Rep. 2016, 6, 30854. [Google Scholar] [CrossRef]
- Ulrich, T.A.; Jain, A.; Tanner, K.; MacKay, J.L.; Kumar, S. Probing cellular mechanobiology in three-dimensional culture with collagen–agarose matrices. Biomaterials 2010, 31, 1875–1884. [Google Scholar] [CrossRef]
- Warner, H.; Wilson, B.J.; Caswell, P.T. Control of adhesion and protrusion in cell migration by Rho GTPases. Curr. Opin. Cell Biol. 2019, 56, 64–70. [Google Scholar] [CrossRef]
- Lawson, C.D.; Burridge, K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases 2014, 5, e27958. [Google Scholar] [CrossRef]
- Lawson, C.D.; Ridley, A.J. Rho GTPase signaling complexes in cell migration and invasion. J. Cell Biol. 2018, 217, 447–457. [Google Scholar] [CrossRef]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.; Wellstein, A.; Pestell, R.G. Altered Rho GTPase Signaling Pathways in Breast Cancer Cells. Breast Cancer Res. Treat. 2004, 84, 43–48. [Google Scholar] [CrossRef]
- Lo, C.-M.; Wang, H.-B.; Dembo, M.; Wang, Y.-L. Cell Movement Is Guided by the Rigidity of the Substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Khademhosseini, A.; Langer, R. Fabrication of Gradient Hydrogels Using a Microfluidics/Photopolymerization Process. Langmuir 2004, 20, 5153–5156. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, A.S.; George, P.A.; Cooper-White, J.J. Directing osteogenic and myogenic differentiation of MSCs: Interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Physiol. 2008, 295, C1037–C1044. [Google Scholar] [CrossRef]
- Tse, J.R.; Engler, A.J. Stiffness Gradients Mimicking In Vivo Tissue Variation Regulate Mesenchymal Stem Cell Fate. PLoS ONE 2011, 6, e15978. [Google Scholar] [CrossRef]
- Ungefroren, H.; Sebens, S.; Seidl, D.; Lehnert, H.; Hass, R. Interaction of tumor cells with the microenvironment. Cell Commun. Signal. 2011, 9, 18. [Google Scholar] [CrossRef]
- Holle, A.W.; Young, J.L.; Spatz, J.P. In vitro cancer cell–ECM interactions inform in vivo cancer treatment. Adv. Drug Deliv. Rev. 2016, 97, 270–279. [Google Scholar] [CrossRef]
- Even-Ram, S.; Yamada, K.M. Cell migration in 3D matrix. Curr. Opin. Cell Biol. 2005, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Prudhomme, S.; Zaman, M.H. Viscoelastic Gel-Strip Model for the Simulation of Migrating Cells. Ann. Biomed. Eng. 2011, 39, 2735–2749. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Guiding cell migration in 3D: A collagen matrix with graded directional stiffness. Cell Motil. Cytoskelet. 2009, 66, 121–128. [Google Scholar] [CrossRef] [PubMed]


| Alginate Concentration (w/v) | Storage Modulus (Pa) | |
|---|---|---|
| RGD-Alginate | Unmodified Alginate | |
| 0.5% | 502 ± 63 | 544 ± 43 |
| 1% | 2832 ± 348 | 3045 ± 338 |
| 2% | 7128 ± 684 | 6882 ± 773 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzos, E.; Asuri, P. Sandwich Culture Platforms to Investigate the Roles of Stiffness Gradients and Cell–Matrix Adhesions in Cancer Cell Migration. Cancers 2023, 15, 1729. https://doi.org/10.3390/cancers15061729
Bouzos E, Asuri P. Sandwich Culture Platforms to Investigate the Roles of Stiffness Gradients and Cell–Matrix Adhesions in Cancer Cell Migration. Cancers. 2023; 15(6):1729. https://doi.org/10.3390/cancers15061729
Chicago/Turabian StyleBouzos, Evangelia, and Prashanth Asuri. 2023. "Sandwich Culture Platforms to Investigate the Roles of Stiffness Gradients and Cell–Matrix Adhesions in Cancer Cell Migration" Cancers 15, no. 6: 1729. https://doi.org/10.3390/cancers15061729
APA StyleBouzos, E., & Asuri, P. (2023). Sandwich Culture Platforms to Investigate the Roles of Stiffness Gradients and Cell–Matrix Adhesions in Cancer Cell Migration. Cancers, 15(6), 1729. https://doi.org/10.3390/cancers15061729







