Molecular Alterations in Cutaneous Squamous Cell Carcinoma in Immunocompetent and Immunosuppressed Hosts—A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
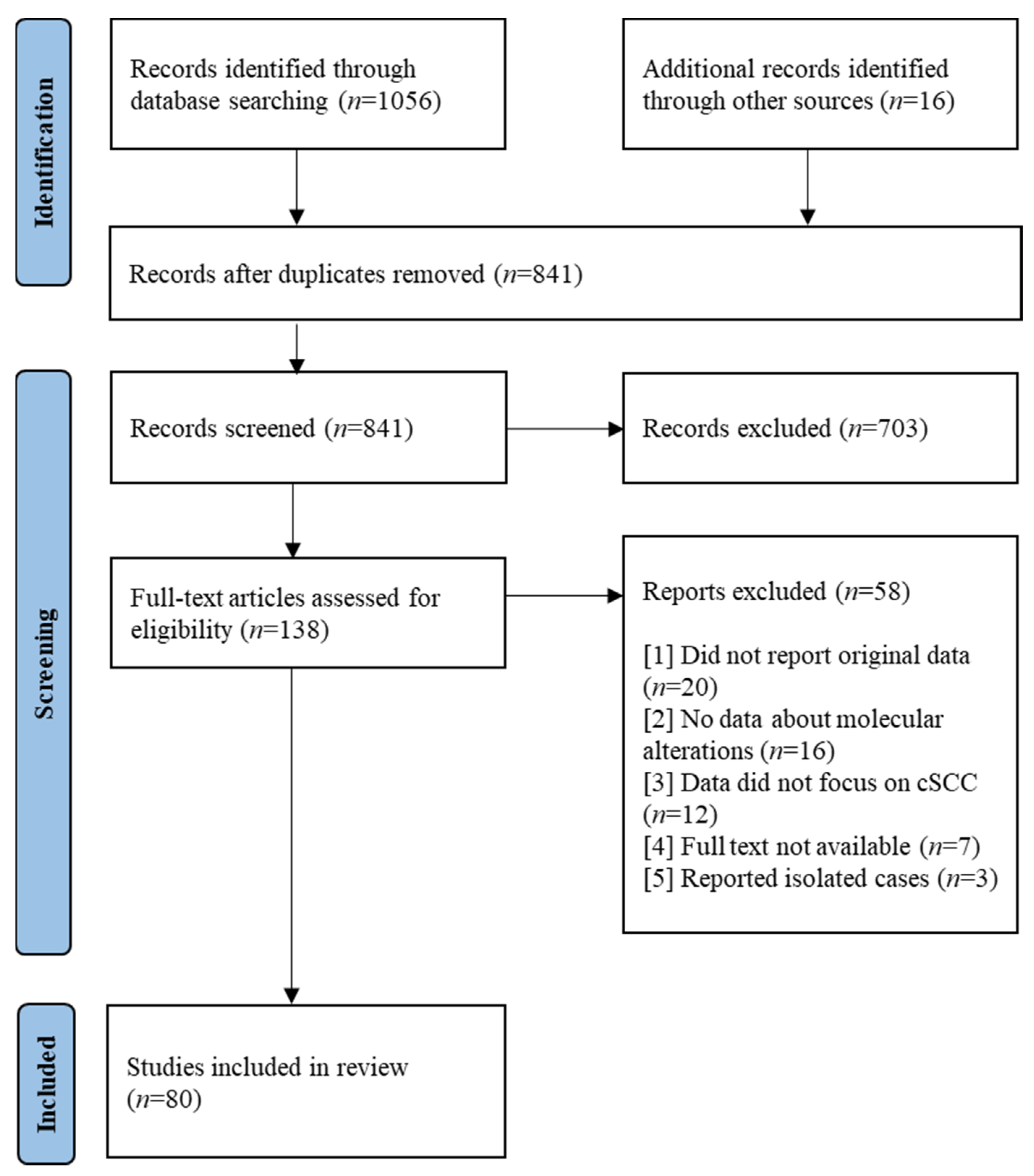
2. Materials and Methods
3. Results
3.1. Molecular Alterations in Immunocompetent Hosts
3.1.1. Genetic Expression
| Gene | Study Population | Results | Author | Year |
|---|---|---|---|---|
| HLA-DQA1 | 7238 cSCC cases and 56,961 controls | An independent association was observed for a threonine to isoleucine change at codon 107 of HLA-DQA1 (OR = 1.14, p = 2.34 × 10−9). Independent cSCC associations with DQA1*05:01 and DQA1*05:05 were identified. | Wang et al. [20] | 2018 |
| HLA-DRB1 | cSCC risk was associated with rs28535317 (OR = 1.20, p = 9.88 × 10−11) corresponding to an amino-acid change from phenylalanine to leucine at codon 26 of HLA-DRB1 (OR = 1.17, p = 2.48 × 10−10). Among the classical HLA alleles, cSCC was associated with DRB1*01 (OR = 1.18, p = 5.86 × 10−10). | |||
| CDC25B | Mouse models, cultured human cSCC cell lines (SCC12B.2, SCC13, SRB1, SRB12, and Colo16) | Primarily cytoplasmic in skin and skin tumours. Increased in cSCC vs. normal skin (p ≤ 0.001) | Al-Matouq et al. [18] | 2019 |
| CDC25C | Primarily nuclear in the skin. Increased cytoplasmic signal in cSCC vs. normal skin (p ≤ 0.001) | |||
| FGFR2 | Human primary (SCC12A and SCC118) and metastatic cSCC cell lines (SCC7). Human normal skin samples (n = 9) AK (n = 9), cSCC (n = 28) and metastatic cSCC (n = 21) | Strong expression of FGFR2 was observed in less than 5% of AK samples. In cSCC and metastatic cSCC, cytoplasmic and perinuclear FGFR2 was noted in tumor cells in the invasive margin and expression was predominantly strong (55% and 60%, respectively) | Khandelwal et al. [19] | 2019 |
| METTL3 | Cell lines: A431, HSC-1 Human cSCC samples from 8 patients | Expression of METTL3 was significantly higher in the cSCC tissues. METTL3 knock down decreased cell proliferation. Less number of colony formation in METTL3 knock down groups vs. control (p < 0.05) | R. Zhou et al. [24] | 2019 |
| NOTCH | CD133+ cells | Inhibiting NOTCH reduced the CD133+ cell population (p < 0.05). NOTCH inhibition decreased DNA-binding activity of canonical NF-κB pathway subunit p65 (RelA), and non-canonical pathway subunits p52, and RelB (64%, 80% and 77% respectively [p < 0.001]) vs. control | Quan et al. [25] | 2019 |
| BPI | Human cSCC samples, uninvolved skin from 3 patients | Novel mutations in cSCC identified via the HitWalker2 prioritization analysis - HGVS DNA reference: g.36938975G > A, variant type: single AA change - HGVS DNA reference: g.36954682C > T, variant type: single AA change | Anderson et al. [23] | 2020 |
| EPHA6 | Mitigated > 30% reductions in cell viability in cSCC | |||
| EPHA7 | Mitigated > 30% reductions in cell viability in cSCC Novel mutations in cSCC identified via the HitWalker2 prioritization analysis - HGVS DNA reference: g.93956676G > A, variant type: single AA change - HGVS DNA reference: g.93953241C > T, variant type: single AA change | |||
| RAC1 | RAC1 was the largest hub within the Eph-ephrin signaling pathway (degree = 14) | |||
| FABP5 | 6 human cSCC samples with matched adjacent skin samples, 3 healthy control skin tissues | Significantly overexpressed in cSCC tissues (p < 0.001). Decrease in cell proliferation measured at 48 h (p = 0.007), increase of cell apoptosis (p < 0.001) after FABP5 knock down | Yan et al. [21] | 2021 |
| S100A9 | Significantly overexpressed in cSCC tissues (p < 0.001). Ability of cell proliferation was significantly inhibited after 24h after S100A9 knock down (p < 0.05) | |||
| FBN2 | Tissue samples of NNS (n = 6), NES (n = 6), AK (n = 6), and cSCC (n = 6) | Upregulated in cSCC vs. normal (p < 0.001) and AK (p < 0.01) | Zou et al. [22] | 2021 |
| HEPHL1 | Upregulated in cSCC vs. normal (p < 0.01) and AK (p < 0.05) | |||
| SULF1 | Upregulated in cSCC vs. normal (p < 0.001) and AK (p < 0.01) | |||
| SULF2 | Upregulated in cSCC vs. normal (p < 0.001) and AK (p < 0.05) | |||
| TCN1 | Upregulated in cSCC vs. normal (p < 0.01) and AK (p < 0.05) | |||
| ZMIZ1 | Mouse models, human cSCC cell lines: A431(CRL–1555), SCC13, COLO16 | ZMIZ1 gene expression significantly increased within cSCC genomes (100-300× normal expression levels). High expression associated with poor outcomes in human cSCC patients, correlation threshold of 0.65 (Cox Proportional Hazards Regression, p = 0.0195; Log-rank Test, p = 0.028 at 50% quintile) | Aiderus et al. [17] | 2021 |
| ZMIZ2 | ZMIZ2 gene expression significantly increased within cSCC genomes (100-300× normal expression levels) | |||
| SERPINE1 | cSCCHN from 50 patients. 21 PRI-, 14 PRI+, 15 MET, matched SES | Upregulated in all tumor cohorts vs. SES (log2FC > 1, Padj < 0.05) | Minaei et al. [17] | 2022 |
| CALR | Matched tumor and blood DNA from 25 patients with regional metastases of cSCCHN | Gene amplification seen most commonly in tumor samples | Thind et al. [16] | 2022 |
| CCND1 | Gene amplification seen most commonly in tumor samples | |||
| EVC | 3′ UTR region of EVC (48%) was significantly functionally altered in cSCC (Q-value < 0.05) | |||
| FGF3 | Gene amplification seen most commonly in tumor samples | |||
| LUM | 3′ UTR region of LUM (16%) was significantly functionally altered in cSCC (Q-value < 0.05) | |||
| PPP1R1A | 3′ UTR region of PPP1R1A (48%) was significantly functionally altered in cSCC (Q-value < 0.05) |
3.1.2. Epigenetic Alterations
3.1.3. Transcriptomic Changes
MicroRNAs
Circular RNAs
Transcription Factors
Long Non-Coding RNAs
3.1.4. Protein Expression
3.1.5. Metabolic Changes
3.1.6. Immune Landscape
| Immune Biomarker | Study Population | Findings | Author | Year |
|---|---|---|---|---|
| AIM2 | Primary (n = 5) and metastatic (n = 3) human cSCC cell lines, NHEK (n = 5) Tissue samples: normal sun-protected skin (n = 15), AK (n = 71), cSCCIS (n = 60), and UV-induced cSCC (n = 81) | Elevated levels of AIM2 mRNA were noted in cSCC in vivo vs. normal skin (p < 0.01). A lower number of proliferating cells was observed in the xenografts established with cSCC cells transfected with AIM2 siRNA (21%) vs. control siRNA tumors (70%), p < 0.001. | Farshchian et al. [65] | 2017 |
| C1r | Human cSCC cell lines (UT-SCC-7, UTSCC- 12A, UT-SCC-59A, and UT-SCC-91) | MMP1, MMP13, MMP10, and MMP12 were significantly downregulated after C1r knockdown (p < 0.001), offering evidence for the role of C1r in promoting the invasion of cSCC cells by increasing MMP production. | K Viiklepp et al. [66] | 2022 |
| C3 | cSCC cell lines A431, Tca8113, SCC13, HSC-5 and HSC-1 and HaCaT | C3 mRNA expression was upregulated in all tumor cell lines and was more than 4.5 times higher in A431 and SCC13 cells. | Fan et al. [68] | 2019 |
| Human cSCC cell lines (n = 8), NHEK (n = 11), mouse cSCC | Mean expression level of C3 mRNAs was higher in cSCC cells (n = 8), as compared to NHEKs (n = 11), p < 0.05. Growth of cSCC xenograft tumors with C3 knockdown was significantly reduced, as compared to control siRNA tumors, p < 0.05. | Riihilä et al. [67] | 2017 | |
| CFB | Mean expression level of CFB mRNAs was higher in cSCC cells (n = 8), as compared to NHEKs (n = 11), p < 0.05. Migration rate of cSCC cells was significantly reduced after CFB knockdown, p < 0.01. | |||
| CFI | Human cSCC cell lines (UT-SCC-1O5, UT-SCC-1O8, UT-SCC-7, and UT-SCC-59A) | Increase in the invasion of cSCC cells through 3D type I collagen (p < 0.001) and 3D Matrigel (p < 0.01) was noted following CFI overexpression. | Nezhad et al. [69] | 2021 |
| CD4+ T cells | Human cSCC from 31 patients: 9 NP, 22 DP. Within the DP group: 5 ISPs and 17 ACIS | Numbers of CD4+ T cells (p = 0.004) and FoxP3+ Tregs (p = 0.001) were higher in the NP group. | Ferguson et al. [71] | 2022 |
| AK (n = 103), KA (n = 43), cSCC (n = 106) | Invasive cSCC showed less CD4+ cells vs. KA (p = 0.0158). | Bauer et al. [70] | 2018 | |
| CD8+ T cells | Invasive cSCC demonstrated more infiltration of CD8+ cells vs. AK and KA (both p < 0.0001). | |||
| Human cSCC from 31 patients: 9 NP, 22 DP. Within the DP group: 5 ISPs and 17 ACIS | CD8+ T cells were greater in the NP group vs. the DP group (p = 0.006). CD8+ T cells were more proliferative (p < 0.0001), and expressed a greater (p < 0.0001) proportion of granzyme B in the NP group vs. DP group. | Ferguson et al. [71] | 2022 | |
| FOXp3+ T cells | AK (n = 103), KA (n = 43), cSCC (n = 106) | Invasive cSCC showed less FOXp3+ T cells in the infiltrate vs. KA (p = 0.0063). | Bauer et al. [70] | 2018 |
| PD-L1 | cSCC expressed significantly more PD-L1 in comparison with AK (p < 0.0001). PD-L1 expression was greater in moderately and poorly differentiated cSCC vs. well-differentiated cSCC (p = 0.0426). | |||
| Podoplanin | Mouse cSCC, human cSCC cell lines | Podoplanin interacts with both CD44s and CD44v (CD44v3-10, CD44v6-10, and CD44v8-10) isoforms expressed in SCC cell lines. | L Montero-Montero et al. [72] | 2020 |
| Tumor-Associated Neutrophils | Mouse models, mouse cSCC cell line: mSCC38 | Significant increase in the proportion of neutrophils within cSCC vs. surrounding skin (p < 0.01), with TANs accounting for 30–80% of tumor-infiltrating CD45+ cells. TANs contribute to cSCC development by limiting effector CD8+ T cell responses. | Khou et al. [64] | 2020 |
3.2. Molecular Alterations in Immunosuppressed Hosts
3.2.1. Tumor Immune Microenvironment
3.2.2. Gene Polymorphisms
3.2.3. Genetic and Epigenetic Alterations
| Gene/RNA of Interest | Study Population | Results | Author | Year |
|---|---|---|---|---|
| FLOT1 | 27 RTRs with SCC and 27 RTRs without SCC | Hypomethylated in patients with cSCC. | Peters et al. [80] | 2018 |
| ZNF577 | Hypermethylated in the group with de novo cSCC after transplantation. | |||
| Signature 32 | 40 cSCC samples from 37 patients; ISPs (n = 30), ICPs (n = 7) | Strong positive correlation with the estimated time of azathioprine exposure and the prevalence of signature 32 (Spearman’s rank order correlation rs(26) = 0.679, p < 0.0001). Most SMG gene mutations observed (including NOTCH1/2, TP53, and CDKN2A) were attributed to azathioprine signature 32 (66.2%). | Inman et al. [83] | 2018 |
| SERPINB9 | Cohort 1: 19 RTRs with cSCC and 19 RTRs without cSCC Cohort 2: 45 RTRs with cSCC and 37 RTRs without cSCC | Higher DNA methylation of SERPINB9 in RTRs who developed cSCC vs. those who did not. Median DNA methylation of SERPINB9 was 58.7% (range: 32.5–81.3%) for region 1 and 54.4% (30.0–78.5%) for region 2 in patients with cSCC and 50.2% (21.8–77.5%) for region 1 and 46.4% (22.1–74.0%) for region 2 in the non-cSCC patients (region 1: p = 0.004 and region 2: p = 0.008). | Peters et al. [81] | 2019 |
| HLA-DRB1*13 | 46 RTRs who developed cSCC after transplant | HLA-DRB1*13 was associated with risk of cSCC in RTRs after transplant (HR = 2.24, 95% CI = 1.12–4.49, p = 0.023). | Kim et al. [84] | 2020 |
| mir-1246 | 8 OTRs with cSCC, 8 OTRs without cSCC | mir-1246 was significantly upregulated in both tumor tissue and serum in OTRs with cSCC vs. those without (p = 0.013). | Geusau et al. [82] | 2020 |
| mir-1290 | mir-1290 was significantly upregulated in both tumor tissue and serum in OTRs with cSCC vs. those without (p = 0.037). |
4. Discussion
4.1. Novel Molecular Targets in CSCC
4.1.1. Genomic Biomarkers
4.1.2. Transcriptomic Biomarkers
4.1.3. Proteomic Biomarkers
4.1.4. Immune Biomarkers
4.2. Key Molecular Alterations in the Immunosuppressed
4.2.1. DNA Methylation—A Novel Risk Factor for CSCC Development
4.2.2. Genetic Polymorphisms May Confer Protection against CSCC Development
4.2.3. Differences in the Immune Landscape between ICPs and ISPs May Influence Responses to Targeted Therapy
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Urban, K.; Mehrmal, S.; Uppal, P.; Giesey, R.L.; Delost, G.R. The global burden of skin cancer: A longitudinal analysis from the Global Burden of Disease Study, 1990–2017. JAAD Int. 2021, 2, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.C.; Jin, A.; Koh, W.-P. Trends of cutaneous basal cell carcinoma, squamous cell carcinoma, and melanoma among the Chinese, Malays, and Indians in Singapore from 1968–2016. JAAD Int. 2021, 4, 39–45. [Google Scholar] [CrossRef]
- Balamucki, C.J.; Mancuso, A.A.; Amdur, R.J.; Kirwan, J.M.; Morris, C.G.; Flowers, F.P.; Stoer, C.B.; Cognetta, A.B.; Mendenhall, W.M. Skin carcinoma of the head and neck with perineural invasion. Am. J. Otolaryngol. 2012, 33, 447–454. [Google Scholar] [CrossRef]
- Zheng, L.; Li, S.; Li, C. Expression profiling analysis of autophagy-related genes in perineural invasion of cutaneous squamous cell carcinoma. Oncol. Lett. 2018, 15, 4837–4848. [Google Scholar] [CrossRef] [PubMed]
- Eviston, T.J.; Minaei, E.; Mueller, S.A.; Ahmadi, N.; Ashford, B.; Clark, J.R.; West, N.; Zhang, P.; Gupta, R.; Ranson, M. Gene expression profiling of perineural invasion in head and neck cutaneous squamous cell carcinoma. Sci. Rep. 2021, 11, 13192. [Google Scholar] [CrossRef]
- Zilberg, C.; Lee, M.W.; Yu, B.; Ashford, B.; Kraitsek, S.; Ranson, M.; Shannon, K.; Cowley, M.; Iyer, N.G.; Palme, C.E.; et al. Analysis of clinically relevant somatic mutations in high-risk head and neck cutaneous squamous cell carcinoma. Mod. Pathol. 2018, 31, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Minaei, E.; Mueller, S.A.; Ashford, B.; Thind, A.S.; Mitchell, J.; Perry, J.R.; Genenger, B.; Clark, J.R.; Gupta, R.; Ranson, M. Cancer Progression Gene Expression Profiling Identifies the Urokinase Plasminogen Activator Receptor as a Biomarker of Metastasis in Cutaneous Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 835929. [Google Scholar] [CrossRef]
- Yilmaz, A.S.; Ozer, H.G.; Gillespie, J.L.; Allain, D.C.; Bernhardt, M.N.; Furlan, K.C.; Castro, L.T.F.; Peters, S.B.; Nagarajan, P.; Kang, S.Y.; et al. Differential mutation frequencies in metastatic cutaneous squamous cell carcinomas versus primary tumors: Mutations in Metastatic and Primary SCCs. Cancer 2017, 123, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Lobl, M.B.; Clarey, D.; Higgins, S.; Sutton, A.; Hansen, L.; Wysong, A. Targeted next-generation sequencing of matched localized and metastatic primary high-risk SCCs identifies driver and co-occurring mutations and novel therapeutic targets. J. Dermatol. Sci. 2020, 99, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Macedo, S.; Fernandes, M.; Pestana, A.; Pardal, J.; Batista, R.; Vinagre, J.; Sanches, A.; Baptista, A.; Lopes, J.M.; et al. TERT promoter mutations are associated with poor prognosis in cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2019, 80, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Alameda, J.P.; García-García, V.A.; López, S.; Hernando, A.; Page, A.; Navarro, M.; Moreno-Maldonado, R.; Paramio, J.M.; Ramírez, Á.; García-Fernández, R.A.; et al. CYLD Inhibits the Development of Skin Squamous Cell Tumors in Immunocompetent Mice. Int. J. Mol. Sci. 2021, 22, 6736. [Google Scholar] [CrossRef]
- Lee, P.; Jiang, S.; Li, Y.; Yue, J.; Gou, X.; Chen, S.; Zhao, Y.; Schober, M.; Tan, M.; Wu, X. Phosphorylation of Pkp1 by RIPK 4 regulates epidermal differentiation and skin tumorigenesis. EMBO J. 2017, 36, 1963–1980. [Google Scholar] [CrossRef] [PubMed]
- Hiller, B.; Hoppe, A.; Haase, C.; Hiller, C.; Schubert, N.; Müller, W.; Reijns, M.A.M.; Jackson, A.P.; Kunkel, T.A.; Wenzel, J.; et al. Ribonucleotide Excision Repair Is Essential to Prevent Squamous Cell Carcinoma of the Skin. Cancer Res. 2018, 78, 5917–5926. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, R.R.; Sarate, R.M.; Setia, P.; Shah, S.; Gupta, S.; Chaturvedi, P.; Gera, P.; Waghmare, S.K. SFRP1 in Skin Tumor Initiation and Cancer Stem Cell Regulation with Potential Implications in Epithelial Cancers. Stem Cell Rep. 2020, 14, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Y.; Zhou, M.; Zhang, Y.; Wang, P.; Li, X.; Yang, J.; Wang, H.; Ding, Z. HOXA9 inhibits HIF-1α-mediated glycolysis through interacting with CRIP2 to repress cutaneous squamous cell carcinoma development. Nat. Commun. 2018, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Thind, A.S.; Ashford, B.; Strbenac, D.; Gupta, R.; Clark, J.R.; Iyer, N.G.; Mitchell, J.; Lee, J.; Mueller, S.A.; Minaei, E.; et al. Whole Genome Analysis Reveals the Genomic Complexity in Metastatic Cutaneous Squamous Cell Carcinoma. Oncology 2022, 12, 919118. [Google Scholar] [CrossRef]
- Aiderus, A.; Newberg, J.Y.; Guzman-Rojas, L.; Contreras-Sandoval, A.M.; Meshey, A.L.; Jones, D.J.; Amaya-Manzanares, F.; Rangel, R.; Ward, J.M.; Lee, S.-C.; et al. Transposon mutagenesis identifies cooperating genetic drivers during keratinocyte transformation and cutaneous squamous cell carcinoma progression. PLoS Genet. 2021, 17, e1009094. [Google Scholar] [CrossRef]
- Al-Matouq, J.; Holmes, T.R.; Hansen, L.A. CDC25B and CDC25C overexpression in nonmelanoma skin cancer suppresses cell death. Mol. Carcinog. 2019, 58, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.R.; Kent, B.; Hillary, S.; Alam, M.M.; Ma, X.; Gu, X.; DiGiovanni, J.; Nathan, C.O. Fibroblast growth factor receptor promotes progression of cutaneous squamous cell carcinoma. Mol. Carcinog. 2019, 58, 1715–1725. [Google Scholar] [CrossRef]
- Wang, W.; Ollila, H.M.; Whittemore, A.S.; Demehri, S.; Ioannidis, N.M.; Jorgenson, E.; Mignot, E.; Asgari, M.M. Genetic variants in the HLA class II region associated with risk of cutaneous squamous cell carcinoma. Cancer Immunol. Immunother. CII 2018, 67, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Li, L.; Zhu, S.; Wu, Y.; Liu, Y.; Zhu, L.; Zhao, Z.; Wu, F.; Jia, N.; Liao, C.; et al. Single-cell transcriptomic analysis reveals the critical molecular pattern of UV-induced cutaneous squamous cell carcinoma. Cell Death Dis. 2021, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.-D.; Xu, D.; Deng, Y.-Y.; Wu, W.-J.; Zhang, J.; Huang, L.; He, L. Identification of key genes in cutaneous squamous cell carcinoma: A transcriptome sequencing and bioinformatics profiling study. Ann. Transl. Med. 2021, 9, 1497. [Google Scholar] [CrossRef]
- Anderson, A.N.; McClanahan, D.; Jacobs, J.; Jeng, S.; Vigoda, M.; Blucher, A.S.; Zheng, C.; Yoo, Y.J.; Hale, C.; Ouyang, X.; et al. Functional genomic analysis identifies drug targetable pathways in invasive and metastatic cutaneous squamous cell carcinoma. Cold Spring Harb. Mol. Case Stud. 2020, 6, a005439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Gao, Y.; Lv, D.; Wang, C.; Wang, D.; Li, Q. METTL3 mediated m6A modification plays an oncogenic role in cutaneous squamous cell carcinoma by regulating ΔNp63. Biochem. Biophys. Res. Commun. 2019, 515, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.X.; Hawk, N.V.; Chen, W.; Coupar, J.; Lee, S.; Petersen, D.W.; Meltzer, P.S.; Montemarano, A.; Braun, M.; Chen, Z.; et al. Targeting Notch1 and IKKα enhanced NF-κB activation in CD133+ Skin Cancer Stem Cells. Mol. Cancer Ther. 2018, 17, 2034–2048. [Google Scholar] [CrossRef] [PubMed]
- Latil, M.; Nassar, D.; Beck, B.; Boumahdi, S.; Wang, L.; Brisebarre, A.; Dubois, C.; Nkusi, E.; Lenglez, S.; Checinska, A.; et al. Cell-Type-Specific Chromatin States Differentially Prime Squamous Cell Carcinoma Tumor-Initiating Cells for Epithelial to Mesenchymal Transition. Cell Stem Cell 2017, 20, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Adhikary, G.; Xu, W.; Kandasamy, S.; Eckert, R.L. ACTL6A suppresses p21Cip1 expression to enhance the epidermal squamous cell carcinoma phenotype. Oncogene 2020, 39, 5855. [Google Scholar] [CrossRef]
- Hervás-Marín, D.; Higgins, F.; Sanmartín, O.; López-Guerrero, J.A.; Bañó, M.C.; Igual, J.C.; Quilis, I.; Sandoval, J. Genome wide DNA methylation profiling identifies specific epigenetic features in high-risk cutaneous squamous cell carcinoma. PLoS ONE 2019, 14, e0223341. [Google Scholar] [CrossRef]
- Li, L.; Li, F.; Xia, Y.; Yang, X.; Lv, Q.; Fang, F.; Wang, Q.; Bu, W.; Wang, Y.; Zhang, K.; et al. UVB induces cutaneous squamous cell carcinoma progression by de novo ID4 methylation via methylation regulating enzymes. EBioMedicine 2020, 57, 102835. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.; Coussement, L.; Knatko, E.V.; Higgins, M.; Steyaert, S.; Proby, C.M.; de Meyer, T.; Dinkova-Kostova, A.T. Clinically relevant aberrant Filip1l DNA methylation detected in a murine model of cutaneous squamous cell carcinoma. EBioMedicine 2021, 67, 103383. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.; Zauner, R.; Ablinger, M.; Piñón-Hofbauer, J.; Guttmann-Gruber, C.; Reisenberger, M.; Lettner, T.; Niklas, N.; Proell, J.; Sajinovic, M.; et al. A cancer stem cell-like phenotype is associated with miR-10b expression in aggressive squamous cell carcinomas. Cell Commun. Signal. 2020, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Ramirez, H.; Pastar, I.; Gordon, K.A.; Stone, R.; Choudhary, S.; Badiavas, E.; Nouri, K.; Tomic-Canic, M. MiR-21 and miR-205 are induced in invasive cutaneous squamous cell carcinomas. Arch. Dermatol. Res. 2017, 309, 133–139. [Google Scholar] [CrossRef]
- Lin, N.; Zhou, Y.; Lian, X.; Tu, Y. MicroRNA-31 functions as an oncogenic microRNA in cutaneous squamous cell carcinoma cells by targeting RhoTBT1. Oncol. Lett. 2017, 13, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Shen, R.; Yan, Y.; Deng, L. miR-186 promotes tumor growth in cutaneous squamous cell carcinoma by inhibiting apoptotic protease activating factor-1. Exp. Ther. Med. 2018, 16, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.-H.; Zhou, F.; Shi, C.; Xiang, T.; Zhou, C.-K.; Wang, Q.-Q.; Jiang, Y.-S.; Gao, S.-F. miRNA-221 promotes cutaneous squamous cell carcinoma progression by targeting PTEN. Cell. Mol. Biol. Lett. 2019, 24, 9. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Li, C.; Das Mahapatra, K.; Lapins, J.; Homey, B.; Sonkoly, E.; Pivarcsi, A. MiR-130a Acts as a Tumor Suppressor MicroRNA in Cutaneous Squamous Cell Carcinoma and Regulates the Activity of the BMP/SMAD Pathway by Suppressing ACVR1. J. Investig. Dermatol. 2021, 141, 1922–1931. [Google Scholar] [CrossRef]
- Neu, J.; Dziunycz, P.J.; Dzung, A.; Lefort, K.; Falke, M.; Denzler, R.; Freiberger, S.N.; Iotzova-Weiss, G.; Kuzmanov, A.; Levesque, M.P.; et al. miR-181a decelerates proliferation in cutaneous squamous cell carcinoma by targeting the proto-oncogene KRAS. PLoS ONE 2017, 12, e0185028. [Google Scholar] [CrossRef]
- Zhou, W.-Y.; Cai, Z.-R.; Liu, J.; Wang, D.-S.; Ju, H.-Q.; Xu, R.-H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Das Mahapatra, K.; Pasquali, L.; Søndergaard, J.N.; Lapins, J.; Nemeth, I.B.; Baltás, E.; Kemény, L.; Homey, B.; Moldovan, L.-I.; Kjems, J.; et al. A comprehensive analysis of coding and non-coding transcriptomic changes in cutaneous squamous cell carcinoma. Sci. Rep. 2020, 10, 3637. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, X.; Gao, L.; Yang, J.; Zheng, L.; Gao, L.; Zhou, X.; Xiang, X.; Zhang, J.; Yi, C. Identification of potential immune-related circRNA-miRNA-mRNA regulatory network in cutaneous squamous cell carcinoma. Am. J. Cancer Res. 2021, 11, 4826–4843. [Google Scholar]
- Rose, A.M.; Spender, L.C.; Stephen, C.; Mitchell, A.; Rickaby, W.; Bray, S.; Evans, A.T.; Dayal, J.; Purdie, K.J.; Harwood, C.A.; et al. Reduced SMAD2/3 activation independently predicts increased depth of human cutaneous squamous cell carcinoma. Oncotarget 2018, 9, 14552–14566. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, R.; Chen, H.; Chen, L.; Zhou, X.; Liu, L.; Ju, M.; Chen, K.; Huang, D. Comprehensive analysis of lncRNA-mRNAs co-expression network identifies potential lncRNA biomarkers in cutaneous squamous cell carcinoma. BMC Genom. 2022, 23, 274. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, L.; Ma, S.; Ma, J.; Wang, Y.; Li, S.; Hu, X.; Han, S.; Zhou, M.; Zhou, L.; et al. MALAT1-KTN1-EGFR regulatory axis promotes the development of cutaneous squamous cell carcinoma. Cell Death Differ. 2019, 26, 2061–2073. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, G.; Rezzonico, R.; Bourget, I.; Allan, R.; Nottet, N.; Popa, A.; Magnone, V.; Rios, G.; Mari, B.; Barbry, P. A new long noncoding RNA (lncRNA) is induced in cutaneous squamous cell carcinoma and down-regulates several anticancer and cell differentiation genes in mouse. J. Biol. Chem. 2017, 292, 12483–12495. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Q.; Kuang, S.; Zhao, C.; Yang, L.; Zhang, Y.; Zhu, H.; Yang, R. USF1-induced upregulation of LINC01048 promotes cell proliferation and apoptosis in cutaneous squamous cell carcinoma by binding to TAF15 to transcriptionally activate YAP1. Cell Death Dis. 2019, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liao, J.; Duan, X.; He, Y.; Liao, Y. Upregulation of LINC00319 indicates a poor prognosis and promotes cell proliferation and invasion in cutaneous squamous cell carcinoma. J. Cell. Biochem. 2018, 119, 10393–10405. [Google Scholar] [CrossRef]
- Lu, D.; Sun, L.; Li, Z.; Mu, Z. lncRNA EZR-AS1 knockdown represses proliferation, migration and invasion of cSCC via the PI3K/AKT signaling pathway. Mol. Med. Rep. 2020, 23, 76. [Google Scholar] [CrossRef]
- Zou, S.; Gao, Y.; Zhang, S. lncRNA HCP5 acts as a ceRNA to regulate EZH2 by sponging miR-138-5p in cutaneous squamous cell carcinoma. Int. J. Oncol. 2021, 59, 56. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.-J.; Sun, Y.; Zhang, D.-W.; Zhang, P. Long non-coding RNA HOTAIR functions as a competitive endogenous RNA to regulate PRAF2 expression by sponging miR-326 in cutaneous squamous cell carcinoma. Cancer Cell Int. 2019, 19, 270. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.-L.; Zhong, S. Long noncoding RNA LINC00520 prevents the progression of cutaneous squamous cell carcinoma through the inactivation of the PI3K/Akt signaling pathway by downregulating EGFR. Chin. Med. J. 2019, 132, 454–465. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, S.; Li, J.; Li, Z.; Wang, Y.; Li, X. lncRNA TINCR participates in ALA-PDT-induced apoptosis and autophagy in cutaneous squamous cell carcinoma. J. Cell. Biochem. 2019, 120, 13893–13902. [Google Scholar] [CrossRef]
- Sun, Y.; Li, A.; Liu, X.; Wang, Q.; Bai, Y.; Liu, Z.; Huang, L.; Wu, M.; Li, H.; Miao, J.; et al. A panel of biomarkers for skin squamous cell carcinoma: Various functional entities and differential responses to resveratrol. Int. J. Clin. Exp. Pathol. 2019, 12, 1363–1377. [Google Scholar] [PubMed]
- Chen, W.; Rao, J.; Liu, Z.; You, X.; Yuan, F.; Le, F.; Tang, M.; Zhou, M.; Xie, T. Integrated tissue proteome and metabolome reveal key elements and regulatory pathways in cutaneous squamous cell carcinoma. J. Proteom. 2021, 247, 104320. [Google Scholar] [CrossRef]
- Azimi, A.; Kaufman, K.L.; Ali, M.; Arthur, J.; Kossard, S.; Fernandez-Penas, P. Differential proteomic analysis of actinic keratosis, Bowen’s disease and cutaneous squamous cell carcinoma by label-free LC–MS/MS. J. Dermatol. Sci. 2018, 91, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Kim, D.; Donahue, L.; White, A. 785 Phenotypic plasticity of cutaneous squamous cell carcinoma mediated by cyclooxygenase-2. J. Investig. Dermatol. 2020, 140, S103. [Google Scholar] [CrossRef]
- Crawford, T.; Fletcher, N.; Veitch, M.; Gonzalez Cruz, J.L.; Pett, N.; Brereton, I.; Wells, J.W.; Mobli, M.; Tesiram, Y. Bacillus anthracis Protective Antigen Shows High Specificity for a UV Induced Mouse Model of Cutaneous Squamous Cell Carcinoma. Front. Med. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Wang, Y.; Wang, N.; Duan, Q.; Wang, S.; Liu, M.; Bilal, M.A.; Zheng, Y. LPCAT1 Promotes Cutaneous Squamous Cell Carcinoma via EGFR-Mediated Protein Kinase B/p38MAPK Signaling Pathways. J. Investig. Dermatol. 2022, 142, 303–313. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, G.; Lin, C.; Guo, H.; Xu, J.; Zhao, T. IGF2BP1 over-expression in skin squamous cell carcinoma cells is essential for cell growth. Biochem. Biophys. Res. Commun. 2018, 501, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Azimi, A.; Lo, K.; Kim, J.; Fernandez-Penas, P. Investigating proteome changes between primary and metastatic cutaneous squamous cell carcinoma using SWATH mass spectrometry. J. Dermatol. Sci. 2020, 99, 119–127. [Google Scholar] [CrossRef]
- Föll, M.C.; Fahrner, M.; Gretzmeier, C.; Thoma, K.; Biniossek, M.L.; Kiritsi, D.; Meiss, F.; Schilling, O.; Nyström, A.; Kern, J.S. Identification of tissue damage, extracellular matrix remodeling and bacterial challenge as common mechanisms associated with high-risk cutaneous squamous cell carcinomas. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 66, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Whitley, M.J.; Suwanpradid, J.; Lai, C.; Jiang, S.W.; Cook, J.L.; Zelac, D.E.; Rudolph, R.; Corcoran, D.L.; Degan, S.; Spasojevic, I.; et al. ENTPD1 (CD39) Expression Inhibits UVR-Induced DNA Damage Repair through Purinergic Signaling and Is Associated with Metastasis in Human Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2021, 141, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.J.; Patel, A.; Purdie, K.J.; Wang, J.; Rizvi, H.; Hufbauer, M.; Ostano, P.; Akgül, B.; Chiorino, G.; Harwood, C.A.; et al. Epigenetic Regulation of iASPP-p63 Feedback Loop in Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2019, 139, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.J.; Tsinkevich, M.; Rodencal, J.; Abbas, H.A.; Su, X.-H.; Gi, Y.-J.; Fang, B.; Rajapakshe, K.; Coarfa, C.; Gunaratne, P.H.; et al. TAp63-Regulated miRNAs Suppress Cutaneous Squamous Cell Carcinoma through Inhibition of a Network of Cell-Cycle Genes. Cancer Res. 2020, 80, 2484–2497. [Google Scholar] [CrossRef]
- Khou, S.; Popa, A.; Luci, C.; Bihl, F.; Meghraoui-Kheddar, A.; Bourdely, P.; Salavagione, E.; Cosson, E.; Rubod, A.; Cazareth, J.; et al. Tumor-Associated Neutrophils Dampen Adaptive Immunity and Promote Cutaneous Squamous Cell Carcinoma Development. Cancers 2020, 12, 1860. [Google Scholar] [CrossRef]
- Farshchian, M.; Nissinen, L.; Siljamäki, E.; Riihilä, P.; Piipponen, M.; Kivisaari, A.; Kallajoki, M.; Grénman, R.; Peltonen, J.; Peltonen, S.; et al. Tumor cell-specific AIM2 regulates growth and invasion of cutaneous squamous cell carcinoma. Oncotarget 2017, 8, 45825–45836. [Google Scholar] [CrossRef] [PubMed]
- Viiklepp, K.; Nissinen, L.; Ojalill, M.; Riihilä, P.; Kallajoki, M.; Meri, S.; Heino, J.; Kähäri, V.-M. C1r Upregulates Production of Matrix Metalloproteinase-13 and Promotes Invasion of Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2022, 142, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Riihilä, P.; Nissinen, L.; Farshchian, M.; Kallajoki, M.; Kivisaari, A.; Meri, S.; Grénman, R.; Peltonen, S.; Peltonen, J.; Pihlajaniemi, T.; et al. Complement Component C3 and Complement Factor B Promote Growth of Cutaneous Squamous Cell Carcinoma. Am. J. Pathol. 2017, 187, 1186–1197. [Google Scholar] [CrossRef]
- Fan, Z.; Qin, J.; Wang, D.; Geng, S. Complement C3a promotes proliferation, migration and stemness in cutaneous squamous cell carcinoma. J. Cell. Mol. Med. 2019, 23, 3097–3107. [Google Scholar] [CrossRef] [PubMed]
- Rahmati Nezhad, P.; Riihilä, P.; Piipponen, M.; Kallajoki, M.; Meri, S.; Nissinen, L.; Kähäri, V.-M. Complement factor I upregulates expression of matrix metalloproteinase-13 and -2 and promotes invasion of cutaneous squamous carcinoma cells. Exp. Dermatol. 2021, 30, 1631–1641. [Google Scholar] [CrossRef]
- Bauer, C.; Abdul Pari, A.A.; Umansky, V.; Utikal, J.; Boukamp, P.; Augustin, H.G.; Goerdt, S.; Géraud, C.; Felcht, M. T-lymphocyte profiles differ between keratoacanthomas and invasive squamous cell carcinomas of the human skin. Cancer Immunol. Immunother. 2018, 67, 1147–1157. [Google Scholar] [CrossRef]
- Ferguson, A.L.; Sharman, A.R.; Allen, R.O.; Ye, T.; Lee, J.H.; Low, H.; Ch’ng, S.; Palme, C.E.; Ashford, B.; Ranson, M.; et al. High-dimensional and spatial analysis reveals immune landscape dependent progression in cutaneous squamous cell carcinoma. bioRxiv 2022. [Google Scholar] [CrossRef]
- Montero-Montero, L.; Renart, J.; Ramírez, A.; Ramos, C.; Shamhood, M.; Jarcovsky, R.; Quintanilla, M.; Martín-Villar, E. Interplay between Podoplanin, CD44s and CD44v in Squamous Carcinoma Cells. Cells 2020, 9, 2200. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ko, J.; Vidimos, A.; Koyfman, S.; Gastman, B. A Distinctive Lineage-Negative Cell Population Produces IL-17A in Cutaneous Squamous Cell Carcinoma. J. Interferon Cytokine Res. 2020, 40, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Frazzette, N.; Khodadadi-Jamayran, A.; Doudican, N.; Santana, A.; Felsen, D.; Pavlick, A.C.; Tsirigos, A.; Carucci, J.A. Decreased cytotoxic T cells and TCR clonality in organ transplant recipients with squamous cell carcinoma. NPJ Precis. Oncol. 2020, 4, 13. [Google Scholar] [CrossRef]
- Varki, V.; Ioffe, O.B.; Bentzen, S.M.; Heath, J.; Cellini, A.; Feliciano, J.; Zandberg, D.P. PD-L1, B7-H3, and PD-1 expression in immunocompetent vs. immunosuppressed patients with cutaneous squamous cell carcinoma. Cancer Immunol. Immunother. 2018, 67, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Frauenfelder, S.R.; Freiberger, S.N.; Bouwes Bavinck, J.N.; Quint, K.D.; Genders, R.; Serra, A.L.; Hofbauer, G.F.L. Prostaglandin E2, Tumor Necrosis Factor α, and Pro-opiomelanocortin Genes as Potential Mediators of Cancer Pain in Cutaneous Squamous Cell Carcinoma of Organ Transplant Recipients. JAMA Dermatol. 2017, 153, 350. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.M.; Toland, A.E.; Arron, S.T. IRF4 Polymorphism Is Associated with Cutaneous Squamous Cell Carcinoma in Organ Transplant Recipients: A Pigment-Independent Phenomenon. J. Investig. Dermatol. 2017, 137, 251–253. [Google Scholar] [CrossRef]
- Wei, L.; Allain, D.C.; Bernhardt, M.N.; Gillespie, J.L.; Peters, S.B.; Iwenofu, O.H.; Nelson, H.H.; Arron, S.T.; Toland, A.E. Variants at the OCA2 / HERC2 locus affect time to first cutaneous squamous cell carcinoma in solid organ transplant recipients collected using two different study designs. Br. J. Dermatol. 2017, 177, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanov, A.; Qi, W.; Stenz, N.; Bochud, P.; Kutalik, Z.; Wojtowicz, A.; Hofbauer, G. rs34567942 a Novel Susceptibility Single Nucleotide Polymorphism for Cutaneous Squamous Cell Carcinoma in Organ Transplant Recipients. Acta Derm. Venereol. 2019, 99, 1303–1304. [Google Scholar] [CrossRef]
- Peters, F.S.; Peeters, A.M.A.; Mandaviya, P.R.; van Meurs, J.B.J.; Hofland, L.J.; van de Wetering, J.; Betjes, M.G.H.; Baan, C.C.; Boer, K. Differentially methylated regions in T cells identify kidney transplant patients at risk for de novo skin cancer. Clin. Epigenet. 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.S.; Peeters, A.M.A.; van den Bosch, T.P.P.; Mooyaart, A.L.; van de Wetering, J.; Betjes, M.G.H.; Baan, C.C.; Boer, K. Disrupted regulation of serpinB9 in circulating T cells is associated with an increased risk for post-transplant skin cancer. Clin. Exp. Immunol. 2019, 197, 341–351. [Google Scholar] [CrossRef]
- Geusau, A.; Borik-Heil, L.; Skalicky, S.; Mildner, M.; Grillari, J.; Hackl, M.; Sunder-Plassmann, R. Dysregulation of tissue and serum microRNAs in organ transplant recipients with cutaneous squamous cell carcinomas. Health Sci. Rep. 2020, 3, e205. [Google Scholar] [CrossRef]
- Inman, G.J.; Wang, J.; Nagano, A.; Alexandrov, L.B.; Purdie, K.J.; Taylor, R.G.; Sherwood, V.; Thomson, J.; Hogan, S.; Spender, L.C.; et al. The genomic landscape of cutaneous SCC reveals drivers and a novel azathioprine associated mutational signature. Nat. Commun. 2018, 9, 3667. [Google Scholar] [CrossRef]
- Kim, Y.; Pattanayak, V.; Levandoski, K.A.; Wojciechowski, D.; Asgari, M. 177 Specific HLA types increase risk of keratinocyte carcinoma in renal transplant recipients. J. Investig. Dermatol. 2018, 138, S30. [Google Scholar] [CrossRef]
- Al-Rohil, R.N.; Tarasen, A.J.; Carlson, J.A.; Wang, K.; Johnson, A.; Yelensky, R.; Lipson, D.; Elvin, J.A.; Vergilio, J.-A.; Ali, S.M.; et al. Evaluation of 122 advanced-stage cutaneous squamous cell carcinomas by comprehensive genomic profiling opens the door for new routes to targeted therapies. Cancer 2016, 122, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Chitsazzadeh, V.; Coarfa, C.; Drummond, J.A.; Nguyen, T.; Joseph, A.; Chilukuri, S.; Charpiot, E.; Adelmann, C.H.; Ching, G.; Nguyen, T.N.; et al. Cross-species identification of genomic drivers of squamous cell carcinoma development across preneoplastic intermediates. Nat. Commun. 2016, 7, 12601. [Google Scholar] [CrossRef] [PubMed]
- Lazo de la Vega, L.; Bick, N.; Hu, K.; Rahrig, S.E.; Silva, C.D.; Matayoshi, S.; Picciarelli, P.; Wang, X.; Sugar, A.; Soong, H.K.; et al. Invasive squamous cell carcinomas and precursor lesions on UV-exposed epithelia demonstrate concordant genomic complexity in driver genes. Mod. Pathol. 2020, 33, 2280–2294. [Google Scholar] [CrossRef]
- Sarin, K.Y.; Lin, Y.; Daneshjou, R.; Ziyatdinov, A.; Thorleifsson, G.; Rubin, A.; Pardo, L.M.; Wu, W.; Khavari, P.A.; Uitterlinden, A.; et al. Genome-wide meta-analysis identifies eight new susceptibility loci for cutaneous squamous cell carcinoma. Nat. Commun. 2020, 11, 820. [Google Scholar] [CrossRef]
- Yoshihara, N.; Takagi, A.; Ueno, T.; Ikeda, S. Inverse correlation between microtubule-associated protein 1A/1B-light chain 3 and p62/sequestosome-1 expression in the progression of cutaneous squamous cell carcinoma. J. Dermatol. 2014, 41, 311–315. [Google Scholar] [CrossRef]
- Hara, Y.; Nakamura, M. Overexpression of autophagy-related beclin-1 in advanced malignant melanoma and its low expression in melanoma-in-situ. Eur. J. Dermatol. 2012, 22, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Zajgla, J.; Pozo, L.D.; Ceballos, G.; Maldonado, V. Tissue Inhibitor of Metalloproteinases-4. The road less traveled. Mol. Cancer 2008, 7, 85. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Hansen, T.B.; Venø, M.T.; Kjems, J. Circular RNAs in cancer: Opportunities and challenges in the field. Oncogene 2018, 37, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Gong, J. Heat Shock Proteins Promote Cancer: It’s a Protection Racket. Trends Biochem. Sci. 2016, 41, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Riihilä, P.M.; Nissinen, L.M.; Ala-Aho, R.; Kallajoki, M.; Grénman, R.; Meri, S.; Peltonen, S.; Peltonen, J.; Kähäri, V.-M. Complement factor H: A biomarker for progression of cutaneous squamous cell carcinoma. J. Investig. Dermatol. 2014, 134, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Garrett, G.L.; Blanc, P.D.; Boscardin, J.; Lloyd, A.A.; Ahmed, R.L.; Anthony, T.; Bibee, K.; Breithaupt, A.; Cannon, J.; Chen, A.; et al. Incidence of and Risk Factors for Skin Cancer in Organ Transplant Recipients in the United States. JAMA Dermatol. 2017, 153, 296–303. [Google Scholar] [CrossRef]
- Jensen, P.; Hansen, S.; Møller, B.; Leivestad, T.; Pfeffer, P.; Geiran, O.; Fauchald, P.; Simonsen, S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J. Am. Acad. Dermatol. 1999, 40, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, R.; Downing, C.; Tyring, S.K. Cutaneous Squamous Cell Carcinomas in Organ Transplant Recipients. J. Clin. Med. 2015, 4, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Garrett, G.L.; Lowenstein, S.E.; Singer, J.P.; He, S.Y.; Arron, S.T. Trends of skin cancer mortality after transplantation in the United States: 1987 to 2013. J. Am. Acad. Dermatol. 2016, 75, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Blue, E.D.; Freeman, S.C.; Lobl, M.B.; Clarey, D.D.; Fredrick, R.L.; Wysong, A.; Whitley, M.J. Cutaneous Squamous Cell Carcinoma Arising in Immunosuppressed Patients: A Systematic Review of Tumor Profiling Studies. JID Innov. 2022, 2, 100126. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Otley, C.C. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J. Am. Acad. Dermatol. 2002, 47, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lanz, J.; Bouwes Bavinck, J.N.; Westhuis, M.; Quint, K.D.; Harwood, C.A.; Nasir, S.; Van-de-Velde, V.; Proby, C.M.; Ferrándiz, C.; Genders, R.E.; et al. Aggressive Squamous Cell Carcinoma in Organ Transplant Recipients. JAMA Dermatol. 2019, 155, 66–71. [Google Scholar] [CrossRef]
- Sheil, A.G.; Disney, A.P.; Mathew, T.H.; Amiss, N. De novo malignancy emerges as a major cause of morbidity and late failure in renal transplantation. Transplant. Proc. 1993, 25, 1383–1384. [Google Scholar] [PubMed]
- Sigalotti, L.; Coral, S.; Nardi, G.; Spessotto, A.; Cortini, E.; Cattarossi, I.; Colizzi, F.; Altomonte, M.; Maio, M. Promoter methylation controls the expression of MAGE2, 3 and 4 genes in human cutaneous melanoma. J. Immunother. 2002, 25, 16–26. [Google Scholar] [CrossRef]
- Tyler, L.N.; Ai, L.; Zuo, C.; Fan, C.-Y.; Smoller, B.R. Analysis of promoter hypermethylation of death-associated protein kinase and p16 tumor suppressor genes in actinic keratoses and squamous cell carcinomas of the skin. Mod. Pathol. 2003, 16, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.M.; Wang, W.; Ioannidis, N.M.; Itnyre, J.; Hoffmann, T.; Jorgenson, E.; Whittemore, A.S. Identification of Susceptibility Loci for Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2016, 136, 930–937. [Google Scholar] [CrossRef]
- Lee, H.E.; Chae, S.W.; Lee, Y.J.; Kim, M.A.; Lee, H.S.; Lee, B.L.; Kim, W.H. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br. J. Cancer 2008, 99, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; Lee, Y.H.; Pan, L.; Lai, L.; Chua, C.; Wasser, M.; Lim, T.K.H.; Yeong, J.; Toh, H.C.; Lee, S.Y.; et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 2019, 68, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Kawai, O.; Ishii, G.; Kubota, K.; Murata, Y.; Naito, Y.; Mizuno, T.; Aokage, K.; Saijo, N.; Nishiwaki, Y.; Gemma, A.; et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 2008, 113, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.; Asaoka, M.; Tokumaru, Y.; Yan, L.; Matsuyama, R.; Ishikawa, T.; Endo, I.; Takabe, K. CD8 T Cell Score as a Prognostic Biomarker for Triple Negative Breast Cancer. Int. J. Mol. Sci. 2020, 21, 6968. [Google Scholar] [CrossRef]
| Gene | Study Population | Results | Author | Year |
|---|---|---|---|---|
| BCL2L1 | 24 human cSCC samples (9 cSCCHN, 7 cSCCHN with incidental PNI, 8 cSCCHN with clinical PNI) | Downregulated in cSCCHN with PNI vs. without PNI (node degree of 36) | Zheng et al. [4] | 2018 |
| ERBB2 | Upregulated in cSCCHN with PNI vs. without PNI (node degree of 31) | |||
| HIF1A | Upregulated in cSCCHN with PNI vs. without PNI (node degree of 30) | |||
| MAPK8 | Upregulated in cSCCHN with PNI vs. without PNI (highest node degree of 41) | |||
| MTOR | Downregulated in cSCCHN with PNI vs. without PNI (node degree of 34) | |||
| MYC | Downregulated in cSCCHN with PNI vs. without PNI (node degree of 42) | |||
| PPARγ | Downregulated in cSCCHN with PNI vs. without PNI (node degree of 32) | |||
| RAB23 | RAB23 gene expression was positively correlated with HIF1A (p = 0.001, r = 0.690), MAPK8 (p = 0.007, r = 0.583) and ARFGAP1 (p = 0.000, r = 0.655), but negatively associated with MTOR (p = 0.002, r = −0.748) and BCL2L1 (p = 0.015, r = −0.528) | |||
| TNF | Downregulated in cSCCHN with PNI vs. without PNI (highest node degree of 44) | |||
| FGFR2 | 10 cases of high-risk head and neck cSCC | Somatic missense mutations in FGFR2 (40%) were exclusively seen in patients with PNI. Two novel mutations, FGFR2 A380D and D528N were observed in this cohort | C Zilberg et al. [6] | 2018 |
| C3 | 45 cases of human HNcSCC stratified into 3 groups (Extensive n = 25, Focal n = 11 and Non PNI n = 9) | Top 10 DEG identified between EXT PNI vs. FOC_NON (Padj < 0.01, log2FC = 3.237621) | Eviston et al. [5] | 2021 |
| FERMT2 | Top 10 DEG identified between EXT PNI vs. FOC_NON (Padj < 0.01, log2FC = 1.717795) | |||
| HGF | Top 10 DEG identified between EXT PNI vs. FOC_NON (Padj < 0.01, log2FC = 2.856216) | |||
| NR4A3 | Top 10 DEG identified between EXT PNI vs. FOC_NON (Padj < 0.01, log2FC = 2.830313) | |||
| PTGIS | Top 10 DEG identified between EXT PNI vs. FOC_NON (Padj < 0.01, log2FC = 4.265134) | |||
| SAMSN1 | Top 10 DEG identified between EXT PNI vs. FOC_NON (Padj < 0.01, log2FC = 1.530389) | |||
| SRGN | Top 10 DEG identified between EXT PNI vs. FOC_NON (Padj < 0.01, log2FC = 2.030587) | |||
| TIMP1 | Top 10 DEG identified between EXT PNI vs. FOC_NON (Padj < 0.01, log2FC = 2.198932) | |||
| THBS4 | Top 10 DEG identified between EXT PNI vs. FOC_NON (Padj < 0.01, log2FC = 4.996716) | |||
| VCAN | Top 10 DEG identified between EXT PNI vs. FOC_NON (Padj < 0.01, log2FC = 1.849012) |
| Gene | Study Population | Results | Author | Year |
|---|---|---|---|---|
| KMT2D | Human metastatic cSCC and primary non-metastatic cSCC | Higher rates of mutation in the metastatic samples (62%) relative to non-metastatic ones (31%) | Yilmaz et al. [8] | 2017 |
| TP53 | Higher mutation frequencies in metastatic disease compared to localized disease (85% vs. 54% respectively; p < 0.0001) | |||
| TERT | 152 cSCC samples from 122 patients (in situ cSCC, n = 31; invasive cSCC, n = 121) | TERTp mutations were significantly more frequent in cases that recurred (13 out of 17 cases [76.5%] vs. 29 of 104 cases [27.9%] [p < 0.001]). TERTp mutation was identified as an independent predictor of recurrence (OR, 8.11; p = 0.002, multivariate analysis) | Campos et al. [10] | 2018 |
| CDKN2A | 20 case-matched localized (10) and metastatic (10) high-risk cSCC | One of the most frequently mutated genes in localized (20%) and metastatic cSCC (40%) | M.B. Lobl et al. [9] | 2020 |
| ERBB4 | Seen only in localized cSCC (20%). ERBB4 and STK11 were found to be significantly co-occurring in localized high-risk SCC (pair wise Fisher’s exact test p < 0.05) | |||
| HRAS | Seen only in metastatic cSCC (20%) | |||
| KDR | One of the most frequently mutated genes in localized (40%) and metastatic cSCC (30%) | |||
| KIT | One of the most frequently mutated genes in localized (10%) and metastatic cSCC (20%) | |||
| NOTCH1 | One of the most frequently mutated genes in localized (20%) and metastatic cSCC (10%) | |||
| PTEN | One of the most frequently mutated genes in localized (10%) and metastatic cSCC (20%) | |||
| SMAD4 | One of the most frequently mutated genes in localized (30%) and metastatic cSCC (20%) | |||
| STK11 | Seen only in localized cSCC (30%). ERBB4 and STK11 were found to be significantly co-occurring in localized high-risk SCC (pair wise Fisher’s exact test p < 0.05) | |||
| TP53 | One of the most frequently mutated genes in localized (70%) and metastatic cSCC (70%) | |||
| ITGA5 | cSCCHN from 50 patients. 21 PRI−, 14 PRI+, 15 MET, matched SES | Upregulated in MET vs. PRI+ (log2FC > 0.58, Padj < 0.05). Upregulated in MET vs. PRI- (log2FC > 0.58, Padj < 0.05) | Minaei et al. [7] | 2022 |
| MMP1 | Upregulated in MET vs. PRI- (log2FC > 0.58, Padj < 0.05) | |||
| MMP10 | Shared upregulated gene in MET vs. SES and PRI+ vs. SES (log2FC > 1, Padj < 0.05) | |||
| MMP13 | Increased in MET vs. SES (log2FC > 1, Padj < 0.05), increased in PRI+ vs. SES | |||
| PLAU | Shared upregulated gene in MET vs. SES and PRI+ vs. SES (log2FC > 1, Padj < 0.05). Upregulated in MET vs. PRI- (log2FC > 0.58, Padj < 0.05) | |||
| PLAUR | Upregulated in MET vs. PRI- (log2FC > 0.58, Padj < 0.05) | |||
| TIMP1 | Upregulated in MET vs. PRI+ (log2FC > 0.58, Padj < 0.05) | |||
| TIMP4 | Downregulated in MET vs. SES (log2FC < 1, Padj < 0.05) and PRI+ vs. SES (log2FC < 1, Padj < 0.05) | |||
| VEGFA | Upregulated in MET vs. PRI+ (log2FC > 0.58, Padj < 0.05). Upregulated in MET vs. PRI- (log2FC > 0.58, Padj < 0.05) |
| Gene | Study Population | Results | Author | Year |
|---|---|---|---|---|
| RIPK4 | Mouse models | The onset of tumors commenced as early as 8 weeks in RIPK4 cKO mice and 13 weeks in WT littermates. At week 11, 100% of cKO animals developed skin lesions, whereas more than 50% of WT animals remained tumor free even after 15 weeks | P Lee et al. [12] | 2017 |
| HOXA9 | Human cSCC cell lines (A431, and HSC-1), control cell line (HaCaT) | HOXA9 was downregulated in all cSCC cell lines compared with the primary keratinocytes and the HaCaT keratinocytes (p < 0.001). MiR-365 expression was inversely correlated with HOXA9 expression in these cell lines | Zhou et al. [15] | 2018 |
| (Genes encoding) RNase H2 | Mouse models | Loss of RNase H2 in the epidermis resulted in spontaneous DNA damage (increased numbers of repair foci, increased transcript levels of p53-inducible genes) and resulted in progression to skin cancer (at least KIN stage) in 100% of cases within the first year of life | Hiller et al. [13] | 2018 |
| SFRP1 | A3886 (skin cutaneous SCC cell line), MCF-10A (control), and MDA-MB-231 (TNBC) cell lines | Sfrp1 −/− and Sfrp1+/− mice papilloma formation appears earlier by 3–4 weeks and 2–3 weeks, respectively, compared with WT mice | Sunkara et al. [14] | 2020 |
| CREBBP | Mouse models, human cSCC cell lines: A431(CRL–1555), SCC13, COLO16 | Forced depletion of CREBBP increased cellular proliferation relative to control (1-factor ANOVA, p < 0.05). Knockdown of CREBBP expression led to larger and significantly more colonies relative to control (p = 0.018) | Aiderus et al. [17] | 2021 |
| KMT2C | Forced depletion of KMT2C increased cellular proliferation relative to control (1-factor ANOVA, p < 0.05). Knockdown of KMT2C accelerated in vitro proliferation and in vivo xenograft growth | |||
| CYLD | Immunocompetent mice | Tumor multiplicity was higher in Control/TgAC mice vs. K5-CYLDwt/TgAC mice (p < 0.01). Lower levels of NF-kB activation found in K5-CYLDwt/TgAC tumors | Alameda et al. [11] | 2021 |
| KANSL1 | Matched tumor and blood DNA from 25 patients with regional metastases of cSCCHN | KANSL1 (Ch17q) showed deletion in 32% of tumor samples | Thind et al. [16] | 2022 |
| PTPRD | Deletion of PTPRD (Chr9p) was observed in 20% of tumor samples |
| Epigenetic Biomarker | Study Population | Results | Author | Year |
|---|---|---|---|---|
| bHLH TFs | Genetically engineered mouse models: Lgr5CreER and K14CreER mice | Upregulated during EMT and enriched in the open chromatin regions of TMCs in 20–45% of targets | Latil et al. [26] | 2017 |
| Ets1 | Upregulated during EMT and positively associated with gene expression in 10% of targets. Upregulated during tumorigenesis in 37% of targets (p = 1 × 10−10) | |||
| Jun/AP1 | Upregulated during EMT and enriched in the open chromatin regions of TMCs in 42% of targets. Enriched in the open chromatin regions during tumorigenesis in 65% of targets (p = 1 × 10−130) | |||
| NF1 | Upregulated during EMT and enriched in the open chromatin regions of TMCs in 45% of targets | |||
| Nf-kb | Enriched in the open chromatin regions during tumorigenesis in 22% of targets (p = 1 × 10−10) | |||
| Nfatc | Upregulated during EMT and enriched in the open chromatin regions of TMCs in 27% of targets | |||
| Runx | Enriched in the open chromatin regions during tumorigenesis in 29% of targets (p = 1 × 10−10) | |||
| Smad2 | Upregulated during EMT and enriched in the open chromatin regions of TMCs in 37% of targets | |||
| TEAD | Enriched in the open chromatin regions during tumorigenesis in 25% of targets (p = 1 × 10−8) | |||
| DNA methylation | 23 human cSCC samples diagnosed at the following stages: AK, early invasive carcinoma, high-risk non-metastatic carcinoma and high-risk carcinoma with nodal metastasis | Initial invasive group showed lower methylation levels than premalignant actinic keratosis. Hypermethylation of all substructures in both high-risk non-metastatic and metastatic groups compared to low-risk initial invasive cSCC samples (p < 0.001, two-sided t-test) | Hervás-Marín et al. [28] | 2019 |
| ID4 | 8 pairs of matched human cSCC and adjacent normal skin tissues; sun-exposed normal skin in the head and neck region (n = 60) from normal patients and distal non-exposed normal skin from cSCC patients (n = 60) | ID4 expression was downregulated in cSCC (p = 0.0111) and correlated with increased levels of promoter methylation (p = 0.00295) | L. Li et al. [29] | 2020 |
| UCHL1 | UCHL1 expression was downregulated in cSCC (p = 0.0205) and correlated with increased levels of promoter methylation (p = 0.0499) | |||
| ACTL6A | SCC-13 and HaCaT cell lines | ACTL6A knockdown reduces SCC cell proliferation, spheroid formation, invasion and migration | Shrestha et al. [27] | 2020 |
| Filip1l | Mouse cSCC, human cSCC, NHEK | In murine cSCC tumours, the Filip1l protein levels were reduced compared to matched controls (paired t-test, p = 0.0026). In human cSCC, FILIP1L protein levels were increased in one cSCC cell line, similar to NHK in 4/12 cSCC cell lines and lower (i.e., below 2/3 of NHK means) than NHEK in 7/12 cSCC cell lines | K. Roth et al. [30] | 2021 |
| ZMIZ1 | Mouse models, human cSCC cell lines: A431(CRL–1555), SCC13, COLO16 | All cSCC tumors with Zmiz1/2 insertions had inactivating insertions in at least one gene involved in chromatin remodeling | Aiderus et al. [17] | 2021 |
| ZMIZ2 |
| Immune Biomarker | Study Population | Results | Author | Year |
|---|---|---|---|---|
| CGRP | cSCC from 34 OTRs; pain-associated (n = 18), without pain (n = 16) | No difference in CGRP expression levels in cSCC with pain vs. cSCC without pain in OTRs. | Frauenfelder et al. [76] | 2017 |
| NGF | No difference in NGF expression levels in cSCC with pain vs. cSCC without pain in OTRs. | |||
| IL-1β | No difference in IL-1β expression levels in cSCC with pain vs. cSCC without pain in OTRs. | |||
| PGE2 | cSCC with pain is associated with increased levels of PGE2 compared with cSCC without pain (OR = 1.9, 95% CI = 1.1–3.4, p = 0.002), adjusted for age and sex. | |||
| POMC | cSCC with pain was associated with increased levels of POMC compared with cSCC without pain (OR = 1.5, 95% CI = 0.99–2.0, p = 0.05), adjusted for age and sex. | |||
| TNF-α | cSCC with pain was associated with increased levels of TNF-α compared with cSCC without pain (adjusted OR = 1.4, 95% CI = 0.99–2.0, p = 0.05). | |||
| AIM2 | Primary (n = 5) and metastatic (n = 3) human cSCC cell lines, NHEK (n = 5). Tissue samples: normal sun-protected skin (n = 15), AK (n = 71), cSCCIS (n = 60), and UV-induced cSCC (n = 81) | In OTR derived tissues, AIM2 expression was significantly more abundant in cSCC (n = 57) compared with cSCCIS (n = 59, p < 0.001) | Farshchian et al. [65] | 2017 |
| B7-H3 | SCC from 42 ICP and 24 ISP (13 OTRs, 8 HIV, and 3 others) | Tumor expression of B7-H3 was higher in in ICP vs. ISP (Median 60 vs. 28%, p = + 0.025) | Varki et al. [75] | 2018 |
| PD-L1 | No difference in PD-L1 expression between ICP and ISP (p = 0.5). | |||
| CD8+ T-cells | cSCC (n = 5), TSCC (n = 6) | OTRs generally exhibited lower levels of CD8+ TILs (n = 6880 ICP; n = 2484 ISP, p < 0.05) | Frazzette et al. [74] | 2020 |
| IL-17A | 14 human cSCC: ISP (n = 3), ICP (n = 11) | CD3−IL-17+ cells consist of over 90% of the total IL-17A-producing cells in the tumor tissue from ISP, while the ratio of CD3+IL-17+ versus CD3−IL-17+ cells vary in ICP | Sun et al. [73] | 2020 |
| B cells | Human cSCC from 31 patients: 9 NP, 22 DP. Within the. DP group: 5 ISP and 17 ACIS | In ACIS patients there was significant increase in total B cell numbers and proliferating B cells in metastases compared to primary DP tumours. This increase was not evident among ISP | Ferguson et al. [71] | 2022 |
| IDO | The increased expression of IDO on all T cells in metastatic cSCC among ACIS patients was absent among ISP | |||
| PD-L1 | The increased expression of PD-L1 on CD8+ T cells in metastatic cSCC among ACIS patients was absent among ISP | |||
| PD-L2 | The increased expression of PD-L2 on CD4+ T cells in metastatic cSCC among ACIS patients was absent among ISP | |||
| TIM3 | TIM3 was increased on non-classical monocytes in metastatic tumours of ACIS patients compared to immunosuppressed patients |
| Genetic Polymorphism | Study Population | Results | Author | Year | |
|---|---|---|---|---|---|
| HERC2 | rs916977, rs12913832 brown eye allele compared with blue eye allele | 386 OTRs with cSCC and without | OTRs homozygous for brown eye alleles rs916977 (GG) and rs12913832 (AA) had significant delays of time to first cSCC after transplant vs. OTRs homozygous for blue eye alleles (HR = 0.34, p < 0.001; HR = 0.54, p = 0.012, respectively). | Wei et al. [78] | 2017 |
| OCA2 | |||||
| IRF4 | rs12203592 T allele | 388 OTRs with cSCC and without | The IRF4 rs12203592 T allele was associated with a significantly increased hazard for time to first cSCC (HR = 1.36, p = 0.02, univariate analysis). This association was maintained when adjusted for age, gender, organ transplanted, and Fitzpatrick skin type (HR = 1.34, p = 0.04). | Asgari et al. [77] | 2017 |
| SLC45A2 | rs16891982 C allele | The SLC45A2 rs16891982 C allele was associated with a decreased hazard for cSCC (HR = 0.58, p = 0.04, univariate analysis); this effect was comparable but not significant using the multivariate model (HR = 0.74, p = 0.06). | |||
| Upstream of RP1163E5.6, FBXO25, and OR4F2 | rs34567942 | 61 OTRs with cSCC and 908 OTRs without cSCC | GWAS identified one SNV, rs34567942, to be significantly associated with cSCC in OTRs (p-value threshold of 5 × 10−8) | Kuzmanov et al. [79] | 2019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsang, D.A.; Tam, S.Y.C.; Oh, C.C. Molecular Alterations in Cutaneous Squamous Cell Carcinoma in Immunocompetent and Immunosuppressed Hosts—A Systematic Review. Cancers 2023, 15, 1832. https://doi.org/10.3390/cancers15061832
Tsang DA, Tam SYC, Oh CC. Molecular Alterations in Cutaneous Squamous Cell Carcinoma in Immunocompetent and Immunosuppressed Hosts—A Systematic Review. Cancers. 2023; 15(6):1832. https://doi.org/10.3390/cancers15061832
Chicago/Turabian StyleTsang, Denise Ann, Steve Y. C. Tam, and Choon Chiat Oh. 2023. "Molecular Alterations in Cutaneous Squamous Cell Carcinoma in Immunocompetent and Immunosuppressed Hosts—A Systematic Review" Cancers 15, no. 6: 1832. https://doi.org/10.3390/cancers15061832
APA StyleTsang, D. A., Tam, S. Y. C., & Oh, C. C. (2023). Molecular Alterations in Cutaneous Squamous Cell Carcinoma in Immunocompetent and Immunosuppressed Hosts—A Systematic Review. Cancers, 15(6), 1832. https://doi.org/10.3390/cancers15061832





