Application of Machine Learning for Differentiating Bone Malignancy on Imaging: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Literature Search Strategy
2.2. The Study Screening and Selection Criteria
2.3. Data Extraction and Reporting
- Research article details: Complete authorship, date of journal or publication, Journal name;
- Main clinical use: Differentiating benign vs. malignant bone lesions, characterization and classification of various bone tumours;
- Patient population: Patients with known bone lesions, who have undergone various imaging investigations (X-ray, CT, PET/CT or MRI) and have subsequently undergone histopathological confirmation;
- Research study details: The type of study, patient or imaging modality sample sizes (for example, internal or external data sets), imaging modalities used (CT, MRI, bone scans or PET/CT), treatment or management information and outcome/prognostic measures;
- Machine Learning techniques used: Radiomics and convolutional neural networks, among others.
3. Results
3.1. Search Results
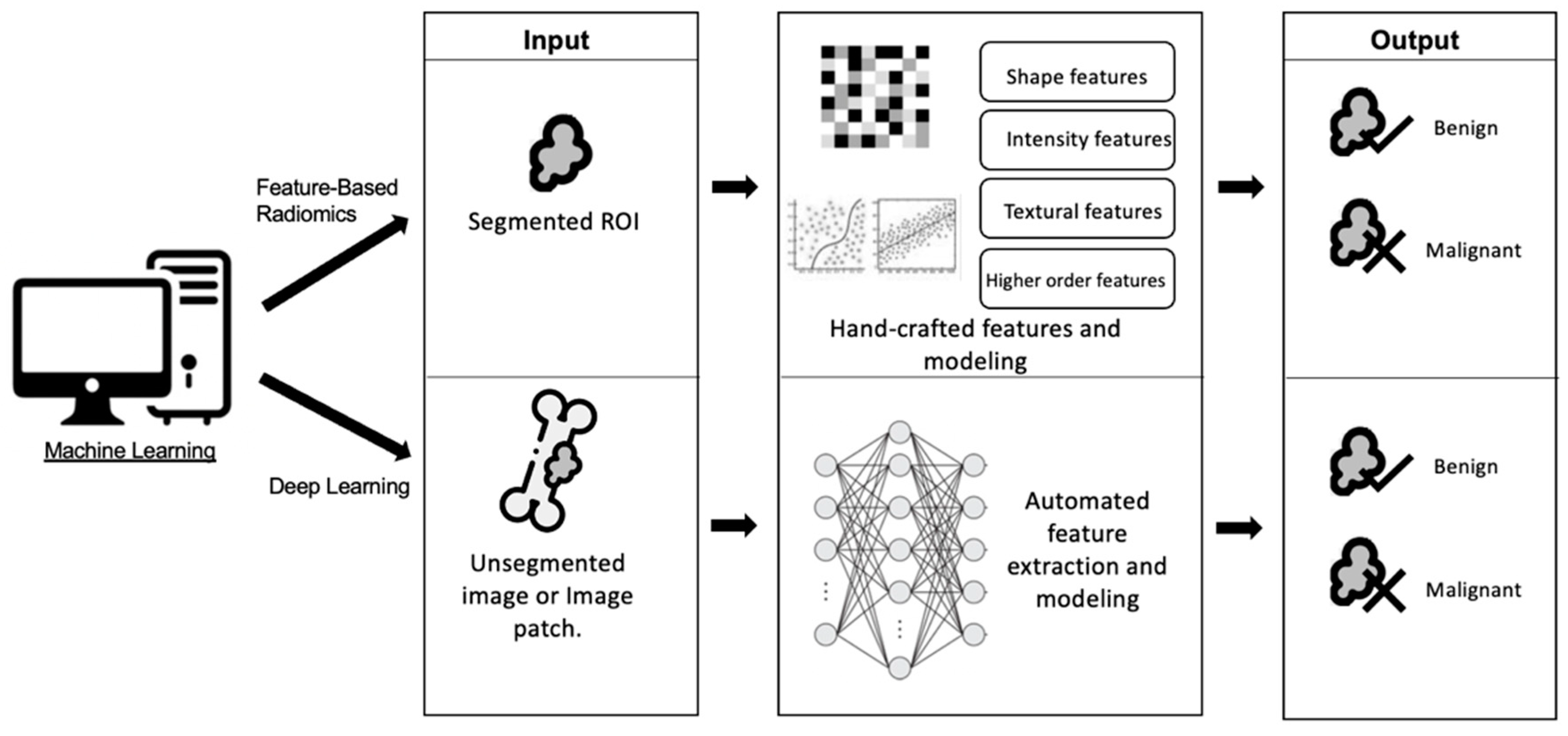
3.2. Machine Learning Techniques
4. Discussion
4.1. Machine Learning on Conventional Radiographs
4.2. Machine Learning on Computed Tomography (CT) Imaging
4.3. Machine Learning on Magnetic Resonance Imaging (MRI)
4.4. Machine Learning on Positiron Emission Tomography with CT (PET/CT) Imaging
4.5. Potential Clinical Impact and Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ladd, L.M.; Roth, T.D. Computed Tomography and Magnetic Resonance Imaging of Bone Tumors. Semin. Roentgenol. 2017, 52, 209–226. [Google Scholar] [CrossRef]
- Piperkova, E.; Mikhaeil, M.; Mousavi, A.; Libes, R.; Viejo-Rullan, F.; Lin, H.; Rosen, G.; Abdel-Dayem, H. Impact of PET and CT in PET/CT studies for staging and evaluating treatment response in bone and soft tissue sarcomas. Clin. Nucl. Med. 2009, 34, 146–150. [Google Scholar] [CrossRef]
- Goyal, N.; Kalra, M.; Soni, A.; Baweja, P.; Ghonghe, N.P. Multi-modality imaging approach to bone tumors—State-of-the art. J. Clin. Orthop. Trauma. 2019, 10, 687–701. [Google Scholar] [CrossRef]
- Hapani, H.; Kalola, J.; Hapani, J. Comparative role of CT scan and MR imaging in primary malignant bone tumors. IOSR J. Dent. Med. Sci. 2014, 13, 29–35. [Google Scholar] [CrossRef]
- Berquist, T.; Dalinka, M.; Alazraki, N.; Daffner, R.; DeSmet, A.; el-Khoury, G.; Goergen, T.; Keats, T.; Manaster, B.; Newberg, A. Bone tumors. American college of radiology. ACR appropriateness criteria. Radiology 2000, 215, 261–264. [Google Scholar]
- Costelloe, C.M.; Rohren, E.M.; Madewell, J.E.; Hamaoka, T.; Theriault, R.L.; Yu, T.-K.; Lewis, V.O.; Ma, J.; Stafford, R.J.; Tari, A.M.; et al. Imaging bone metastases in breast cancer: Techniques and recommendations for diagnosis. Lancet Oncol. 2009, 10, 606–614. [Google Scholar] [CrossRef]
- Karanian, M.; Coindre, J.M. Fourth edition of WHO classification tumours of soft tissue. Ann. Pathol. 2015, 35, 71–85. [Google Scholar] [CrossRef]
- Vanel, D. General principles of imaging. In Diagnosis of Musculoskeletal Tumors and Tumor-like Conditions: Clinical, Radiological and Histological Correlations—The Rizzoli Case Archive; Picci, P., Manfrini, M., Donati, D.M., Gambarotti, M., Righi, A., Vanel, D., Dei Tos, A.P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 27–30. [Google Scholar]
- Huang, P.-Y.; Wu, P.-K.; Chen, C.-F.; Lee, F.-T.; Wu, H.-T.; Liu, C.-L.; Chen, T.-H.; Chen, W.-M. Osteomyelitis of the femur mimicking bone tumors: A review of 10 cases. World J. Surg. Oncol. 2013, 11, 283. [Google Scholar] [CrossRef]
- Gerber, E.; Said-Hartley, Q.; Gamieldien, R.; Hartley, T.; Candy, S. Accuracy of plain radiographs in diagnosing biopsy-proven malignant bone lesions. SA J. Radiol. 2019, 23, 1768. [Google Scholar] [CrossRef]
- Priolo, F.; Cerase, A. The current role of radiography in the assessment of skeletal tumors and tumor-like lesions. Eur. J. Radiol. 1998, 27 (Suppl. S1), S77–S85. [Google Scholar] [CrossRef]
- Picci, P. Epidemiology of Bone Lesions. In Diagnosis of Musculoskeletal Tumors and Tumor-like Conditions: Clinical, Radiological and Histological Correlations—The Rizzoli Case Archive; Picci, P., Manfrini, M., Donati, D.M., Gambarotti, M., Righi, A., Vanel, D., Dei Tos, A.P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–9. [Google Scholar]
- Wu, J.S.; Hochman, M.G. Bone Tumors: A Practical Guide to Imaging; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yang, J.; Li, K.; Deng, H.; Feng, J.; Fei, Y.; Jin, Y.; Liao, C.; Li, Q. CT cinematic rendering for pelvic primary tumor photorealistic visualization. Quant. Imaging Med. Surg. 2018, 8, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Zampa, V.; Roselli, G.; Beltrami, G. MRI of bone tumors: Advances in diagnosis and treatment assessment. Imaging Med. 2010, 2, 325–340. [Google Scholar] [CrossRef]
- Abdel Razek, A.A.; Castillo, M. Imaging appearance of primary bony tumors and pseudo-tumors of the spine. J. Neuroradiol. 2010, 37, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Faiella, E.; Santucci, D.; Calabrese, A.; Russo, F.; Vadala, G.; Zobel, B.B.; Soda, P.; Iannello, G.; de Felice, C.; Denaro, V. Artificial Intelligence in Bone Metastases: An MRI and CT Imaging Review. Int. J. Env. Res. Public Health 2022, 19, 1880. [Google Scholar] [CrossRef]
- Salamipour, H.; Jimenez, R.M.; Brec, S.L.; Chapman, V.M.; Kalra, M.K.; Jaramillo, D. Multidetector row CT in pediatric musculoskeletal imaging. Pediatr. Radiol. 2005, 35, 555–564. [Google Scholar] [CrossRef]
- Zimmer, W.D.; Berquist, T.H.; McLeod, R.A.; Sim, F.H.; Pritchard, D.J.; Shives, T.C.; Wold, L.E.; May, G.R. Bone tumors: Magnetic resonance imaging vs. computed tomography. Radiology 1985, 155, 709–718. [Google Scholar] [CrossRef]
- Nascimento, D.; Suchard, G.; Hatem, M.; de Abreu, A. The role of magnetic resonance imaging in the evaluation of bone tumours and tumour-like lesions. Insights Imaging 2014, 5, 419–440. [Google Scholar] [CrossRef] [PubMed]
- Vande Berg, B.C.; Malghem, J.; Lecouvet, F.E.; Maldague, B. Classification and detection of bone marrow lesions with magnetic resonance imaging. Skelet. Radiol. 1998, 27, 529–545. [Google Scholar] [CrossRef]
- Davies, A.M.; Sundaram, M.; James, S.L. Imaging of bone tumors and tumor-like lesions: Techniques and applications; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Rijswijk, C.S.P.v.; Geirnaerdt, M.J.A.; Hogendoorn, P.C.W.; Taminiau, A.H.M.; Coevorden, F.v.; Zwinderman, A.H.; Pope, T.L.; Bloem, J.L. Soft-Tissue Tumors: Value of Static and Dynamic Gadopentetate Dimeglumine–enhanced MR Imaging in Prediction of Malignancy. Radiology 2004, 233, 493–502. [Google Scholar] [CrossRef]
- Tokuda, O.; Hayashi, N.; Taguchi, K.; Matsunaga, N. Dynamic contrast-enhanced perfusion MR imaging of diseased vertebrae: Analysis of three parameters and the distribution of the time-intensity curve patterns. Skelet. Radiol. 2005, 34, 632–638. [Google Scholar] [CrossRef]
- Kajihara, M.; Sugawara, Y.; Sakayama, K.; Kikuchi, K.; Mochizuki, T.; Murase, K. Evaluation of tumor blood flow in musculoskeletal lesions: Dynamic contrast-enhanced MR imaging and its possibility when monitoring the response to preoperative chemotherapy—Work in progress. Radiat. Med. 2007, 25, 94–105. [Google Scholar] [CrossRef]
- Costa, F.M.; Ferreira, E.C.; Vianna, E.M. Diffusion-Weighted Magnetic Resonance Imaging for the Evaluation of Musculoskeletal Tumors. Magn. Reson. Imaging Clin. N. Am. 2011, 19, 159–180. [Google Scholar] [CrossRef]
- van Rijswijk, C.S.; Kunz, P.; Hogendoorn, P.C.; Taminiau, A.H.; Doornbos, J.; Bloem, J.L. Diffusion-weighted MRI in the characterization of soft-tissue tumors. J. Magn. Reason. Imaging 2002, 15, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.; Bischoff, G.; Buck, A.; von Baer, A.; Pauls, S.; Scheffold, F.; Schultheiss, M.; Gebhard, F.; Reske, S.N. Integrated FDG-PET-CT: Its role in the assessment of bone and soft tissue tumors. Arch. Orthop. Trauma Surg. 2010, 130, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Charest, M.; Hickeson, M.; Lisbona, R.; Novales-Diaz, J.A.; Derbekyan, V.; Turcotte, R.E. FDG PET/CT imaging in primary osseous and soft tissue sarcomas: A retrospective review of 212 cases. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.; Brecht-Krauss, D.; Heymer, B.; Guhlmann, A.; Hartwig, E.; Sarkar, M.R.; Diederichs, C.G.; Von Baer, A.; Kotzerke, J.; Reske, S.N. Grading of tumors and tumorlike lesions of bone: Evaluation by FDG PET. J. Nucl. Med. 2000, 41, 1695–1701. [Google Scholar] [PubMed]
- Dimitrakopoulou-Strauss, A.; Strauss, L.G.; Heichel, T.; Wu, H.; Burger, C.; Bernd, L.; Ewerbeck, V. The role of quantitative (18)F-FDG PET studies for the differentiation of malignant and benign bone lesions. J. Nucl. Med. 2002, 43, 510–518. [Google Scholar] [PubMed]
- Choi, H.S.; Yoo Ie, R.; Park, H.L.; Choi, E.K.; Kim, S.H.; Lee, W.H. Role of ¹⁸F-FDG PET/CT in differentiation of a benign lesion and metastasis on the ribs of cancer patients. Clin. Imaging 2014, 38, 109–114. [Google Scholar] [CrossRef]
- Wang, L.J.; Wu, H.B.; Zhou, W.L.; Yu, S.R.; Wang, Q.S. Gummatous Syphilis Mimicking Malignant Bone Tumor on FDG PET/CT. Clin. Nucl. Med. 2019, 44, 313–316. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, H.; Yin, Y.; Zhang, J.; Yang, M.; Qin, S.; Zhang, X.; Yu, F. Texture Analysis of (18)F-FDG PET/CT for Differential Diagnosis Spinal Metastases. Front. Med. 2020, 7, 605746. [Google Scholar] [CrossRef]
- Ma, L.D.; Frassica, F.J.; Scott, W.W., Jr.; Fishman, E.K.; Zerbouni, E.A. Differentiation of benign and malignant musculoskeletal tumors: Potential pitfalls with MR imaging. Radiographics 1995, 15, 349–366. [Google Scholar] [CrossRef]
- Tran, K.A.; Kondrashova, O.; Bradley, A.; Williams, E.D.; Pearson, J.V.; Waddell, N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021, 13, 152. [Google Scholar] [CrossRef]
- Liu, B.; Chi, W.; Li, X.; Li, P.; Liang, W.; Liu, H.; Wang, W.; He, J. Evolving the pulmonary nodules diagnosis from classical approaches to deep learning-aided decision support: Three decades’ development course and future prospect. J. Cancer Res. Clin. Oncol. 2020, 146, 153–185. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, K.; Zhong, Y.; Liang, M.; Qin, P.; Li, H.; Zhang, R.; Li, S.; Liu, X. Assessing the predictive accuracy of lung cancer, metastases, and benign lesions using an artificial intelligence-driven computer aided diagnosis system. Quant Imaging Med. Surg. 2021, 11, 3629–3642. [Google Scholar] [CrossRef]
- Raya-Povedano, J.L.; Romero-Martín, S.; Elías-Cabot, E.; Gubern-Mérida, A.; Rodríguez-Ruiz, A.; Álvarez-Benito, M. AI-based Strategies to Reduce Workload in Breast Cancer Screening with Mammography and Tomosynthesis: A Retrospective Evaluation. Radiology 2021, 300, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Graewingholt, A.; Duffy, S. Retrospective comparison between single reading plus an artificial intelligence algorithm and two-view digital tomosynthesis with double reading in breast screening. J. Med. Screen. 2021, 28, 365–368. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Sotiras, A.; Milchenko, M.; LaMontagne, P.; Hileman, M.; Marcus, D. MRI-based Identification and Classification of Major Intracranial Tumor Types by Using a 3D Convolutional Neural Network: A Retrospective Multi-institutional Analysis. Radiol. Artif. Intell. 2021, 3, e200301. [Google Scholar] [CrossRef] [PubMed]
- Deepak, S.; Ameer, P.M. Brain tumor classification using deep CNN features via transfer learning. Comput. Biol. Med. 2019, 111, 103345. [Google Scholar] [CrossRef]
- Díaz-Pernas, F.J.; Martínez-Zarzuela, M.; Antón-Rodríguez, M.; González-Ortega, D. A Deep Learning Approach for Brain Tumor Classification and Segmentation Using a Multiscale Convolutional Neural Network. Healthcare 2021, 9, 153. [Google Scholar] [CrossRef]
- Dmitriev, K.; Kaufman, A.E.; Javed, A.A.; Hruban, R.H.; Fishman, E.K.; Lennon, A.M.; Saltz, J.H. Classification of Pancreatic Cysts in Computed Tomography Images Using a Random Forest and Convolutional Neural Network Ensemble. Med. Image Comput. Comput. Assist. Interv. 2017, 10435, 150–158. [Google Scholar] [CrossRef]
- Massafra, R.; Fanizzi, A.; Amoroso, N.; Bove, S.; Comes, M.C.; Pomarico, D.; Didonna, V.; Diotaiuti, S.; Galati, L.; Giotta, F.; et al. Analyzing breast cancer invasive disease event classification through explainable artificial intelligence. Front. Med. 2023, 10, 1116354. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Lee, V.H.; Yuan, H.; Lam, K.O.; Pang, H.H.; Chen, Y.; Lam, E.Y.; Khong, P.L.; Lee, A.W.; Kwong, D.L.; et al. Radiomics Model to Predict Early Progression of Nonmetastatic Nasopharyngeal Carcinoma after Intensity Modulation Radiation Therapy: A Multicenter Study. Radiol. Artif. Intell. 2019, 1, e180075. [Google Scholar] [CrossRef] [PubMed]
- Bibault, J.E.; Giraud, P.; Housset, M.; Durdux, C.; Taieb, J.; Berger, A.; Coriat, R.; Chaussade, S.; Dousset, B.; Nordlinger, B.; et al. Deep Learning and Radiomics predict complete response after neo-adjuvant chemoradiation for locally advanced rectal cancer. Sci. Rep. 2018, 8, 12611. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.S.; Hsu, Y. A Meta-Analysis for Using Radiomics to Predict Complete Pathological Response in Esophageal Cancer Patients Receiving Neoadjuvant Chemoradiation. Vivo 2021, 35, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- DiCenzo, D.; Quiaoit, K.; Fatima, K.; Bhardwaj, D.; Sannachi, L.; Gangeh, M.; Sadeghi-Naini, A.; Dasgupta, A.; Kolios, M.C.; Trudeau, M.; et al. Quantitative ultrasound radiomics in predicting response to neoadjuvant chemotherapy in patients with locally advanced breast cancer: Results from multi-institutional study. Cancer Med. 2020, 9, 5798–5806. [Google Scholar] [CrossRef]
- Lin, M.; Momin, S.; Lei, Y.; Wang, H.; Curran, W.J.; Liu, T.; Yang, X. Fully automated segmentation of brain tumor from multiparametric MRI using 3D context deep supervised U-Net. Med. Phys. 2021, 48, 4365–4374. [Google Scholar] [CrossRef]
- Primakov, S.P.; Ibrahim, A.; van Timmeren, J.E.; Wu, G.; Keek, S.A.; Beuque, M.; Granzier, R.W.Y.; Lavrova, E.; Scrivener, M.; Sanduleanu, S.; et al. Automated detection and segmentation of non-small cell lung cancer computed tomography images. Nat. Commun. 2022, 13, 3423. [Google Scholar] [CrossRef]
- Chang, C.Y.; Buckless, C.; Yeh, K.J.; Torriani, M. Automated detection and segmentation of sclerotic spinal lesions on body CTs using a deep convolutional neural network. Skelet. Radiol. 2022, 51, 391–399. [Google Scholar] [CrossRef]
- Goehler, A.; Harry Hsu, T.M.; Lacson, R.; Gujrathi, I.; Hashemi, R.; Chlebus, G.; Szolovits, P.; Khorasani, R. Three-Dimensional Neural Network to Automatically Assess Liver Tumor Burden Change on Consecutive Liver MRIs. J. Am. Coll. Radiol. 2020, 17, 1475–1484. [Google Scholar] [CrossRef]
- Anderson, B.M.; Rigaud, B.; Lin, Y.-M.; Jones, A.K.; Kang, H.C.; Odisio, B.C.; Brock, K.K. Automated segmentation of colorectal liver metastasis and liver ablation on contrast-enhanced CT images. Front. Oncol. 2022, 12, 886517. [Google Scholar] [CrossRef]
- Fathi Kazerooni, A.; Bagley, S.J.; Akbari, H.; Saxena, S.; Bagheri, S.; Guo, J.; Chawla, S.; Nabavizadeh, A.; Mohan, S.; Bakas, S.; et al. Applications of Radiomics and Radiogenomics in High-Grade Gliomas in the Era of Precision Medicine. Cancers 2021, 13, 5921. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Jena, B.; Gupta, N.; Das, S.; Sarmah, D.; Bhattacharya, P.; Nath, T.; Paul, S.; Fouda, M.M.; Kalra, M.; et al. Role of Artificial Intelligence in Radiogenomics for Cancers in the Era of Precision Medicine. Cancers 2022, 14, 2860. [Google Scholar] [CrossRef]
- Ren, M.; Yang, H.; Lai, Q.; Shi, D.; Liu, G.; Shuang, X.; Su, J.; Xie, L.; Dong, Y.; Jiang, X. MRI-based radiomics analysis for predicting the EGFR mutation based on thoracic spinal metastases in lung adenocarcinoma patients. Med. Phys. 2021, 48, 5142–5151. [Google Scholar] [CrossRef] [PubMed]
- Darvish, L.; Bahreyni-Toossi, M.-T.; Roozbeh, N.; Azimian, H. The role of radiogenomics in the diagnosis of breast cancer: A systematic review. Egypt. J. Med. Hum. Genet. 2022, 23, 99. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, B.-J.; Yue, W.-W.; Liu, Y.; Xu, Z.-F.; Xing, W.; Xu, Z.; Yao, J.-C.; Wang, S.-R.; Xu, D. Differentiating non-lactating mastitis and malignant breast tumors by deep-learning based AI automatic classification system: A preliminary study. Front. Oncol. 2022, 12, 997306. [Google Scholar] [CrossRef]
- Kayode, A.A.; Akande, N.O.; Adegun, A.A.; Adebiyi, M.O. An automated mammogram classification system using modified support vector machine. Med. Devices 2019, 12, 275–284. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Senapati, M.R.; Beberta, S.; Lenka, S.K. Texture-based features for classification of mammograms using decision tree. Neural Comput. Appl. 2013, 23, 1011–1017. [Google Scholar] [CrossRef]
- Liberini, V.; Laudicella, R.; Balma, M.; Nicolotti, D.G.; Buschiazzo, A.; Grimaldi, S.; Lorenzon, L.; Bianchi, A.; Peano, S.; Bartolotta, T.V.; et al. Radiomics and artificial intelligence in prostate cancer: New tools for molecular hybrid imaging and theragnostics. Eur. Radiol. Exp. 2022, 6, 27. [Google Scholar] [CrossRef]
- Van Booven, D.J.; Kuchakulla, M.; Pai, R.; Frech, F.S.; Ramasahayam, R.; Reddy, P.; Parmar, M.; Ramasamy, R.; Arora, H. A Systematic Review of Artificial Intelligence in Prostate Cancer. Res. Rep. Urol. 2021, 13, 31–39. [Google Scholar] [CrossRef]
- Wan, Y.L.; Wu, P.W.; Huang, P.C.; Tsay, P.K.; Pan, K.T.; Trang, N.N.; Chuang, W.Y.; Wu, C.Y.; Lo, S.B. The Use of Artificial Intelligence in the Differentiation of Malignant and Benign Lung Nodules on Computed Tomograms Proven by Surgical Pathology. Cancers 2020, 12, 2211. [Google Scholar] [CrossRef]
- Heuvelmans, M.A.; van Ooijen, P.M.A.; Ather, S.; Silva, C.F.; Han, D.; Heussel, C.P.; Hickes, W.; Kauczor, H.-U.; Novotny, P.; Peschl, H.; et al. Lung cancer prediction by Deep Learning to identify benign lung nodules. Lung Cancer 2021, 154, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, B.; Li, H.; You, B. Application of artificial intelligence in the diagnosis of multiple primary lung cancer. Thorac. Cancer 2019, 10, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Xiong, J.; Wang, D.; She, D.; Kuai, X.; Geng, D.; Yin, B. Presurgical differentiation between malignant haemangiopericytoma and angiomatous meningioma by a radiomics approach based on texture analysis. J. Neuroradiol. 2019, 46, 281–287. [Google Scholar] [CrossRef]
- Ullah, N.; Khan, J.A.; Khan, M.S.; Khan, W.; Hassan, I.; Obayya, M.; Negm, N.; Salama, A.S. An Effective Approach to Detect and Identify Brain Tumors Using Transfer Learning. Appl. Sci. 2022, 12, 5645. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, J.; Hu, S.; Dai, Y.; Zhang, Y.; Hu, C. Differentiating between Multiple Myeloma and Metastasis Subtypes of Lumbar Vertebra Lesions Using Machine Learning-Based Radiomics. Front. Oncol. 2021, 11, 601699. [Google Scholar] [CrossRef]
- Eweje, F.R.; Bao, B.; Wu, J.; Dalal, D.; Liao, W.H.; He, Y.; Luo, Y.; Lu, S.; Zhang, P.; Peng, X.; et al. Deep Learning for Classification of Bone Lesions on Routine MRI. EBioMedicine 2021, 68, 103402. [Google Scholar] [CrossRef]
- Zhong, X.; Li, L.; Jiang, H.; Yin, J.; Lu, B.; Han, W.; Li, J.; Zhang, J. Cervical spine osteoradionecrosis or bone metastasis after radiotherapy for nasopharyngeal carcinoma? The MRI-based radiomics for characterization. BMC Med. Imaging 2020, 20, 104. [Google Scholar] [CrossRef]
- Sun, W.; Liu, S.; Guo, J.; Liu, S.; Hao, D.; Hou, F.; Wang, H.; Xu, W. A CT-based radiomics nomogram for distinguishing between benign and malignant bone tumours. Cancer Imaging 2021, 21, 20. [Google Scholar] [CrossRef]
- Reinus, W.R.; Wilson, A.J.; Kalman, B.; Kwasny, S. Diagnosis of focal bone lesions using neural networks. Investig. Radiol. 1994, 29, 606–611. [Google Scholar] [CrossRef]
- Filograna, L.; Lenkowicz, J.; Cellini, F.; Dinapoli, N.; Manfrida, S.; Magarelli, N.; Leone, A.; Colosimo, C.; Valentini, V. Identification of the most significant magnetic resonance imaging (MRI) radiomic features in oncological patients with vertebral bone marrow metastatic disease: A feasibility study. Radiol. Med. 2019, 124, 50–57. [Google Scholar] [CrossRef]
- Yin, P.; Mao, N.; Zhao, C.; Wu, J.; Chen, L.; Hong, N. A Triple-Classification Radiomics Model for the Differentiation of Primary Chordoma, Giant Cell Tumor, and Metastatic Tumor of Sacrum Based on T2-Weighted and Contrast-Enhanced T1-Weighted MRI. J. Magn. Reason. Imaging 2019, 49, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Acar, E.; Leblebici, A.; Ellidokuz, B.E.; Basbinar, Y.; Kaya, G.C. Machine learning for differentiating metastatic and completely responded sclerotic bone lesion in prostate cancer: A retrospective radiomics study. Br. J. Radiol. 2019, 92, 20190286. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Pan, I.; Bao, B.; Halsey, K.; Chang, M.; Liu, H.; Peng, S.; Sebro, R.A.; Guan, J.; Yi, T.; et al. Deep learning-based classification of primary bone tumors on radiographs: A preliminary study. EBioMedicine 2020, 62, 103121. [Google Scholar] [CrossRef] [PubMed]
- Reicher, J.J.; Alto, P.; Do, B.H.; Nguyen, M.; Beaulieu, C.F. Single-input bone tumor diagnosis based on convolutional neural network classification of bone tumor matrix. In Proceedings of the SIIM Annual Meeting 2018, National Harbor, MD, USA, 31 May–2 June 2018. [Google Scholar]
- Li, Y.; Zhou, W.; Lv, G.; Luo, G.; Zhu, Y.; Liu, J. Classification of Bone Tumor on CT Images Using Deep Convolutional Neural Network. In Proceedings of the Artificial Neural Networks and Machine Learning—ICANN 2018, Rhodes, Greece, 4–7 October 2018; Lecture Notes in Computer Science. pp. 127–136. [Google Scholar]
- Park, C.W.; Oh, S.J.; Kim, K.S.; Jang, M.C.; Kim, I.S.; Lee, Y.K.; Chung, M.J.; Cho, B.H.; Seo, S.W. Artificial intelligence-based classification of bone tumors in the proximal femur on plain radiographs: System development and validation. PLoS ONE 2022, 17, e0264140. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Pan, D.; Xu, Y.; Zeng, H.; He, Z.; Lin, J.; Zeng, W.; Wu, Z.; Luo, Z.; Qin, G.; et al. A deep learning-machine learning fusion approach for the classification of benign, malignant, and intermediate bone tumors. Eur. Radiol. 2022, 32, 1371–1383. [Google Scholar] [CrossRef]
- Pan, D.; Liu, R.; Zheng, B.; Yuan, J.; Zeng, H.; He, Z.; Luo, Z.; Qin, G.; Chen, W. Using Machine Learning to Unravel the Value of Radiographic Features for the Classification of Bone Tumors. Biomed. Res. Int. 2021, 2021, 8811056. [Google Scholar] [CrossRef]
- Hong, J.H.; Jung, J.Y.; Jo, A.; Nam, Y.; Pak, S.; Lee, S.Y.; Park, H.; Lee, S.E.; Kim, S. Development and Validation of a Radiomics Model for Differentiating Bone Islands and Osteoblastic Bone Metastases at Abdominal CT. Radiology 2021, 299, 626–632. [Google Scholar] [CrossRef]
- Von Schacky, C.E.; Wilhelm, N.J.; Schafer, V.S.; Leonhardt, Y.; Jung, M.; Jungmann, P.M.; Russe, M.F.; Foreman, S.C.; Gassert, F.G.; Gassert, F.T.; et al. Development and evaluation of machine learning models based on X-ray radiomics for the classification and differentiation of malignant and benign bone tumors. Eur. Radiol. 2022, 32, 6247–6257. [Google Scholar] [CrossRef]
- Perk, T.; Bradshaw, T.; Chen, S.; Im, H.J.; Cho, S.; Perlman, S.; Liu, G.; Jeraj, R. Automated classification of benign and malignant lesions in (18)F-NaF PET/CT images using machine learning. Phys. Med. Biol. 2018, 63, 225019. [Google Scholar] [CrossRef] [PubMed]
- Do, B.H.; Langlotz, C.; Beaulieu, C.F. Bone Tumor Diagnosis Using a Naive Bayesian Model of Demographic and Radiographic Features. J. Digit. Imaging 2017, 30, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.E., Jr.; Laur, J.J.; Carrera, G.F. A Bayesian network for diagnosis of primary bone tumors. J. Digit. Imaging 2001, 14, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Cuocolo, R.; van Langevelde, K.; van de Sande, M.A.J.; Parafioriti, A.; Luzzati, A.; Imbriaco, M.; Sconfienza, L.M.; Bloem, J.L. MRI radiomics-based machine learning classification of atypical cartilaginous tumour and grade II chondrosarcoma of long bones. EBioMedicine 2022, 75, 103757. [Google Scholar] [CrossRef]
- Gitto, S.; Cuocolo, R.; Albano, D.; Chianca, V.; Messina, C.; Gambino, A.; Ugga, L.; Cortese, M.C.; Lazzara, A.; Ricci, D.; et al. MRI radiomics-based machine-learning classification of bone chondrosarcoma. Eur. J. Radiol. 2020, 128, 109043. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Mao, N.; Chen, H.; Sun, C.; Wang, S.; Liu, X.; Hong, N. Machine and Deep Learning Based Radiomics Models for Preoperative Prediction of Benign and Malignant Sacral Tumors. Front. Oncol. 2020, 10, 564725. [Google Scholar] [CrossRef] [PubMed]
- Georgeanu, V.A.; Mamuleanu, M.; Ghiea, S.; Selisteanu, D. Malignant Bone Tumors Diagnosis Using Magnetic Resonance Imaging Based on Deep Learning Algorithms. Medicina 2022, 58, 636. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Kido, S.; Suga, K.; Hirano, Y.; Tachibana, R.; Muramatsu, K.; Chagawa, K.; Tanaka, S. Texture analysis on (18)F-FDG PET/CT images to differentiate malignant and benign bone and soft-tissue lesions. Ann. Nucl. Med. 2014, 28, 926–935. [Google Scholar] [CrossRef]
- Chianca, V.; Cuocolo, R.; Gitto, S.; Albano, D.; Merli, I.; Badalyan, J.; Cortese, M.C.; Messina, C.; Luzzati, A.; Parafioriti, A.; et al. Radiomic Machine Learning Classifiers in Spine Bone Tumors: A Multi-Software, Multi-Scanner Study. Eur. J. Radiol. 2021, 137, 109586. [Google Scholar] [CrossRef]
- Gitto, S.; Bologna, M.; Corino, V.D.A.; Emili, I.; Albano, D.; Messina, C.; Armiraglio, E.; Parafioriti, A.; Luzzati, A.; Mainardi, L.; et al. Diffusion-weighted MRI radiomics of spine bone tumors: Feature stability and machine learning-based classification performance. Radiol. Med. 2022, 127, 518–525. [Google Scholar] [CrossRef]
- Consalvo, S.; Hinterwimmer, F.; Neumann, J.; Steinborn, M.; Salzmann, M.; Seidl, F.; Lenze, U.; Knebel, C.; Rueckert, D.; Burgkart, R.H.H. Two-Phase Deep Learning Algorithm for Detection and Differentiation of Ewing Sarcoma and Acute Osteomyelitis in Paediatric Radiographs. Anticancer Res. 2022, 42, 4371–4380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, M.; Xie, Z.; Yan, X.; Wu, S.; Liao, P.; Lu, H.; Shen, W.; Fu, C.; Cui, H.; et al. Deep Learning Assisted Diagnosis of Musculoskeletal Tumors Based on Contrast-Enhanced Magnetic Resonance Imaging. J. Magn. Reason. Imaging 2022, 56, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.; Perk, T.; Chen, S.; Im, H.-J.; Cho, S.; Perlman, S.; Jeraj, R. Deep learning for classification of benign and malignant bone lesions in [F-18]NaF PET/CT images. J. Nucl. Med. 2018, 59, 327. [Google Scholar]
- Do, N.-T.; Jung, S.-T.; Yang, H.-J.; Kim, S.-H. Multi-Level Seg-Unet Model with Global and Patch-Based X-ray Images for Knee Bone Tumor Detection. Diagnostics 2021, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, S.; Mehralivand, S.; Harmon, S.A.; Lay, N.; Lindenberg, L.; Mena, E.; Pinto, P.A.; Citrin, D.E.; Gulley, J.L.; Wood, B.J.; et al. Deep Learning Based Staging of Bone Lesions from Computed Tomography Scans. IEEE Access 2021, 9, 87531–87542. [Google Scholar] [CrossRef]
- Gitto, S.; Cuocolo, R.; Annovazzi, A.; Anelli, V.; Acquasanta, M.; Cincotta, A.; Albano, D.; Chianca, V.; Ferraresi, V.; Messina, C.; et al. CT radiomics-based machine learning classification of atypical cartilaginous tumours and appendicular chondrosarcomas. EBioMedicine 2021, 68, 103407. [Google Scholar] [CrossRef]
- Schacky, C.E.v.; Wilhelm, N.J.; Schäfer, V.S.; Leonhardt, Y.; Gassert, F.G.; Foreman, S.C.; Gassert, F.T.; Jung, M.; Jungmann, P.M.; Russe, M.F.; et al. Multitask Deep Learning for Segmentation and Classification of Primary Bone Tumors on Radiographs. Radiology 2021, 301, 398–406. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Thrall, J.H.; Li, X.; Li, Q.; Cruz, C.; Do, S.; Dreyer, K.; Brink, J. Artificial Intelligence and Machine Learning in Radiology: Opportunities, Challenges, Pitfalls, and Criteria for Success. J. Am. Coll. Radiol. 2018, 15, 504–508. [Google Scholar] [CrossRef]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef]
- Ong, W.; Zhu, L.; Zhang, W.; Kuah, T.; Lim, D.S.W.; Low, X.Z.; Thian, Y.L.; Teo, E.C.; Tan, J.H.; Kumar, N.; et al. Application of Artificial Intelligence Methods for Imaging of Spinal Metastasis. Cancers 2022, 14, 4025. [Google Scholar] [CrossRef]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine Learning for Medical Imaging. Radiographics 2017, 37, 505–515. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging-“how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Mannil, M.; von Spiczak, J.; Manka, R.; Alkadhi, H. Texture Analysis and Machine Learning for Detecting Myocardial Infarction in Noncontrast Low-Dose Computed Tomography: Unveiling the Invisible. Investig. Radiol. 2018, 53, 338–343. [Google Scholar] [CrossRef]
- Aerts, H.J. The Potential of Radiomic-Based Phenotyping in Precision Medicine: A Review. JAMA Oncol. 2016, 2, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Shur, J.D.; Doran, S.J.; Kumar, S.; ap Dafydd, D.; Downey, K.; O’Connor, J.P.B.; Papanikolaou, N.; Messiou, C.; Koh, D.-M.; Orton, M.R. Radiomics in Oncology: A Practical Guide. RadioGraphics 2021, 41, 1717–1732. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef]
- Ciresan, D.C.; Meier, U.; Masci, J.; Gambardella, L.M.; Schmidhuber, J. Flexible, high performance convolutional neural networks for image classification. In Proceedings of the 22nd International Joint Conference on Artificial Intelligence, Catalonia, Spain, 16–22 July 2011. [Google Scholar]
- Zaharchuk, G.; Gong, E.; Wintermark, M.; Rubin, D.; Langlotz, C.P. Deep Learning in Neuroradiology. AJNR Am. J. Neuroradiol. 2018, 39, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Kaka, H.; Zhang, E.; Khan, N. Artificial intelligence and deep learning in neuroradiology: Exploring the new frontier. Can. Assoc. Radiol. J. 2021, 72, 35–44. [Google Scholar] [CrossRef]
- Ziyad, S.R.; Radha, V.; Vayyapuri, T. Overview of Computer Aided Detection and Computer Aided Diagnosis Systems for Lung Nodule Detection in Computed Tomography. Curr. Med. Imaging Rev. 2020, 16, 16–26. [Google Scholar] [CrossRef]
- Roodman, G.D. Skeletal imaging and management of bone disease. Hematol. Am. Soc. Hematol. Educ. Program 2008, 2008, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.; Hasan, O.H.A.; Khan, D.; Uddin, N.; Noordin, S. Systematic approach to musculoskeletal benign tumors. Int. J. Surg. Oncol. 2017, 2, e46. [Google Scholar] [CrossRef]
- Remotti, F.; Feldman, F. Nonneoplastic lesions that simulate primary tumors of bone. Arch. Pathol. Lab. Med. 2012, 136, 772–788. [Google Scholar] [CrossRef]
- Teo, H.E.; Peh, W.C. Primary bone tumors of adulthood. Cancer Imaging 2004, 4, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.T. Bone tumors and tumorlike conditions: Analysis with conventional radiography. Radiology 2008, 246, 662–674. [Google Scholar] [CrossRef]
- Costelloe, C.M.; Madewell, J.E. Radiography in the initial diagnosis of primary bone tumors. AJR Am. J. Roentgenol. 2013, 200, 3–7. [Google Scholar] [CrossRef]
- Gemescu, I.N.; Thierfelder, K.M.; Rehnitz, C.; Weber, M.-A. Imaging Features of Bone Tumors: Conventional Radiographs and MR Imaging Correlation. Magn. Reson. Imaging Clin. N. Am. 2019, 27, 753–767. [Google Scholar] [CrossRef]
- Hayes, C.W.; Conway, W.F.; Sundaram, M. Misleading aggressive MR imaging appearance of some benign musculoskeletal lesions. Radiographics 1992, 12, 1119–1134; discussion 1135–1136. [Google Scholar] [CrossRef] [PubMed]
- Camargo, O.P.; Croci, A.T.; Oliveira, C.R.; Baptista, A.M.; Caiero, M.T. Functional and radiographic evaluation of 214 aggressive benign bone lesions treated with curettage, cauterization, and cementation: 24 years of follow-up. Clinics 2005, 60, 439–444. [Google Scholar] [CrossRef]
- Fletcher, C.D.; Unni, K.; Mertens, F. World Health Organization classification of tumours. In Pathology and Genetics of Tumours of Soft Tissue and Bone; IARC Press: Lyon, France, 2002. [Google Scholar]
- Weber, M.A.; Papakonstantinou, O.; Nikodinovska, V.V.; Vanhoenacker, F.M. Ewing’s Sarcoma and Primary Osseous Lymphoma: Spectrum of Imaging Appearances. Semin. Musculoskelet Radiol. 2019, 23, 36–57. [Google Scholar] [CrossRef]
- McCarville, M.B.; Chen, J.Y.; Coleman, J.L.; Li, Y.; Li, X.; Adderson, E.E.; Neel, M.D.; Gold, R.E.; Kaufman, R.A. Distinguishing Osteomyelitis From Ewing Sarcoma on Radiography and MRI. AJR Am. J. Roentgenol. 2015, 205, 640–650; quiz 651. [Google Scholar] [CrossRef]
- Kuleta-Bosak, E.; Kluczewska, E.; Machnik-Broncel, J.; Madziara, W.; Ciupinska-Kajor, M.; Sojka, D.; Rogala, W.; Juszczyk, J.; Wilk, R. Suitability of imaging methods (X-ray, CT, MRI) in the diagnostics of Ewing’s sarcoma in children—Analysis of own material. Pol. J. Radiol. 2010, 75, 18–28. [Google Scholar]
- Mar, W.A.; Taljanovic, M.S.; Bagatell, R.; Graham, A.R.; Speer, D.P.; Hunter, T.B.; Rogers, L.F. Update on imaging and treatment of Ewing sarcoma family tumors: What the radiologist needs to know. J. Comput. Assist. Tomogr. 2008, 32, 108–118. [Google Scholar] [CrossRef]
- Vanel, D.; Ruggieri, P.; Ferrari, S.; Picci, P.; Gambarotti, M.; Staals, E.; Alberghini, M. The incidental skeletal lesion: Ignore or explore? Cancer Imaging 2009, 9, S38–S43. [Google Scholar] [CrossRef] [PubMed]
- Caballes, R.L.; Caballes, R.A., Jr. Polyostotic giant enostoses with strongly positive radionuclide bone scan. Ann. Diagn. Pathol. 2004, 8, 247–251. [Google Scholar] [CrossRef]
- Ulano, A.; Bredella, M.A.; Burke, P.; Chebib, I.; Simeone, F.J.; Huang, A.J.; Torriani, M.; Chang, C.Y. Distinguishing Untreated Osteoblastic Metastases From Enostoses Using CT Attenuation Measurements. Am. J. Roentgenol. 2016, 207, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.; Dapoto, A.; Morzenti, C.; Firetto, M.C.; Valle, C.; Tomasoni, A.; Sironi, S. Bone islands incidentally detected on computed tomography: Frequency of enostosis and differentiation from untreated osteoblastic metastases based on CT attenuation value. Br. J. Radiol. 2019, 92, 20190249. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zheng, S.; Machida, H.; Wang, B.; Liu, A.; Liu, Y.; Zhang, X. Differential diagnosis of osteoblastic metastases from bone islands in patients with lung cancer by single-source dual-energy CT: Advantages of spectral CT imaging. Eur. J. Radiol. 2015, 84, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Schajowicz, F.; Sissons, H.A.; Sobin, L.H. The World Health Organization’s histologic classification of bone tumors. A commentary on the second edition. Cancer 1995, 75, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol. Rev. 2021, 101, 797–855. [Google Scholar] [CrossRef]
- Rajiah, P.; Ilaslan, H.; Sundaram, M. Imaging of primary malignant bone tumors (nonhematological). Radiol. Clin. N. Am. 2011, 49, 1135–1161. [Google Scholar] [CrossRef] [PubMed]
- Strobel, K.; Exner, U.E.; Stumpe, K.D.; Hany, T.F.; Bode, B.; Mende, K.; Veit-Haibach, P.; von Schulthess, G.K.; Hodler, J. The additional value of CT images interpretation in the differential diagnosis of benign vs. malignant primary bone lesions with 18F-FDG-PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, L.; Albano, D.; Messina, C.; Corazza, A.; Rapisarda, S.; Pozzi, G.; Di Bernardo, A.; Parafioriti, A.; Scotto, G.; Perrucchini, G.; et al. Accuracy of CT and MRI to assess resection margins in primary malignant bone tumours having histology as the reference standard. Clin. Radiol. 2019, 74, e713–e736. [Google Scholar] [CrossRef]
- James, S.L.; Panicek, D.M.; Davies, A.M. Bone marrow oedema associated with benign and malignant bone tumours. Eur. J. Radiol. 2008, 67, 11–21. [Google Scholar] [CrossRef]
- Oh, E.; Yoon, Y.C.; Kim, J.H.; Kim, K. Multiparametric approach with diffusion-weighted imaging and dynamic contrast-enhanced MRI: A comparison study for differentiating between benign and malignant bone lesions in adults. Clin. Radiol. 2017, 72, 552–559. [Google Scholar] [CrossRef]
- Hillengass, J.; Stieltjes, B.; Bäuerle, T.; McClanahan, F.; Heiss, C.; Hielscher, T.; Wagner-Gund, B.; Habetler, V.; Goldschmidt, H.; Schlemmer, H.P.; et al. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and diffusion-weighted imaging of bone marrow in healthy individuals. Acta Radiol. 2011, 52, 324–330. [Google Scholar] [CrossRef]
- Suh, C.H.; Yun, S.J.; Jin, W.; Lee, S.H.; Park, S.Y.; Ryu, C.-W. ADC as a useful diagnostic tool for differentiating benign and malignant vertebral bone marrow lesions and compression fractures: A systematic review and meta-analysis. Eur. Radiol. 2018, 28, 2890–2902. [Google Scholar] [CrossRef]
- Pozzi, G.; Albano, D.; Messina, C.; Angileri, S.A.; Al-Mnayyis, A.a.; Galbusera, F.; Luzzati, A.; Perrucchini, G.; Scotto, G.; Parafioriti, A.; et al. Solid bone tumors of the spine: Diagnostic performance of apparent diffusion coefficient measured using diffusion-weighted MRI using histology as a reference standard. J. Magn. Reson. Imaging 2018, 47, 1034–1042. [Google Scholar] [CrossRef]
- Sharma, G.; Saran, S.; Saxena, S.; Goyal, T. Multiparametric evaluation of bone tumors utilising diffusion weighted imaging and dynamic contrast enhanced magnetic resonance imaging. J. Clin. Orthop. Trauma. 2022, 30, 101899. [Google Scholar] [CrossRef] [PubMed]
- Kayhan, A.; Yang, C.; Soylu, F.N.; Lakadamyalı, H.; Sethi, I.; Karczmar, G.; Stadler, W.; Oto, A. Dynamic contrast-enhanced MR imaging findings of bone metastasis in patients with prostate cancer. World J. Radiol. 2011, 3, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-S.; Jee, W.-H.; McCauley, T.R.; Ha, K.-Y.; Choi, K.-H. Discrimination of Metastatic from Acute Osteoporotic Compression Spinal Fractures with MR Imaging1. RadioGraphics 2003, 23, 179–187. [Google Scholar] [CrossRef]
- Board, W. Classification of Tumours Editorial. Soft Tissue and Bone Tumours; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Van Praag Veroniek, V.M.; Rueten-Budde, A.J.; Ho, V.; Dijkstra, P.D.S.; Fiocco, M.; van de Sande, M.A.J. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg. Oncol. 2018, 27, 402–408. [Google Scholar] [CrossRef]
- Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of Histopathologic and Radiologic Grading of Cartilaginous Neoplasms in Long Bones. J. Bone Jt. Surg. 2007, 89, 2113–2123. [Google Scholar] [CrossRef]
- Eefting, D.; Schrage, Y.M.; Geirnaerdt, M.J.; Le Cessie, S.; Taminiau, A.H.; Bovée, J.V.; Hogendoorn, P.C. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am. J. Surg. Pathol. 2009, 33, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, K.L.; Lang, P. Bone and soft tissue tumors: The role of contrast agents for MR imaging. Eur. J. Radiol. 2000, 34, 229–246. [Google Scholar] [CrossRef]
- Kransdorf, M.J. The use of gadolinium in the MR evaluation of musculoskeletal tumors. Top. Magn. Reason. Imaging 1996, 8, 15–23. [Google Scholar] [CrossRef]
- May, D.A.; Good, R.B.; Smith, D.K.; Parsons, T.W. MR imaging of musculoskeletal tumors and tumor mimickers with intravenous gadolinium: Experience with 242 patients. Skelet. Radiol. 1997, 26, 2–15. [Google Scholar] [CrossRef]
- Sundaram, M. The use of gadolinium in the MR imaging of bone tumors. Semin Ultrasound CT MR 1997, 18, 307–311. [Google Scholar] [CrossRef]
- Stacy, G.S.; Mahal, R.S.; Peabody, T.D. Staging of Bone Tumors: A Review with Illustrative Examples. Am. J. Roentgenol. 2006, 186, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Mithal, L.B.; Patel, P.S.; Mithal, D.; Palac, H.L.; Rozenfeld, M.N. Use of gadolinium-based magnetic resonance imaging contrast agents and awareness of brain gadolinium deposition among pediatric providers in North America. Pediatr. Radiol. 2017, 47, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Hahn, G.; Hirsch, W.; Kim, M.J.; Mentzel, H.J.; Olsen, O.E.; Stokland, E.; Triulzi, F.; Vazquez, E. Contrast-enhanced magnetic resonance imaging in pediatric patients: Review and recommendations for current practice. Magn. Reason. Insights 2013, 6, 95–111. [Google Scholar] [CrossRef]
- Strauss, L.G.; Conti, P.S. The applications of PET in clinical oncology. J. Nucl. Med. 1991, 32, 623–648; discussion 649–650. [Google Scholar] [PubMed]
- Hoh, C.K.; Schiepers, C.; Seltzer, M.A.; Gambhir, S.S.; Silverman, D.H.; Czernin, J.; Maddahi, J.; Phelps, M.E. PET in oncology: Will it replace the other modalities? Semin. Nucl. Med. 1997, 27, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Brock, C.S.; Meikle, S.R.; Price, P. Does fluorine-18 fluorodeoxyglucose metabolic imaging of tumours benefit oncology? Eur. J. Nucl. Med. 1997, 24, 691–705. [Google Scholar] [CrossRef]
- Alauddin, M.M. Positron emission tomography (PET) imaging with (18)F-based radiotracers. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 55–76. [Google Scholar] [PubMed]
- Weber, D.A.; Greenberg, E.J.; Dimich, A.; Kenny, P.J.; Rothschild, E.O.; Myers, W.P.; Laughlin, J.S. Kinetics of radionuclides used for bone studies. J. Nucl. Med. 1969, 10, 8–17. [Google Scholar]
- Blake, G.M.; Park-Holohan, S.J.; Cook, G.J.; Fogelman, I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin. Nucl. Med. 2001, 31, 28–49. [Google Scholar] [CrossRef]
- Schlonsky, J. Radioisotope scanning of bone. A review of the literature. Ohio. State Med. J. 1972, 68, 128–133. [Google Scholar]
- Bastawrous, S.; Bhargava, P.; Behnia, F.; Djang, D.S.W.; Haseley, D.R. Newer PET Application with an Old Tracer: Role of 18F-NaF Skeletal PET/CT in Oncologic Practice. RadioGraphics 2014, 34, 1295–1316. [Google Scholar] [CrossRef]
- Kern, K.A.; Brunetti, A.; Norton, J.A.; Chang, A.E.; Malawer, M.; Lack, E.; Finn, R.D.; Rosenberg, S.A.; Larson, S.M. Metabolic imaging of human extremity musculoskeletal tumors by PET. J. Nucl. Med. 1988, 29, 181–186. [Google Scholar] [PubMed]
- Adler, L.P.; Blair, H.F.; Makley, J.T.; Williams, R.P.; Joyce, M.J.; Leisure, G.; al-Kaisi, N.; Miraldi, F. Noninvasive grading of musculoskeletal tumors using PET. J. Nucl. Med. 1991, 32, 1508–1512. [Google Scholar] [PubMed]
- Griffeth, L.K.; Dehdashti, F.; McGuire, A.H.; McGuire, D.J.; Perry, D.J.; Moerlein, S.M.; Siegel, B.A. PET evaluation of soft-tissue masses with fluorine-18 fluoro-2-deoxy-D-glucose. Radiology 1992, 182, 185–194. [Google Scholar] [CrossRef]
- Dehdashti, F.; Siegel, B.A.; Griffeth, L.K.; Fusselman, M.J.; Trask, D.D.; McGuire, A.H.; McGuire, D.J. Benign vs. malignant intraosseous lesions: Discrimination by means of PET with 2-[F-18]fluoro-2-deoxy-D-glucose. Radiology 1996, 200, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Palmer, W.E.; Rosenthal, D.I.; Schoenberg, O.I.; Fischman, A.J.; Simon, L.S.; Rubin, R.H.; Polisson, R.P. Quantification of inflammation in the wrist with gadolinium-enhanced MR imaging and PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology 1995, 196, 647–655. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Yu, H.; Shi, H. The value of skeletal standardized uptake values obtained by quantitative SPECT/CT in differential diagnosis of bone metastases. J. Nucl. Med. 2020, 61, 1527. [Google Scholar]
- Even-Sapir, E.; Metser, U.; Mishani, E.; Lievshitz, G.; Lerman, H.; Leibovitch, I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J. Nucl. Med. 2006, 47, 287–297. [Google Scholar]
- Aoki, J.; Endo, K.; Watanabe, H.; Shinozaki, T.; Yanagawa, T.; Ahmed, A.R.; Takagishi, K. FDG-PET for evaluating musculoskeletal tumors: A review. J. Orthop. Sci. 2003, 8, 435–441. [Google Scholar] [CrossRef]
- Schwarzbach, M.H.; Dimitrakopoulou-Strauss, A.; Willeke, F.; Hinz, U.; Strauss, L.G.; Zhang, Y.M.; Mechtersheimer, G.; Attigah, N.; Lehnert, T.; Herfarth, C. Clinical value of [18-F]] fluorodeoxyglucose positron emission tomography imaging in soft tissue sarcomas. Ann. Surg. 2000, 231, 380–386. [Google Scholar] [CrossRef]
- Shin, D.-S.; Shon, O.-J.; Han, D.-S.; Choi, J.-H.; Chun, K.-A.; Cho, I.-H. The clinical efficacy of (18)F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann. Nucl. Med. 2008, 22, 603–609. [Google Scholar] [CrossRef]
- Li, Y.; Schiepers, C.; Lake, R.; Dadparvar, S.; Berenji, G.R. Clinical utility of (18)F-fluoride PET/CT in benign and malignant bone diseases. Bone 2012, 50, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.J.; Lind, T.; Antoch, G.; Bockisch, A. False-positive FDG PET uptake--the role of PET/CT. Eur. Radiol. 2006, 16, 1054–1065. [Google Scholar] [CrossRef]
- Long, N.M.; Smith, C.S. Causes and imaging features of false positives and false negatives on F-PET/CT in oncologic imaging. Insights Imaging 2011, 2, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, X.; Zhang, Z.; Jiang, Y. Deep Learning-Based Identification of Spinal Metastasis in Lung Cancer Using Spectral CT Images. Sci. Program. 2021, 2021, 2779390. [Google Scholar] [CrossRef]
- Orlhac, F.; Thézé, B.; Soussan, M.; Boisgard, R.; Buvat, I. Multiscale Texture Analysis: From 18F-FDG PET Images to Histologic Images. J. Nucl. Med. 2016, 57, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Orlhac, F.; Soussan, M.; Maisonobe, J.-A.; Garcia, C.A.; Vanderlinden, B.; Buvat, I. Tumor Texture Analysis in 18F-FDG PET: Relationships between Texture Parameters, Histogram Indices, Standardized Uptake Values, Metabolic Volumes, and Total Lesion Glycolysis. J. Nucl. Med. 2014, 55, 414. [Google Scholar] [CrossRef]
- Stacy, G.S.; Dixon, L.B. Pitfalls in MR image interpretation prompting referrals to an orthopedic oncology clinic. Radiographics 2007, 27, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Trieu, J.; Sinnathamby, M.; Di Bella, C.; Pianta, M.; Perera, W.; Slavin, J.L.; Schlicht, S.M.; Choong, P.F. Biopsy and the diagnostic evaluation of musculoskeletal tumours: Critical but often missed in the 21st century. ANZ J. Surg. 2016, 86, 133–138. [Google Scholar] [CrossRef]
- Meek, R.D.; Mills, M.K.; Hanrahan, C.J.; Beckett, B.R.; Leake, R.L.; Allen, H.; Williams, D.D.; Tommack, M.; Schmahmann, S.; Hansford, B.G. Pearls and Pitfalls for Soft-Tissue and Bone Biopsies: A Cross-Institutional Review. RadioGraphics 2020, 40, 266–290. [Google Scholar] [CrossRef]
- Traina, F.; Errani, C.; Toscano, A.; Pungetti, C.; Fabbri, D.; Mazzotti, A.; Donati, D.; Faldini, C. Current concepts in the biopsy of musculoskeletal tumors: AAOS exhibit selection. J. Bone Jt. Surg. Am. 2015, 97, e7. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.E.; Ng, D.C. Differentiating osteoradionecrosis from nasopharyngeal carcinoma tumour recurrence using 99Tcm-sestamibi SPECT/CT. Br. J. Radiol. 2011, 84, e172–e175. [Google Scholar] [CrossRef] [PubMed]
- Alhilali, L.; Reynolds, A.R.; Fakhran, S. Osteoradionecrosis after radiation therapy for head and neck cancer: Differentiation from recurrent disease with CT and PET/CT imaging. AJNR Am. J. Neuroradiol. 2014, 35, 1405–1411. [Google Scholar] [CrossRef]
- Lipkova, J.; Chen, R.J.; Chen, B.; Lu, M.Y.; Barbieri, M.; Shao, D.; Vaidya, A.J.; Chen, C.; Zhuang, L.; Williamson, D.F.K.; et al. Artificial intelligence for multimodal data integration in oncology. Cancer Cell 2022, 40, 1095–1110. [Google Scholar] [CrossRef]
- Antropova, N.; Huynh, B.Q.; Giger, M.L. A deep feature fusion methodology for breast cancer diagnosis demonstrated on three imaging modality datasets. Med. Phys. 2017, 44, 5162–5171. [Google Scholar] [CrossRef]
- Sedghi, A.; Mehrtash, A.; Jamzad, A.; Amalou, A.; Wells, W.M.; Kapur, T.; Kwak, J.T.; Turkbey, B.; Choyke, P.; Pinto, P.; et al. Improving detection of prostate cancer foci via information fusion of MRI and temporal enhanced ultrasound. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1215–1223. [Google Scholar] [CrossRef] [PubMed]


| Authors | Artificial Intelligence Method | Publication Year | Main Objectives | Title of Journal | Main Imaging Modality Used | Performance |
|---|---|---|---|---|---|---|
| Xiong et al. [71] | Machine learning–based classifiers (ANN) | 2021 | Differentiate Multiple Myeloma from Metastases | Frontiers in Oncology | MRI | MCC = 0.605; Accuracy: 0.815; Sensitivity 0.879; Specificity: 0.790 |
| Eweje et al. [72] | EfficientNet-B0 architecture and a logistic regression model (CNN) | 2020 | Differentiate between benign and malignant bone lesion | EBioMedicine | MRI | Accuracy: 0.760; Sensitivity: 0.790; Specificity: 0.750; AUC: 0.820 |
| Zhong et al. [73] | Radiomics using MRI machine learning techniques (SVM) | 2020 | Differentiate between osteoradionecrosis from spinal metastases in Nasopharyngeal Carcinoma | BMC Medical Imaging | MRI | Accuracy: 0.737; Sensitivity: 0.843; Specificity 0.614; AUC of 0.725 |
| Sun et al. [74] | Radiomics using CT machine learning techniques (SVM) | 2021 | Distinguish between benign and malignant bone tumours | Cancer Imaging | MRI | AUC of 0.892; NRI of 0.238; IDI of 0.163 |
| Reinus et al. [75] | Two layer feed-forward neural network (ANN) | 1994 | Diagnosis of focal bone lesion | Investigative Radiology | X-ray | Accuracy 0.835 |
| Filograna et al. [76] | Radiomics using MRI machine learning techniques (SVM) | 2019 | Differentiate between metastatic vs. non-metastatic vertebral bodies | La Radiologia Medica | MRI | AUC: 0.841–0.912 |
| Yin P. et al. [77] | Radiomics using MRI machine learning techniques | 2019 | Differentiation of lesions in the sacrum, for example, chordoma, giant cell tumour, or metastatic lesions) | Journal of Magnetic Resonance Imaging | MRI | Accuracy: 0.810; AUC: 0.840 |
| Acar E. et al. [78] | Texture analysis and PET CT machine learning techniques (Weighted KNN algorithm) | 2019 | Differentiating metastatic and completely responded sclerotic bone lesions in prostate cancer | British Journal of Radiology | PET/CT | Accuracy: 0.735; Sensitivity: 0.735; Specificity: 0.737; AUC: 0.76 |
| He et al. [79] | EfficientNet-B0 convolutional neural network architecture | 2020 | Differentiating benign between benign and malignant bone lesion | EbioMedicine | X-ray | Accuracy: 0.734 AUC: 0.877 (benign); 0.916 (malignant) Accuracy: 0.99 Average mean IoU: 0.848 |
| Reicher et al. [80] | TensorFlow Inception-v3 recurrent convolutional neural network (CNN) trained to the ImageNet model | 2018 | Classifying bone tumour matrix | SIIM (Society for Imaging Informatics in Medicine) | X-ray | Accuracy 0.93 |
| Y. Li et al. [81] | Super label guided convolutional neural network | 2018 | Distinguish between benign and malignant bone tumours and classifies bone tumour matrix | Artificial Neural Networks and Machine Learning | CT | Accuracy: 0.740 |
| Park et al. [82] | ResNet 50, GoogleNet Inception v3, and EfficientNet-b1, b2, and b3 | 2022 | Distinguish between benign, malignant or no tumour over the femur | PLoS ONE | X-ray | Accuracy: 0.853; Sensitivity: 0.822; Specificity: 0.912; Precision: 0.821; AUC: 0.953 |
| Liu et al. [83] | PyTorch 1.2.0 with Python 3.7.3, XGBoost and SHapley ad- ditive exPlanations value | 2021 | Classification of benign, intermediate and malignant bone lesions | European Radiology | X-ray | AUC: 0.898 (benign); 0.894 (malignant); 0.865 (intermediate) Macroaverage AUC: 0.872 |
| Pan D. et al. [84] | Radiomics using X-ray machine learning techniques, SHapley Additive exPlanations (SHAP) | 2021 | Classification of benign, intermediate and malignant bone lesions | Biomed Research International | X-ray | AUC: 0.970 (binary), 0.940 (tertiary); Accuracy: 0.947 (binary); 0.828 (tertiary) |
| Hong J. H. et al. [85] | Radiomics using CT machine learning techniques | 2021 | Distinguish between benign bone island and osteoblastic metastasis | Radiology | CT | AUC: 0.960; Sensitivity: 0.800; Specificity: 0.960; Accuracy: 0.860 |
| von Schacky et al. [86] | Radiomics using X-ray machine learning techniques, Python 3.7.7, scikit- learn 0.22.2 andfastai library | 2022 | Distinguish between benign and malignant bone tumours | European Radiology | X-ray | Accuracy: 0.80 (internal), 0.75 (external); Sensitivity: 0.75, 0.90; AUC: 0.79, 0.90 |
| T. Perk et al. [87] | Radiomics using PET/CT machine learning techniques | 2018 | Distinguish between benign and malignant bone tumours | Physics in Medicine and Biology | PET/CT | AUC: 0.950; Sensitivity: 0.880; Specificity: 0.890 |
| Bao H. D. et al. [88] | Radiomics using X-ray machine learning techniques | 2017 | Classification of different bone tumours | Journal of Digital Imaging | X-ray | Primary accuracy: 0.440–0.620; Differential accuracy: 0.620–0.800 |
| Kahn et al. [89] | Bayesian network using X-ray machine learning techniques | 2001 | Classification of different bone tumours | Journal of Digital Imaging | X-ray | Accuracy: 0.890 (binary), 0.680 (tertiary) |
| Gitto et al. [90] | Radiomics using MRI machine learning techniques | 2022 | Distinguish between benign vs. malignant cartilaginous lesions | EBioMedicine | MRI | Accuracy: 0.98 (ACT), 0.80 (CS2); AUC: 0.94 (ACT), 0.90 (CS2) |
| Gitto et al. [91] | Radiomics using MRI machine learning techniques | 2020 | Distinguish between low-grade vs. high-grade cartilaginous lesions | European Journal of Radiology | MRI | Accuracy: 0.857 (training), 0.750 (test); AUC: 0.850 (training), 0.78 (test) |
| Yin et al. [92] | Radiomics using CT machine learning techniques, Pyradiomics python package, | 2020 | Distinguish between benign vs. malignant sacral tumour | Frontier Oncology | CT | Accuracy: 0.81 (Clinical-LR), 0.81 (Clinical-DNN); AUC: 0.84 (Clinical-LR), 0.83 (Clinical-DNN) |
| Georgeanu et al. [93] | Radiomics using MRI machine learning techniques, ResNet50 image classifiers | 2022 | Distinguish between benign and malignant bone tumours | Medicina (Kaunas) | MRI | Accuracy: 0.808 (training), 0.805 (test); AUC: 0.885 (training), 0.879 (test) |
| Fan et al. [34] | Radiomics using PET/CT machine learning techniques | 2021 | Distinguish between benign and metastatic vertebral lesions | Frontiers in Medicine | PET/CT | Accuracy: 0.875 (LR), 0.834 (SVM), 0.750 (Decision Tree) |
| Xu et al. [94] | Radiomics using PET/CT machine learning techniques | 2014 | Distinguish between malignant and benign bone and soft-tissue lesions | Annals of Nuclear Medicine | PET/CT | Accuracy 0.825; Sensitivity 0.864; Specificity 0.772 |
| Chianca et al. [95] | Radiomics using MRI machine learning techniques, 3D Slicer heterogeneityCAD module (hCAD) and PyRadiomics | 2021 | Distinguish between different benign, primary malignant vs. metastatic vertebral lesions | European Journal of Radiology | MRI | 2-Label Classification—Accuracy: 0.94 (training), 0.86 (test); 3-Label Classification—Accuracy: 0.80 (training), 0.69 (test). |
| Gitto et al. [96] | Radiomics using MRI machine learning techniques | 2022 | Distinguish between benign and metastatic vertebral lesions | La Radiologica Medica | MRI | Accuracy—0.76; Sensitivity: 0.78; Specificity: 0.68; AUC 0.78 |
| Consalvo et al. [97] | PyTorch 1.9.0 and cuda toolkit 11.1 & ResNet18 architecture | 2022 | Distinguish between Ewing Sarcoma (ES) vs. acute osteomyelitis (OM) | Anticancer Research | X-ray | Accuracy: 0.867 (ES), 0.903 (OM); Sensitivity: 1.00 (ES), 0.930 (OM); Specificity: 0.76 (ES), 0.844 |
| Zhao et al. [98] | Radiomics using MRI machine learning techniques | 2022 | Distinguish between benign vs. malignant bone lesions | Journal of Magnetic Resonance Imaging | MRI | Improved Sensitivities 0.12 to 0.36 as compared to manual. |
| Bradshaw et al. [99] | Deep convolutional neural network via VGG19 architecture | 2018 | Classifying benign and malignant bone lesion | Journal of Nuclear Medicine | PET/CT | Accuracy: 0.88; Sensitivity: 0.90; Specificity 0.85. |
| Do et al. [100] | Deep convolutional neural network with combination of global and patch-based models | 2021 | Classifying bone tumours in the knee into benign vs. malignant | Diagnostics | X-ray | Accuracy: 0.99 Average mean IoU: 0.848 |
| Masoudi et al. [101] | Deep convolutional neural network with 2D ResNet- 50 & 3D ResNet-18 | 2021 | Classify benign or malignant bone lesions in prostate cancer | IEEE Access | CT | Accuracy: 0.922; F1: 92.3% |
| Gitto et al. [102] | Machine-learning classifier (LogitBoost) | 2021 | Classification of atypical cartilaginous tumours and higher-grade chondrosarcoma, of long bones. | EBioMedicine | CT | Accuracy 0.750, AUC 0.78 (Validation set) |
| von Schacky et al. [103] | Mask region–based convolutional neural network (Mask-RCNN-X101) | 2021 | Classify benign or malignant bone lesions | Radiology | X-ray | Accuracy: 80.2%, Sensitivity: 62.9%, Specificity: 88.2% |
| Imaging Modality | Accuracy | Sensitivity | Specificity | Area under Curve (AUC) |
|---|---|---|---|---|
| X-ray | 0.44–0.99 | 0.75–1.00 | 0.78–0.91 | 0.79–0.95 |
| Computed Tomography (CT) | 0.74–0.92 | 0.80 | 0.96 | 0.78–0.96 |
| Magnetic Resonance Imaging (MRI) | 0.74–0.98 | 0.78–0.88 | 0.61–0.79 | 0.73–0.94 |
| Positron Emission Tomography with CT (PET/CT) | 0.74–0.88 | 0.84–0.90 | 0.74–0.85 | 0.76–0.95 |
| Overall | 0.44–0.99 | 0.63–1.00 | 0.73–0.96 | 0.73–0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, W.; Zhu, L.; Tan, Y.L.; Teo, E.C.; Tan, J.H.; Kumar, N.; Vellayappan, B.A.; Ooi, B.C.; Quek, S.T.; Makmur, A.; et al. Application of Machine Learning for Differentiating Bone Malignancy on Imaging: A Systematic Review. Cancers 2023, 15, 1837. https://doi.org/10.3390/cancers15061837
Ong W, Zhu L, Tan YL, Teo EC, Tan JH, Kumar N, Vellayappan BA, Ooi BC, Quek ST, Makmur A, et al. Application of Machine Learning for Differentiating Bone Malignancy on Imaging: A Systematic Review. Cancers. 2023; 15(6):1837. https://doi.org/10.3390/cancers15061837
Chicago/Turabian StyleOng, Wilson, Lei Zhu, Yi Liang Tan, Ee Chin Teo, Jiong Hao Tan, Naresh Kumar, Balamurugan A. Vellayappan, Beng Chin Ooi, Swee Tian Quek, Andrew Makmur, and et al. 2023. "Application of Machine Learning for Differentiating Bone Malignancy on Imaging: A Systematic Review" Cancers 15, no. 6: 1837. https://doi.org/10.3390/cancers15061837
APA StyleOng, W., Zhu, L., Tan, Y. L., Teo, E. C., Tan, J. H., Kumar, N., Vellayappan, B. A., Ooi, B. C., Quek, S. T., Makmur, A., & Hallinan, J. T. P. D. (2023). Application of Machine Learning for Differentiating Bone Malignancy on Imaging: A Systematic Review. Cancers, 15(6), 1837. https://doi.org/10.3390/cancers15061837






