Development of a 2-(2-Hydroxyphenyl)-1H-benzimidazole-Based Fluorescence Sensor Targeting Boronic Acids for Versatile Application in Boron Neutron Capture Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
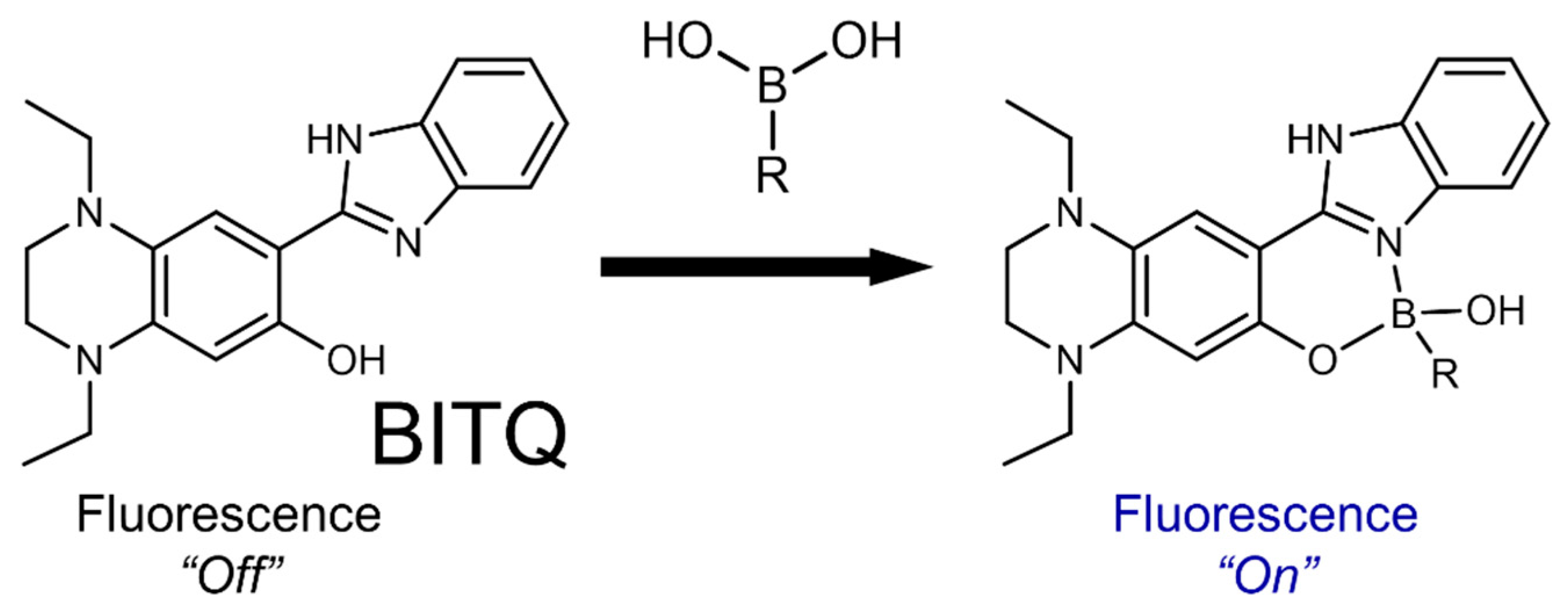
2.1. Preparation of BITQ
2.2. Fluorescence Properties
2.3. Reactivity for Boron-Containing Compounds
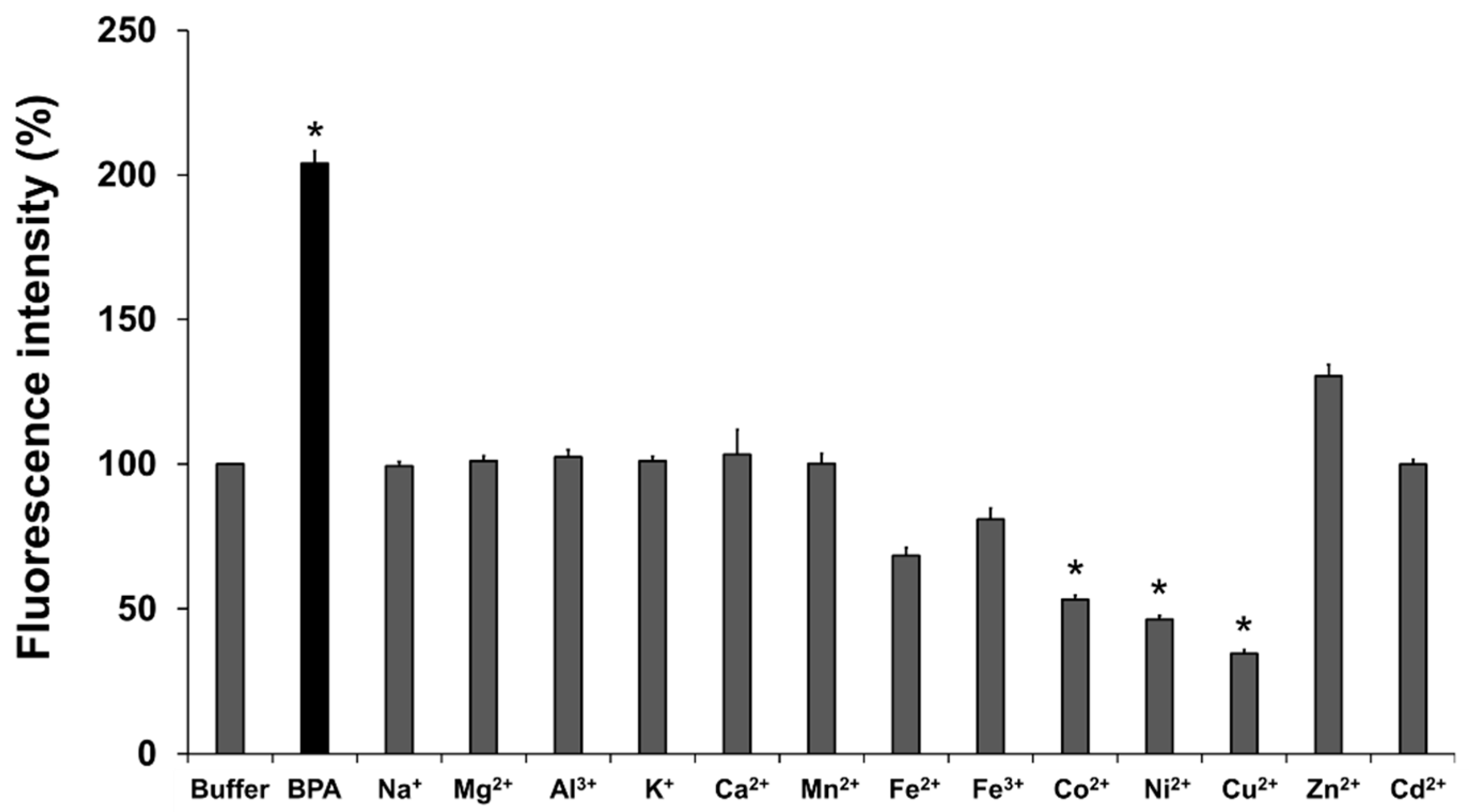
2.4. Selectivity Assay
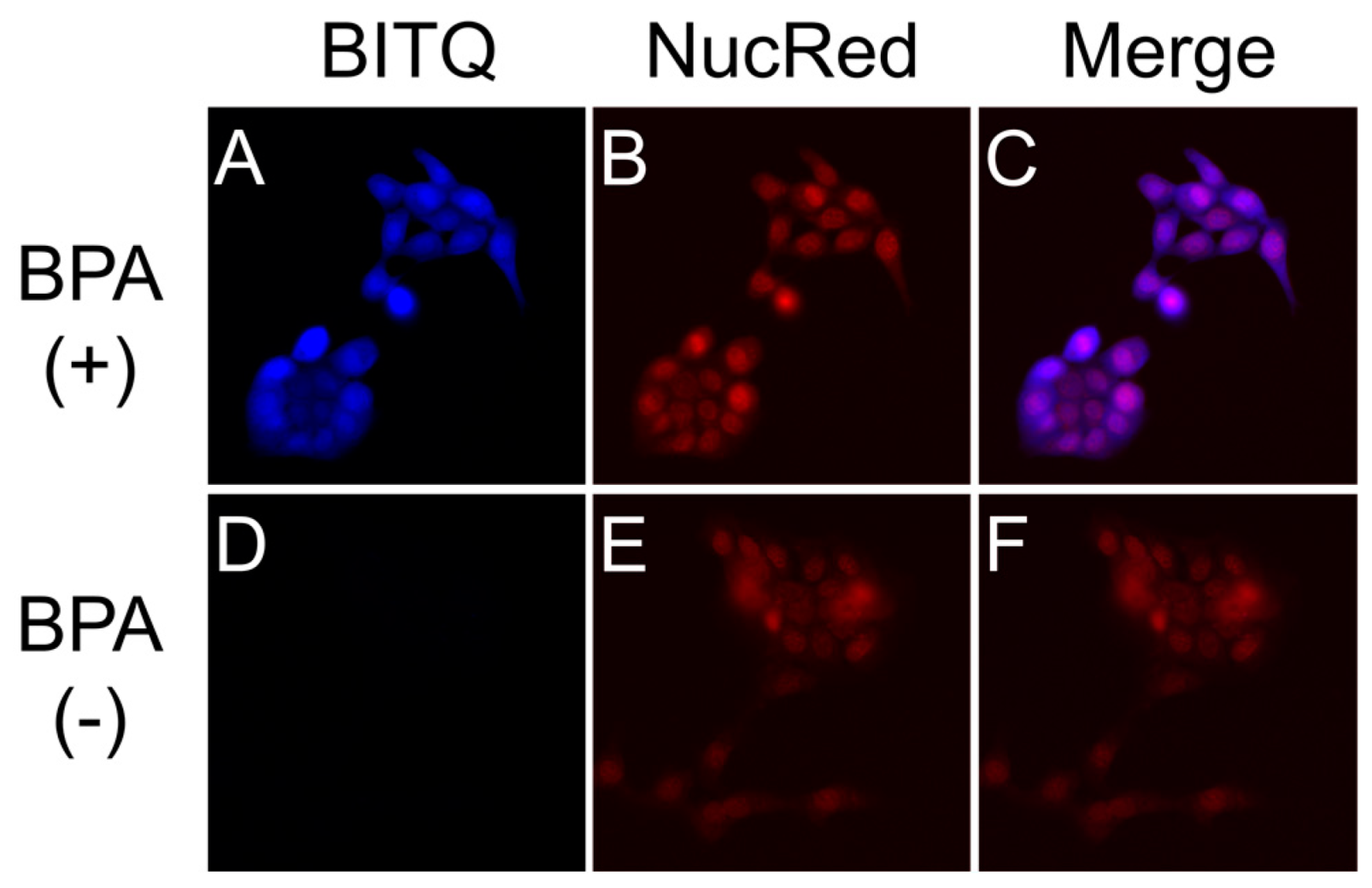
2.5. Fluorescence Microscopy
2.6. Quantification of BPA Concentration in Mice Plasma
2.7. Statistics
3. Results
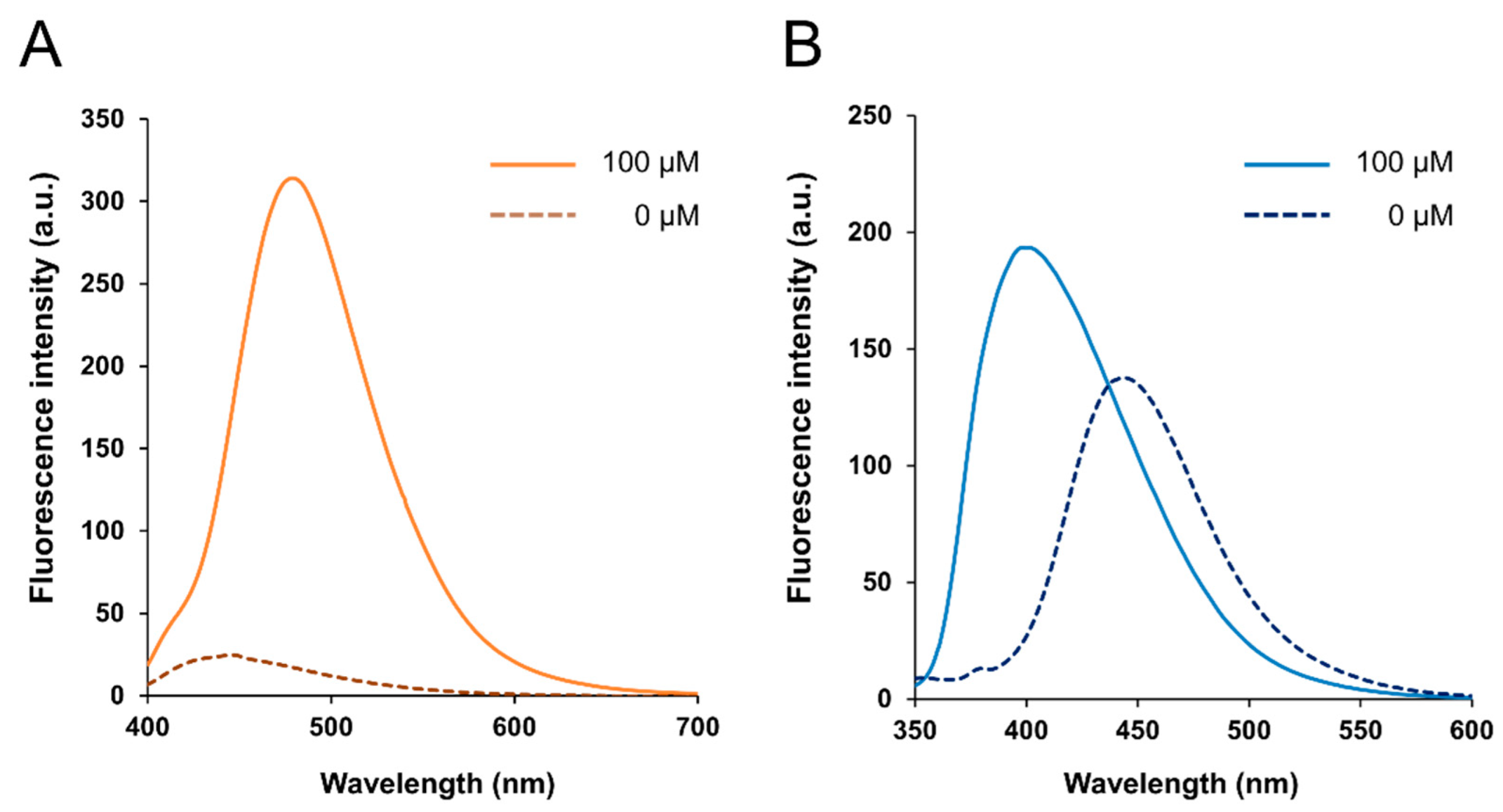
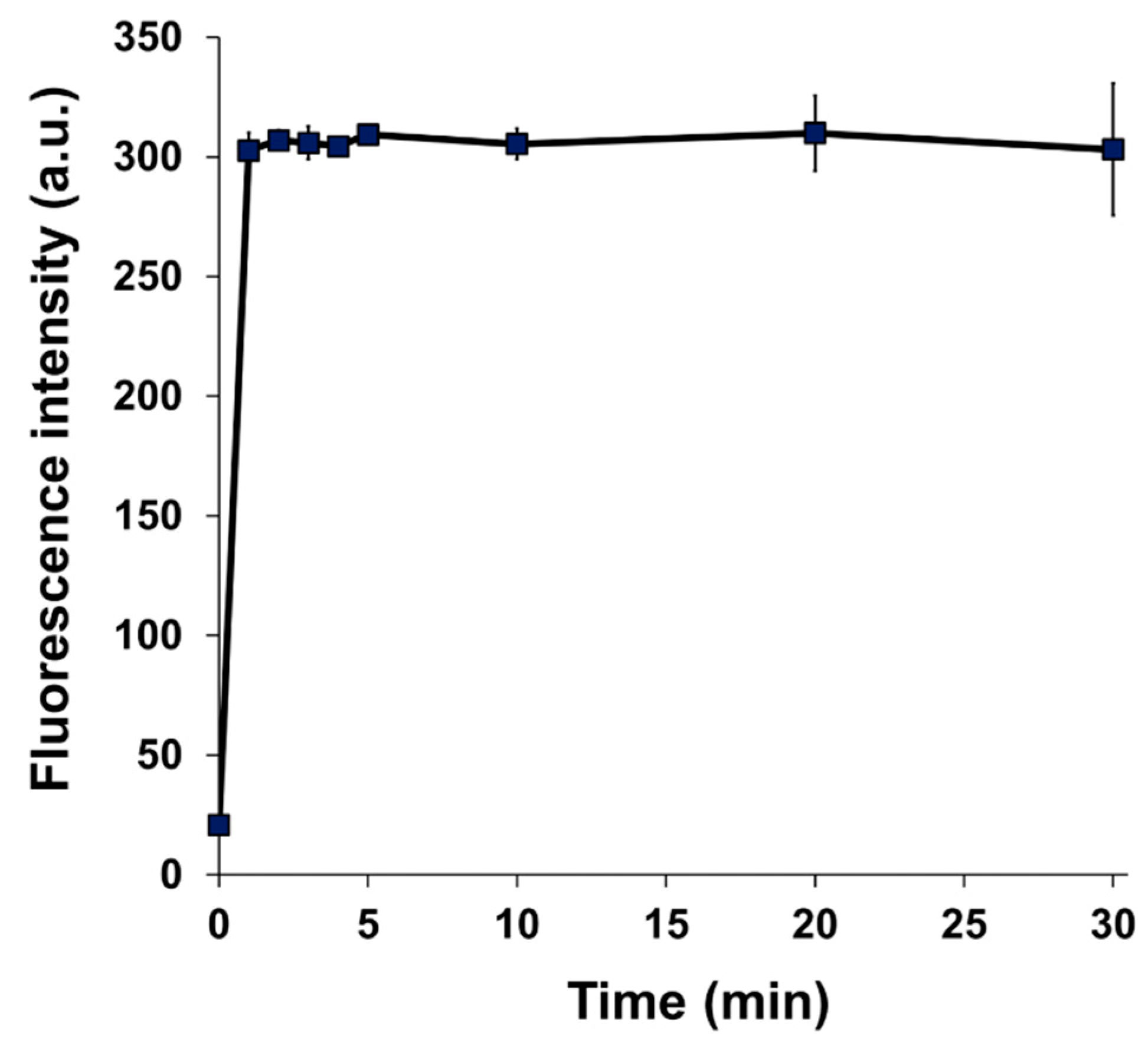
3.1. Fluorescence Properties of BITQ
3.2. Selectivity Assay
3.3. Fluorescence Microscopy Study
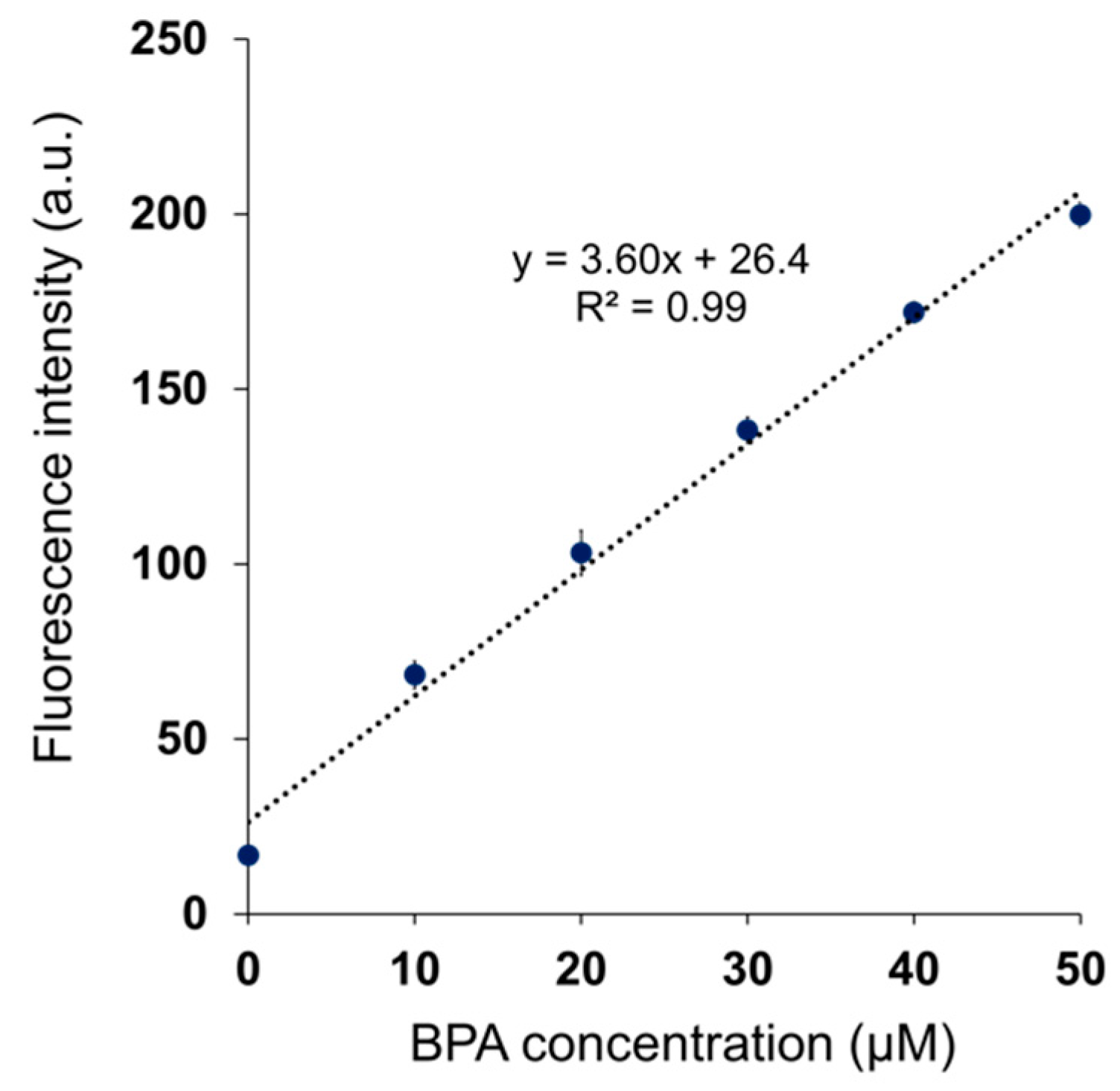
3.4. Quantification of BPA Concentration in Mouse Blood
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moss, R.L. Critical review, with an optimistic outlook, on Boron Neutron Capture Therapy (BNCT). Appl. Radiat. Isot. 2014, 88, 2–11. [Google Scholar] [CrossRef]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef]
- Mishima, Y.; Ichihashi, M.; Hatta, S.; Honda, C.; Yamamura, K.; Nakagawa, T. New thermal neutron capture therapy for malignant melanoma: Melanogenesis-seeking 10B molecule-melanoma cell interaction from in vitro to first clinical trial. Pigment. Cell Res. 1989, 2, 226–234. [Google Scholar] [CrossRef]
- Snyder, H.R.; Reedy, A.J.; Lennarz, W.J. Synthesis of Aromatic Boronic Acids. Aldehydo Boronic Acids and a Boronic Acid Analog of Tyrosine1. J. Am. Chem. Soc. 2002, 80, 835–838. [Google Scholar] [CrossRef]
- Kato, I.; Ono, K.; Sakurai, Y.; Ohmae, M.; Maruhashi, A.; Imahori, Y.; Kirihata, M.; Nakazawa, M.; Yura, Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl. Radiat. Isot. 2004, 61, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Detta, A.; Cruickshank, G.S. L-amino acid transporter-1 and boronophenylalanine-based boron neutron capture therapy of human brain tumors. Cancer Res. 2009, 69, 2126–2132. [Google Scholar] [CrossRef]
- Wongthai, P.; Hagiwara, K.; Miyoshi, Y.; Wiriyasermkul, P.; Wei, L.; Ohgaki, R.; Kato, I.; Hamase, K.; Nagamori, S.; Kanai, Y. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015, 106, 279–286. [Google Scholar] [CrossRef]
- Kanno, H.; Nagata, H.; Ishiguro, A.; Tsuzuranuki, S.; Nakano, S.; Nonaka, T.; Kiyohara, K.; Kimura, T.; Sugawara, A.; Okazaki, Y.; et al. Designation Products: Boron Neutron Capture Therapy for Head and Neck Carcinoma. Oncologist 2021, 26, e1250–e1255. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Hirano, F.; Temma, T. Evaluation of 3-Borono-L-Phenylalanine as a Water-Soluble Boron Neutron Capture Therapy Agent. Pharmaceutics 2022, 14, 1106. [Google Scholar] [CrossRef]
- Nomoto, T.; Inoue, Y.; Yao, Y.; Suzuki, M.; Kanamori, K.; Takemoto, H.; Matsui, M.; Tomoda, K.; Nishiyama, N. Poly(vinyl alcohol) boosting therapeutic potential of p-boronophenylalanine in neutron capture therapy by modulating metabolism. Sci. Adv. 2020, 6, eaaz1722. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Suzuki, Y.; Nagasaki, Y. Molecular design of a high-performance polymeric carrier for delivery of a variety of boronic acid-containing drugs. Acta Biomater. 2021, 121, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Suzuki, M.; Matsumoto, Y.; Fukumitsu, N.; Nagasaki, Y. Non-isotope enriched phenylboronic acid-decorated dual-functional nano-assembles for an actively targeting BNCT Drug. Biomaterials 2021, 268, 120551. [Google Scholar] [CrossRef]
- Linko, S.; Revitzer, H.; Zilliacus, R.; Kortesniemi, M.; Kouri, M.; Savolainen, S. Boron detection from blood samples by ICP-AES and ICP-MS during boron neutron capture therapy. Scand. J. Clin. Lab. Investig. 2008, 68, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Kobayashi, T.; Kanda, K. Analytical Evaluating Method of 10B(n,αγ)7Li Prompt Gamma-Ray Spectrum for Measuring Absorbed Dose Distribution in Boron Neutron Capture Therapy. Frontiers in Neutron Capture Therapy. Front. Neutron Capture Ther. 2001, 1, 1145–1149. [Google Scholar] [CrossRef]
- Skwierawska, D.; Lopez-Valverde, J.A.; Balcerzyk, M.; Leal, A. Clinical Viability of Boron Neutron Capture Therapy for Personalized Radiation Treatment. Cancers 2022, 14, 2865. [Google Scholar] [CrossRef]
- Maliszewska-Olejniczak, K.; Kaniowski, D.; Araszkiewicz, M.; Tyminska, K.; Korgul, A. Molecular Mechanisms of Specific Cellular DNA Damage Response and Repair Induced by the Mixed Radiation Field During Boron Neutron Capture Therapy. Front. Oncol. 2021, 11, 676575. [Google Scholar] [CrossRef]
- Chandra, S.; Lorey, D.R. SIMS ion microscopy imaging of boronophenylalanine (BPA) and 13C15N-labeled phenylalanine in human glioblastoma cells: Relevance of subcellular scale observations to BPA-mediated boron neutron capture therapy of cancer. Int. J. Mass Spectrom. 2007, 260, 90–101. [Google Scholar] [CrossRef]
- Aldossari, S.; McMahon, G.; Lockyer, N.P.; Moore, K.L. Microdistribution and quantification of the boron neutron capture therapy drug BPA in primary cell cultures of human glioblastoma tumour by NanoSIMS. Analyst 2019, 144, 6214–6224. [Google Scholar] [CrossRef]
- Espain, M.S.; Dattoli Viegas, A.M.; Trivillin, V.A.; Saint Martin, G.; Thorp, S.I.; Curotto, P.; Pozzi, E.C.C.; Gonzalez, S.J.; Portu, A.M. Neutron autoradiography to study the microdistribution of boron in the lung. Appl. Radiat Isot. 2020, 165, 109331. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Ishimura, M.; Ohta, Y.; Takenaka, H.; Watanabe, T.; Tanaka, H.; Ono, K.; Kirihata, M. Detection of boronic acid derivatives in cells using a fluorescent sensor. Org. Biomol. Chem. 2015, 13, 6927–6930. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Ishimura, M.; Ohta, Y.; Takenaka, H.; Kirihata, M. Visualization of Boronic Acid Containing Pharmaceuticals in Live Tumor Cells Using a Fluorescent Boronic Acid Sensor. ACS Sens. 2016, 1, 1394–1397. [Google Scholar] [CrossRef]
- Takada, S.; Kondo, N.; Hagimori, M.; Temma, T. Development of a switching-type fluorescence sensor for the detection of boronic acid-containing agents. Anal. Sci. 2022, 38, 1289–1296. [Google Scholar] [CrossRef]
- Martinez-Aguirre, M.A.; Flores Alamo, M.; Elisa Trejo-Huizar, K.; Yatsimirsky, A.K. Boronic acid complexes with amino phenolic N,O-ligands and their use for non-covalent protein fluorescence labeling. Bioorg. Chem. 2021, 113, 104993. [Google Scholar] [CrossRef]
- Konoshima, H.; Nagao, S.; Kiyota, I.; Amimoto, K.; Yamamoto, N.; Sekine, M.; Nakata, M.; Furukawa, K.; Sekiya, H. Excited-state intramolecular proton transfer and charge transfer in 2-(2′-hydroxyphenyl)benzimidazole crystals studied by polymorphs-selected electronic spectroscopy. Phys. Chem. Chem. Phys. 2012, 14, 16448–16457. [Google Scholar] [CrossRef]
- Vazquez, S.R.; Rodriguez, M.C.; Mosquera, M.; Rodriguez-Prieto, F. Rotamerism, tautomerism, and excited-state intramolecular proton transfer in 2-(4′-N,N-diethylamino-2′-hydroxyphenyl)benzimidazoles: Novel benzimidazoles undergoing excited-state intramolecular coupled proton and charge transfer. J. Phys. Chem. A 2008, 112, 376–387. [Google Scholar] [CrossRef]
- Ren, T.B.; Xu, W.; Zhang, W.; Zhang, X.X.; Wang, Z.Y.; Xiang, Z.; Yuan, L.; Zhang, X.B. A General Method To Increase Stokes Shift by Introducing Alternating Vibronic Structures. J. Am. Chem. Soc. 2018, 140, 7716–7722. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Luo, H.; Liao, L.; Song, X.; Chen, W. Red-emitting boron difluoride complexes with a mega-large Stokes shift and unexpectedly high fluorescence quantum yield. Chem. Commun. 2020, 56, 2159–2162. [Google Scholar] [CrossRef]
- Hou, P.; Chen, S.; Wang, H.; Wang, J.; Voitchovsky, K.; Song, X. An aqueous red emitting fluorescent fluoride sensing probe exhibiting a large Stokes shift and its application in cell imaging. Chem. Commun. 2014, 50, 320–322. [Google Scholar] [CrossRef]
- Kondo, N.; Aoki, E.; Takada, S.; Temma, T.A. Red-Emitting Fluorescence Sensor for Detecting Boronic Acid-Containing Agents in Cells. Sensors 2022, 22, 7671. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, E.; Tomaszewska, B.; Wator, K.; Bodzek, M. Selected problems with boron determination in water treatment processes. Part I: Comparison of the reference methods for ICP-MS and ICP-OES determinations. Environ. Sci. Pollut. Res. Int. 2016, 23, 11658–11667. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Ogaki, T.; Ishimura, M.; Ohta, Y.; Kirihata, M. Development and Elucidation of a Novel Fluorescent Boron-Sensor for the Analysis of Boronic Acid-Containing Compounds. Sensors 2017, 17, 2436. [Google Scholar] [CrossRef]
- Michiue, H.; Sakurai, Y.; Kondo, N.; Kitamatsu, M.; Bin, F.; Nakajima, K.; Hirota, Y.; Kawabata, S.; Nishiki, T.; Ohmori, I.; et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014, 35, 3396–3405. [Google Scholar] [CrossRef]
- Armstrong, C.M. The Na/K pump, Cl ion, and osmotic stabilization of cells. Proc. Natl. Acad. Sci. USA 2003, 100, 6257–6262. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Nishikubo, T.; Wakamatsu, T.; Ishikawa, H.; Nakagawa, N.; Kuramitsu, S.; Masui, R. Insights into different dependence of dNTP triphosphohydrolase on metal ion species from intracellular ion concentrations in Thermus thermophilus. Extremophiles 2008, 12, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, K. 4-Borono-2-(18)F-fluoro-L-phenylalanine PET for boron neutron capture therapy-oriented diagnosis: Overview of a quarter century of research. Ann. Nucl. Med. 2019, 33, 223–236. [Google Scholar] [CrossRef]
- Wang, L.W.; Liu, Y.H.; Chou, F.I.; Jiang, S.H. Clinical trials for treating recurrent head and neck cancer with boron neutron capture therapy using the Tsing-Hua Open Pool Reactor. Cancer Commun. 2018, 38, 37. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Nandwana, N.K.; Das, S.; Nandwana, V.; Shareef, M.A.; Das, Y.; Saito, M.; Weiss, L.M.; Almaguel, F.; Hosmane, N.S.; et al. Boron Chemicals in Drug Discovery and Development: Synthesis and Medicinal Perspective. Molecules 2022, 27, 2615. [Google Scholar] [CrossRef]
- Jinna, S.; Finch, J. Spotlight on tavaborole for the treatment of onychomycosis. Drug Des. Devel. Ther. 2015, 9, 6185–6190. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Denny, W.A.; Dos Santos, J.L. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef]

| Stokes Shift | φboron | φfree | φboron /φfree | |||
|---|---|---|---|---|---|---|
| BITQ | 390 nm | 480 nm | 90 nm | 0.53 | 0.094 | 5.6 |
| DAHMI | 411 nm | 431 nm | 20 nm | 0.053 | 0.0034 | 15.7 |
| HPBI | 337 nm | 399 nm | 62 nm | 0.89 | 0.71 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, N.; Takada, S.; Hagimori, M.; Temma, T. Development of a 2-(2-Hydroxyphenyl)-1H-benzimidazole-Based Fluorescence Sensor Targeting Boronic Acids for Versatile Application in Boron Neutron Capture Therapy. Cancers 2023, 15, 1862. https://doi.org/10.3390/cancers15061862
Kondo N, Takada S, Hagimori M, Temma T. Development of a 2-(2-Hydroxyphenyl)-1H-benzimidazole-Based Fluorescence Sensor Targeting Boronic Acids for Versatile Application in Boron Neutron Capture Therapy. Cancers. 2023; 15(6):1862. https://doi.org/10.3390/cancers15061862
Chicago/Turabian StyleKondo, Naoya, Shinya Takada, Masayori Hagimori, and Takashi Temma. 2023. "Development of a 2-(2-Hydroxyphenyl)-1H-benzimidazole-Based Fluorescence Sensor Targeting Boronic Acids for Versatile Application in Boron Neutron Capture Therapy" Cancers 15, no. 6: 1862. https://doi.org/10.3390/cancers15061862
APA StyleKondo, N., Takada, S., Hagimori, M., & Temma, T. (2023). Development of a 2-(2-Hydroxyphenyl)-1H-benzimidazole-Based Fluorescence Sensor Targeting Boronic Acids for Versatile Application in Boron Neutron Capture Therapy. Cancers, 15(6), 1862. https://doi.org/10.3390/cancers15061862







