Systemic Inflammation Indices and Association with Prostate Cancer Survival in a Diverse Patient Cohort
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
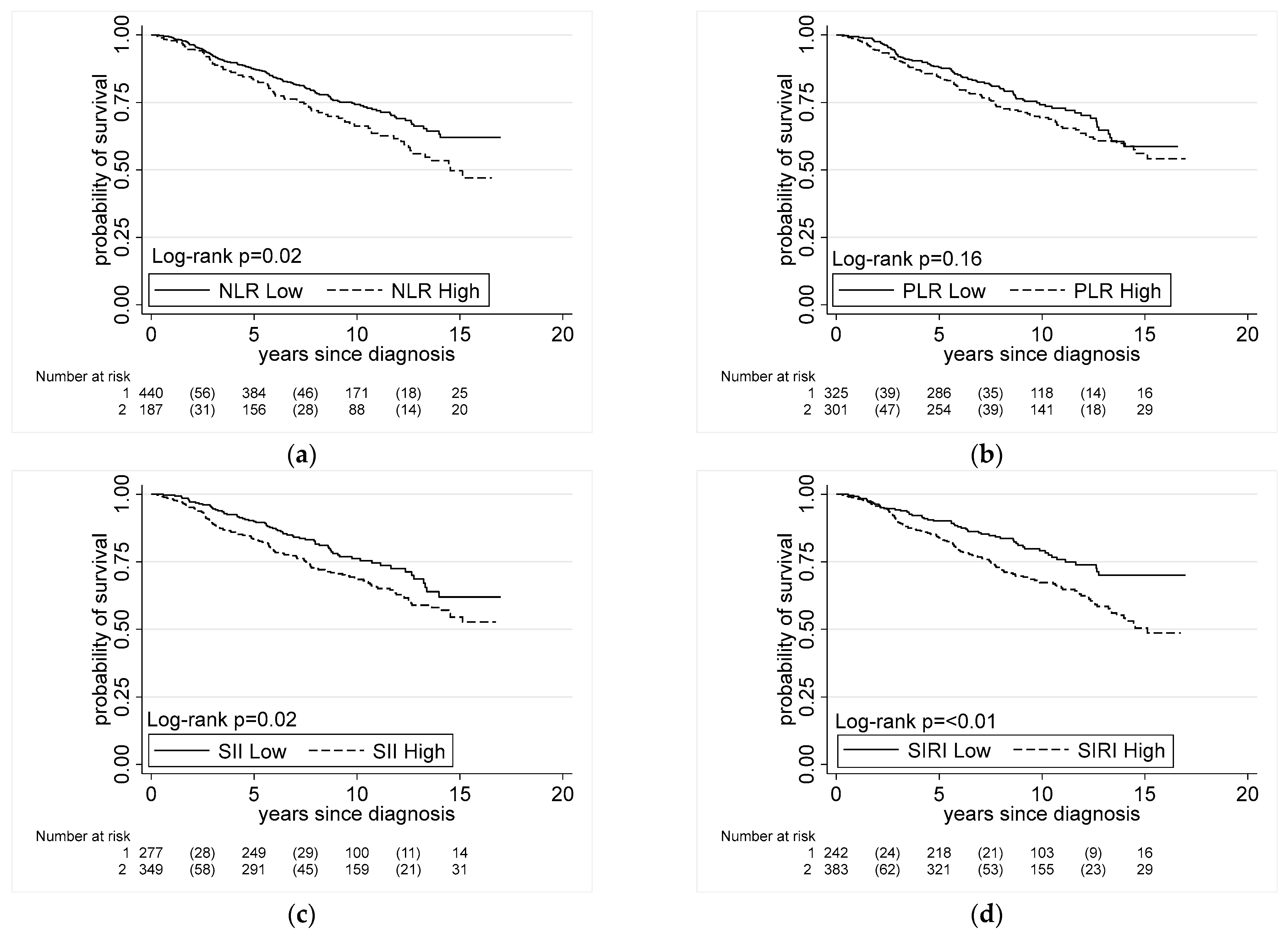
3. Results
3.1. ROC Analysis of Systemic Inflammation Indices
3.2. Clinical and Demographic Characteristics of Participants
3.3. Systemic Inflammation Indices and All-Cause Mortality in Men with Prostate Cancer
3.4. Systemic Inflammation Indices and Prostate Cancer-Specific Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butler, E.N.; Kelly, S.P.; Coupland, V.H.; Rosenberg, P.S.; Cook, M.B. Fatal prostate cancer incidence trends in the United States and England by race, stage, and treatment. Br. J. Cancer 2020, 123, 487–494. [Google Scholar] [CrossRef]
- Cook, M.B.; Hurwitz, L.M.; Geczik, A.M.; Butler, E.N. An Up-to-date Assessment of US Prostate Cancer Incidence Rates by Stage and Race: A Novel Approach Combining Multiple Imputation with Age and Delay Adjustment. Eur. Urol. 2021, 79, 33–41. [Google Scholar] [CrossRef]
- Kiely, M.; Ambs, S. Immune Inflammation Pathways as Therapeutic Targets to Reduce Lethal Prostate Cancer in African American Men. Cancers 2021, 13, 2874. [Google Scholar] [CrossRef]
- Wallace, T.A.; Prueitt, R.L.; Yi, M.; Howe, T.M.; Gillespie, J.W.; Yfantis, H.G.; Stephens, R.M.; Caporaso, N.E.; Loffredo, C.A.; Ambs, S. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008, 68, 927–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Wallace, T.A.; Yi, M.; Magi-Galluzzi, C.; Dorsey, T.H.; Onabajo, O.O.; Obajemu, A.; Jordan, S.V.; Loffredo, C.A.; Stephens, R.M. IFNL4-ΔG allele is associated with an interferon signature in tumors and survival of African-American men with prostate cancer. Clin. Cancer Res. 2018, 24, 5471–5481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.J.; Dorsey, T.H.; Tang, W.; Jordan, S.V.; Loffredo, C.A.; Ambs, S. Aspirin use reduces the risk of aggressive prostate cancer and disease recurrence in African-American men. Cancer Epidemiol. Prev. Biomark. 2017, 26, 845–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Fowke, J.H.; Hurwitz, L.M.; Steinwandel, M.; Blot, W.J.; Ambs, S. Aspirin Use and Prostate Cancer among African-American Men in the Southern Community Cohort Study. Cancer Epidemiol. Prev. Biomark. 2021, 30, 539–544. [Google Scholar] [CrossRef]
- Kiely, M.; Milne, G.L.; Minas, T.Z.; Dorsey, T.H.; Tang, W.; Smith, C.J.; Baker, F.; Loffredo, C.A.; Yates, C.; Cook, M.B. Urinary Thromboxane B2 and Lethal Prostate Cancer in African American Men. JNCI J. Natl. Cancer Inst. 2021, 114, 123–129. [Google Scholar] [CrossRef]
- Guo, W.; Lu, X.; Liu, Q.; Zhang, T.; Li, P.; Qiao, W.; Deng, M. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med. 2019, 8, 4135–4148. [Google Scholar] [CrossRef] [Green Version]
- Hua, X.; Long, Z.-Q.; Huang, X.; Deng, J.-P.; Wen, W.; He, Z.-Y.; Guo, L.; Zhang, W.-W.; Lin, H.-X. The preoperative systemic inflammation response index (SIRI) independently predicts survival in postmenopausal women with breast cancer. Curr. Probl. Cancer 2020, 44, 100560. [Google Scholar] [CrossRef]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef]
- Peng, H.; Luo, X. Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: A meta-analysis. Cancer Cell Int. 2019, 19, 70. [Google Scholar] [CrossRef]
- Rajwa, P.; Schuettfort, V.M.; D’Andrea, D.; Quhal, F.; Mori, K.; Katayama, S.; Laukhtina, E.; Pradere, B.; Motlagh, R.S.; Mostafaei, H. Impact of systemic Immune–inflammation Index on oncologic outcomes in patients treated with radical prostatectomy for clinically nonmetastatic prostate cancer. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Lolli, C.; Caffo, O.; Scarpi, E.; Aieta, M.; Conteduca, V.; Maines, F.; Bianchi, E.; Massari, F.; Veccia, A.; Chiuri, V.E. Systemic immune-inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone. Front. Pharmacol. 2016, 7, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Wang, R.; Chi, C.; Cai, W.; Zhang, Y.; Qian, H.; Shao, X.; Wang, Y.; Xu, F.; Pan, J. Systemic immune-inflammation index predicts the combined clinical outcome after sequential therapy with abiraterone and docetaxel for metastatic castration-resistant prostate cancer patients. Prostate 2018, 78, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, S.; Huang, X.; Liu, X.; Liu, J.; Tian, G. Prognostic value of systemic immune-inflammation index in patients with urinary system cancers: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1302–1310. [Google Scholar] [PubMed]
- Wallace, K.; Li, H.; Brazeal, J.G.; Lewin, D.N.; Sun, S.; Ba, A.; Paulos, C.M.; Rachidi, S.; Li, Z.; Alekseyenko, A.V. Platelet and hemoglobin count at diagnosis are associated with survival in African American and Caucasian patients with colorectal cancer. Cancer Epidemiol. 2020, 67, 101746. [Google Scholar] [CrossRef]
- Minas, T.Z.; Tang, W.; Smith, C.J.; Onabajo, O.O.; Obajemu, A.; Dorsey, T.H.; Jordan, S.V.; Obadi, O.M.; Ryan, B.M.; Prokunina-Olsson, L.; et al. IFNL4-ΔG is associated with prostate cancer among men at increased risk of sexually transmitted infections. Commun. Biol. 2018, 1, 191. [Google Scholar] [CrossRef] [Green Version]
- Murray, N.P.; Fuentealba, C.; Salazar, A.; Reyes, E. Platelet-to-lymphocyte ratio and systemic immune-inflammation index versus circulating prostate cells to predict significant prostate cancer at first biopsy. Turk. J. Urol. 2020, 46, 115–122. [Google Scholar] [CrossRef]
- Sonmez, G.; Demirtas, T.; Tombul, S.; Akgun, H.; Demirtas, A. Diagnostic efficiency of systemic immune-inflammation index in fusion prostate biopsy. Actas Urológicas Españolas 2021, 45, 359–365. [Google Scholar] [CrossRef]
- Man, Y.-N.; Chen, Y.-F. Systemic immune-inflammation index, serum albumin, and fibrinogen impact prognosis in castration-resistant prostate cancer patients treated with first-line docetaxel. Int. Urol. Nephrol. 2019, 51, 2189–2199. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wang, K.; Lu, H.; Xue, D.; Fan, M.; Zhuang, Q.; Yin, S.; He, X.; Xu, R. Systemic inflammation response index predicts prognosis in patients with clear cell renal cell carcinoma: A propensity score-matched analysis. Cancer Manag. Res. 2019, 11, 909. [Google Scholar] [CrossRef] [Green Version]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.J.; Ciampricotti, M.; Hawinkels, L.J.; Jonkers, J. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Bekes, E.M.; Schweighofer, B.; Kupriyanova, T.A.; Zajac, E.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.I. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am. J. Pathol. 2011, 179, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 2015, 528, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Kitayama, J.; Yasuda, K.; Kawai, K.; Sunami, E.; Nagawa, H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer 2011, 11, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N. Platelets in cancer metastasis: To help the “villain” to do evil. Int. J. Cancer 2016, 138, 2078–2087. [Google Scholar] [CrossRef]
- Lucotti, S.; Cerutti, C.; Soyer, M.; Gil-Bernabé, A.M.; Gomes, A.L.; Allen, P.D.; Smart, S.; Markelc, B.; Watson, K.; Armstrong, P.C. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A 2. J. Clin. Investig. 2019, 129, 1845–1862. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.M.; Cembrowski, G.; Cembrowski, M.; Clarke, G. Race-specific WBC and neutrophil count reference intervals. Int. J. Lab. Hematol. 2010, 32, 590–597. [Google Scholar] [CrossRef]
- Guzman-Esquivel, J.; Mendoza-Hernandez, M.A.; Tiburcio-Jimenez, D.; Avila-Zamora, O.N.; Delgado-Enciso, J.; De-Leon-Zaragoza, L.; Casarez-Price, J.C.; Rodriguez-Sanchez, I.P.; Martinez-Fierro, M.L.; Meza-Robles, C. Decreased biochemical progression in patients with castration-resistant prostate cancer using a novel mefenamic acid anti-inflammatory therapy: A randomized controlled trial. Oncol. Lett. 2020, 19, 4151–4160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kast, R.E. High Neutrophil-to-Lymphocyte Ratio Facilitates Cancer Growth—Currently Marketed Drugs Tadalafil, Isotretinoin, Colchicine, and Omega-3 to Reduce It: The TICO Regimen. Cancers 2022, 14, 4965. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, L.M.; Joshu, C.E.; Barber, J.R.; Prizment, A.E.; Vitolins, M.Z.; Jones, M.R.; Folsom, A.R.; Han, M.; Platz, E.A. Aspirin and non-aspirin NSAID use and prostate cancer incidence, mortality, and case fatality in the atherosclerosis risk in communities study. Cancer Epidemiol. Prev. Biomark. 2019, 28, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cases a | NLR | PLR | SII | SIRI | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | |||||||||||||
| ≤2.9 | >2.9 | ≤133.7 | >133.7 | ≤430.8 | >430.8 | ≤0.9 | >0.9 | |||||||||||||
| Demographics of Cases | All m (n = 680) | AA b (n = 305) | EA c (n = 172) | AA (n = 86) | EA (n = 117) | All (n = 679) | AA b (n = 212) | EA c (n = 144) | AA c (n = 178) | EA c (n = 145) | All (n = 679) | AA b (n = 203) | EA c (n = 97) | AA c (n = 187) | EA c (n = 192) | All (n = 678) | AA b (n = 190) | EA c (n = 70) | AA c (n = 200) | EA c (n = 217) |
| Age d | ||||||||||||||||||||
| Median (IQR e) in years | 63 (10) | 63 (9) | 63 (9) | 64 (11) | 65 (10) | 63 (10) | 63 (10) | 64 (10) | 64 (10) | 64 (11) | 63 (10) | 63 (10) | 63 (9) | 63 (10) | 64 (11) | 63 (10) | 63 (9) | 60 (9) | 63 (10) | 64 (11) |
| BMI | ||||||||||||||||||||
| Mean (SD f) in kg/m2 | 28.2 (5.0) | 28.3 (5.6) | 28.4 (4.1) | 27.1 (4.9) | 28.2 (4.9) | 28.2 (5.0) | 28.8 (5.6) | 28.8 (4.3) | 27.2 (5.3) | 27.9 (4.6) | 28.2 (5.0) | 28.4 (5.2) | 28.2 (3.9) | 27.7 (5.8) | 28.4 (4.7) | 28.2 (5.0) | 28.0 (5.0) | 27.1 (3.3) | 28.2 (6.0) | 28.7 (4.7) |
| Education, N (%) | ||||||||||||||||||||
| High school or less | 264 (39) | 137 (45) | 51 (30) | 36 (42) | 40 (34) | 263 (39) | 90 (42) | 47 (32) | 82 (46) | 44 (30) | 263 (39) | 93 (46) | 29 (30) | 79 (42) | 62 (32) | 263 (39) | 81 (43) | 18 (26) | 91 (46) | 73 (34) |
| Some college | 230 (34) | 117 (38) | 59 (34) | 27 (31) | 27 (23) | 230 (34) | 81 (38) | 48 (33) | 63 (35) | 38 (26) | 230 (34) | 76 (37) | 30 (31) | 68 (36) | 56 (29) | 230 (34) | 75 (39) | 19 (27) | 69 (34) | 66 (30) |
| College | 84 (12) | 25 (8) | 26 (15) | 10 (12) | 23 (20) | 84 (12) | 18 (8) | 24 (17) | 17 (10) | 25 (17) | 84 (12) | 18 (9) | 17 (18) | 17 (9) | 32 (17) | 84 (12) | 19 (10) | 13 (19) | 16 (8) | 36 (17) |
| Graduate | 47 (7) | 11 (4) | 15 (9) | 4 (5) | 17 (15) | 47 (7) | 9 (4) | 11 (8) | 6 (3) | 21 (14) | 47 (7) | 7 (3) | 6 (6) | 8 (4) | 26 (14) | 47 (7) | 5 (3) | 8 (11) | 10 (5) | 24 (11) |
| Did not provide | 55 (8) | 15 (5) | 21 (12) | 9 (10) | 10 (8) | 55 (8) | 14 (7) | 14 (10) | 10 (6) | 17 (12) | 55 (8) | 9 (4) | 15 (15) | 15 (8) | 16 (8) | 54 (8) | 10 (5) | 12 (17) | 14 (7) | 18 (8) |
| Baseline Health Factors | ||||||||||||||||||||
| Family history of prostate cancer g, N (%) | ||||||||||||||||||||
| No | 546 (80) | 255 (84) | 125 (73) | 68 (79) | 98 (84) | 545 (80) | 172 (81) | 109 (76) | 150 (84) | 114 (79) | 545 (80) | 169 (83) | 66 (68) | 153 (82) | 157 (82) | 545 (80) | 161 (85) | 46 (66) | 161 (81) | 176 (81) |
| Yes | 78 (11) | 33 (11) | 25 (14) | 9 (10.5) | 11 (9) | 78 (11) | 24 (11) | 21 (14) | 18 (10) | 15 (10) | 78 (12) | 23 (11) | 16 (16) | 19 (10) | 20 (10) | 78 (11) | 19 (10) | 12 (17) | 23 (11) | 24 (11) |
| Did not provide | 56 (8) | 17 (6) | 22 (13) | 9 (10.5) | 8 (7) | 56 (8) | 16 (8) | 14 (10) | 10 (6) | 16 (11) | 56 (8) | 11 (5) | 15 (15) | 15 (8) | 15 (8) | 55 (8) | 10 (5) | 12 (17) | 16 (8) | 17 (8) |
| Smoking status h, N (%) | ||||||||||||||||||||
| Current | 190 (28) | 106 (35) | 33 (19) | 30 (35) | 21 (18) | 189 (28) | 77 (36) | 32 (22) | 58 (33) | 22 (15) | 189 (28) | 66 (32) | 16 (16) | 69 (37) | 38 (20) | 190 (28) | 67 (35) | 12 (17) | 69 (34) | 42 (19) |
| Former | 254 (37) | 100 (33) | 68 (40) | 28 (33) | 58 (50) | 254 (37) | 67 (32) | 61 (42) | 61 (34) | 65 (45) | 254 (37) | 67 (33) | 42 (43) | 61 (33) | 84 (44) | 254 (37) | 62 (33) | 29 (41) | 66 (33) | 96 (44) |
| Never | 182 (27) | 83 (27) | 52 (30) | 19 (22) | 28 (24) | 182 (27) | 54 (25) | 39 (27) | 48 (27) | 41 (28) | 182 (27) | 60 (30) | 26 (27) | 42 (22) | 54 (28) | 181 (27) | 50 (26) | 18 (26) | 51 (26) | 62 (29) |
| Did not provide | 54 (8) | 16 (5) | 19 (11) | 9 (10) | 10 (8) | 54 (8) | 14 (7) | 12 (8) | 11 (6) | 17 (12) | 54 (8) | 10 (5) | 13 (13) | 15 (8) | 16 (8) | 53 (8) | 11 (6) | 11 (16) | 14 (7) | 17 (8) |
| Stage i, N (%) | ||||||||||||||||||||
| T1 | 117 (17) | 50 (16) | 37 (22) | 14 (16) | 16 (14) | 117 (17) | 39 (18) | 30 (21) | 25 (14) | 23 (16) | 117 (17) | 37 (18) | 22 (23) | 27 (14) | 31 (16) | 116 (17) | 35 (18) | 20 (28) | 29 (15) | 32 (15) |
| T2 | 461 (68) | 220 (72) | 108 (63) | 54 (63) | 79 (68) | 460 (68) | 147 (69) | 92 (64) | 126 (71) | 95 (65) | 460 (68) | 144 (71) | 57 (59) | 129 (69) | 130 (68) | 461 (68) | 131 (69) | 41 (59) | 143 (71) | 145 (67) |
| T3 | 54 (8) | 16 (5) | 22 (13) | 7 (8) | 9 (8) | 54 (8) | 17 (8) | 17 (12) | 6 (3) | 14 (10) | 54 (8) | 12 (6) | 13 (13) | 11 (6) | 18 (9) | 54 (8) | 15 (8) | 7 (10) | 8 (4) | 24 (11) |
| T4 | 44 (6) | 17 (6) | 4 (2) | 10 (12) | 13 (11) | 44 (6) | 6 (3) | 4 (3) | 21 (12) | 13 (9) | 44 (6) | 9 (4) | 4 (4) | 18 (10) | 13 (7) | 43 (6) | 8 (4) | 2 (3) | 18 (9) | 15 (7) |
| Missing | 4 (1) | 2 (1) | 1 (<1) | 1 (1) | 4 (1) | 3 (1) | 1 (1) | 4 (1) | 1 (<1) | 1 (1) | 2 (1) | 4 (1) | 1 (1) | 2 (1) | 1 (<1) | |||||
| Gleason score, N (%) | ||||||||||||||||||||
| ≤7 | 549 (81) | 251 (82) | 142 (83) | 64 (74) | 92 (79) | 548 (81) | 175 (83) | 116 (81) | 139 (78) | 118 (81) | 548 (81) | 171 (84) | 77 (79) | 143 (76) | 157 (82) | 548 (81) | 158 (83) | 62 (89) | 157 (78) | 170 (78) |
| >7 | 126 (18) | 51 (17) | 30 (17) | 20 (23) | 25 (21) | 126 (18) | 33 (16) | 28 (19) | 38 (21) | 27 (19) | 126 (18) | 31 (15) | 20 (21) | 40 (21) | 35 (18) | 125 (18) | 30 (16) | 8 (11) | 40 (20) | 47 (22) |
| Missing | 5 (1) | 3 (1) | 2 (2) | 5 (1) | 2 (2) | 1 (1) | 5 (1) | 1 (1) | 4 (2) | 5 (1) | 2 (1) | 3 (2) | ||||||||
| Disease aggressiveness, N (%) | ||||||||||||||||||||
| Nonaggressive disease j | 491 (72) | 230 (75) | 125 (73) | 58 (68) | 78 (67) | 490 (72) | 159 (75) | 104 (72) | 128 (72) | 99 (68) | 490 (72) | 158 (78) | 67 (69) | 129 (69) | 136 (71) | 490 (72) | 144 (76) | 55 (79) | 144 (72) | 146 (67) |
| Aggressive disease k | 185 (27) | 73 (24) | 46 (27) | 27 (31) | 39 (33) | 185 (27) | 50 (24) | 39 (27) | 50 (28) | 46 (32) | 185 (27) | 44 (22) | 29 (30) | 56 (30) | 56 (29) | 184 (27) | 45 (24) | 15 (21) | 54 (27) | 70 (32) |
| Missing | 4 (1) | 2 (1) | 1 (<1) | 1 (1) | 4 (1) | 3 (1) | 1 (1) | 4 (1) | 1 (1) | 1 (1) | 2 (1) | 4 (1) | 1 (1) | 2 (1) | 1 (1) | |||||
| PSA l | ||||||||||||||||||||
| Median (IQR) in ng/mL | 6.8 (7.0) | 7.0 (7.3) | 5.8 (4.4) | 7.5 (13.9) | 7.4 (9.5) | 6.8 (7.0) | 6.9 (6.5) | 6.1 (4.6) | 7.5 (10.9) | 6.8 (6.6) | 6.8 (7.0) | 6.8 (6.6) | 6 (4.25) | 7.4 (11.2) | 6.6 (7.3) | 6.8 (7.0) | 6.5 (6.7) | 5.8 (4.2) | 7.6 (11.1) | 6.7 (6.6) |
| All Cases | African American | European American | P Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NLR | Alive N (%) | Dead N (%) | * HR (95% CI) | Alive N (%) | Dead N (%) | * HR (95% CI) | Alive N (%) | Dead N (%) | * HR (95% CI) | |
| Low ≤ 2.9 | 228 (73) | 97 (61) | Ref | 142 (82) | 68 (73) | Ref | 86 (62) | 29 (44) | Ref | |
| High > 2.9 | 84 (27) | 62 (39) | 1.34 (0.94–1.89) | 32 (18) | 25 (27) | 1.33 (0.80–2.21) | 52 (38) | 37 (56) | 1.27 (0.74–2.17) | |
| Continuous | 1.23 (1.03–1.48) | 1.26 (1.00–1.59) | 1.25 (0.89–1.76) | |||||||
| PLR | ||||||||||
| Low ≤133.7 | 163 (52) | 70 (44) | Ref | 96 (55) | 40 (43) | Ref | 67 (49) | 30 (45) | Ref | |
| High >133.7 | 149 (48) | 88 (56) | 1.10 (0.78–1.53) | 78 (45) | 52 (57) | 1.48 (0.92–2.38) | 71 (51) | 36 (55) | 0.88 (0.52–1.50) | |
| Continuous | 1.13 (0.89–1.43) | 1.33 (0.94–1.88) | 1.14 (0.90–1.44) | |||||||
| SII | ||||||||||
| Low ≤ 430.8 | 142 (46) | 51 (32) | Ref | 98 (56) | 34 (37) | Ref | 44 (32) | 17 (26) | Ref | |
| High > 430.8 | 170 (54) | 107 (68) | 1.47 (1.04–2.09) | 76 (44) | 58 (63) | 1.66 (1.06–2.60) | 94 (68) | 49 (74) | 1.07 (0.60–1.91) | 0.01 |
| Continuous | 1.20 (1.02–1.41) | 1.23 (0.99–1.52) | 1.16 (0.88–1.54) | |||||||
| SIRI | ||||||||||
| Low ≤ 0.9 | 134 (43) | 45 (28) | Ref | 94 (54) | 37 (40) | Ref | 40 (29) | 8 (12) | Ref | |
| High > 0.9 | 177 (57) | 113 (72) | 1.65 (1.14–2.41) | 80 (46) | 55 (60) | 1.55 (0.99–2.43) | 97 (71) | 58 (88) | 1.87 (0.83–4.22) | |
| Continuous | 1.17 (1.02–1.33) | 1.22 (1.02–1.46) | 1.14 (0.90–1.44) | <0.01 | ||||||
| All Cases | African American | European American | P Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NLR | Alive N (%) | Dead N (%) | * HR (95% CI) | Alive N (%) | Dead N (%) | * HR (95% CI) | Alive N (%) | Dead N (%) | * HR (95% CI) | |
| Low ≤ 2.9 | 305 (71) | 20 (50) | Ref | 194 (80) | 16 (64) | Ref | 111 (59) | 4 (27) | Ref | |
| High > 2.9 | 126 (29) | 20 (50) | 2.56 (1.25–5.25) | 48 (20) | 9 (36) | 2.96 (1.10–7.98) | 78 (41) | 11 (73) | 2.43 (0.57–10.42) | <0.01 |
| Continuous | 1.51 (1.05–2.18) | 1.40 (0.89–2.22) | 1.84 (0.94–3.59) | |||||||
| PLR | ||||||||||
| Low ≤133.7 | 219 (51) | 14 (35) | Ref | 126 (52) | 10 (40) | Ref | 93 (49) | 4 (27) | Ref | |
| High >133.7 | 211 (49) | 26 (65) | 1.55 (0.74–3.27) | 115 (48) | 15 (60) | 1.75 (0.65–4.74) | 96 (51) | 11 (73) | 1.87 (0.41–8.49) | |
| Continuous | 1.11 (0.68–1.81) | 0.89 (0.44–1.80) | 1.47 (0.71–3.05) | |||||||
| SII | ||||||||||
| Low ≤430.8 | 184 (43) | 9 (22) | Ref | 124 (51) | 8 (32) | Ref | 60 (32) | 1 (7) | Ref | |
| High >430.8 | 246 (57) | 31 (78) | 3.13 (1.37–7.16) | 117 (49) | 17 (68) | 2.63 (1.0–6.97) | 129 (68) | 14 (93) | 11.63 (1.03–131.02) | |
| Continuous | 1.34 (0.96–1.87) | 1.14 (0.77–1.68) | 1.77 (1.00–3.13) | |||||||
| SIRI | ||||||||||
| Low ≤0.9 | 171 (40) | 8 (21) | Ref | 123 (51) | 8 (33) | Ref | 48 (26) | Ref | ||
| High >0.9 | 259 (60) | 31 (79) | 3.24 (1.31–8.06) | 119 (49) | 16 (67) | 3.19 (1.12–9.04) | 140 (74) | 15 (100) | Not enough cases | |
| Continuous | 1.43 (1.11–1.84) | 1.58 (1.11–2.25) | 1.32 (0.84–2.08) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey-Whyte, M.; Minas, T.Z.; Dorsey, T.H.; Smith, C.J.; Loffredo, C.A.; Ambs, S. Systemic Inflammation Indices and Association with Prostate Cancer Survival in a Diverse Patient Cohort. Cancers 2023, 15, 1869. https://doi.org/10.3390/cancers15061869
Bailey-Whyte M, Minas TZ, Dorsey TH, Smith CJ, Loffredo CA, Ambs S. Systemic Inflammation Indices and Association with Prostate Cancer Survival in a Diverse Patient Cohort. Cancers. 2023; 15(6):1869. https://doi.org/10.3390/cancers15061869
Chicago/Turabian StyleBailey-Whyte, Maeve, Tsion Z. Minas, Tiffany H. Dorsey, Cheryl J. Smith, Christopher A. Loffredo, and Stefan Ambs. 2023. "Systemic Inflammation Indices and Association with Prostate Cancer Survival in a Diverse Patient Cohort" Cancers 15, no. 6: 1869. https://doi.org/10.3390/cancers15061869
APA StyleBailey-Whyte, M., Minas, T. Z., Dorsey, T. H., Smith, C. J., Loffredo, C. A., & Ambs, S. (2023). Systemic Inflammation Indices and Association with Prostate Cancer Survival in a Diverse Patient Cohort. Cancers, 15(6), 1869. https://doi.org/10.3390/cancers15061869







