Novel Generation of FAP Inhibitor-Based Homodimers for Improved Application in Radiotheranostics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Organic Synthesis
2.2.1. Synthesis of Glu.(FAPi)2
tert-Butyl ((S)-1,5-bis((4-((4-((2-((S)-2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)carbamoyl)-quinolin-6-yl)oxy)butyl)amino)-1,5-dioxopentan-2-yl)carbamate (3, Boc-Glu.(FAPi)2)
(S)-2-Amino-N1,N5-bis(4-((4-((2-((S)-2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)carbamoyl)-quinolin-6-yl)oxy)butyl)pentanediamide (4, Glu.(FAPi)2)
2.2.2. Synthesis of DOTAGA.Glu.(FAPi)2
2.2.3. Synthesis of DO3A.Glu.(FAPi)2
2.2.4. Synthesis of natLu-Complexes
[natLu]Lu-DOTAGA.Glu.(FAPi)2 (natLu-7)
[natLu]Lu-DO3A.Glu.(FAPi)2 (natLu-11)
2.3. Radiosynthesis
2.3.1. 68Ga-Radiolabeling
2.3.2. 177Lu-Radiolabeling
2.3.3. 90Y-Radiolabeling
2.3.4. 225Ac-Radiolabeling
2.3.5. Complex Stability Measurements
2.3.6. Determination of logD7.4 (Lipophilicity Measurement)
2.4. In Vitro Inhibition Assays (IC50 Measurements)
2.5. Patient Study
2.5.1. Radiosynthesis
2.5.2. Clinical Image Acquisition and Analysis
3. Results and Discussion
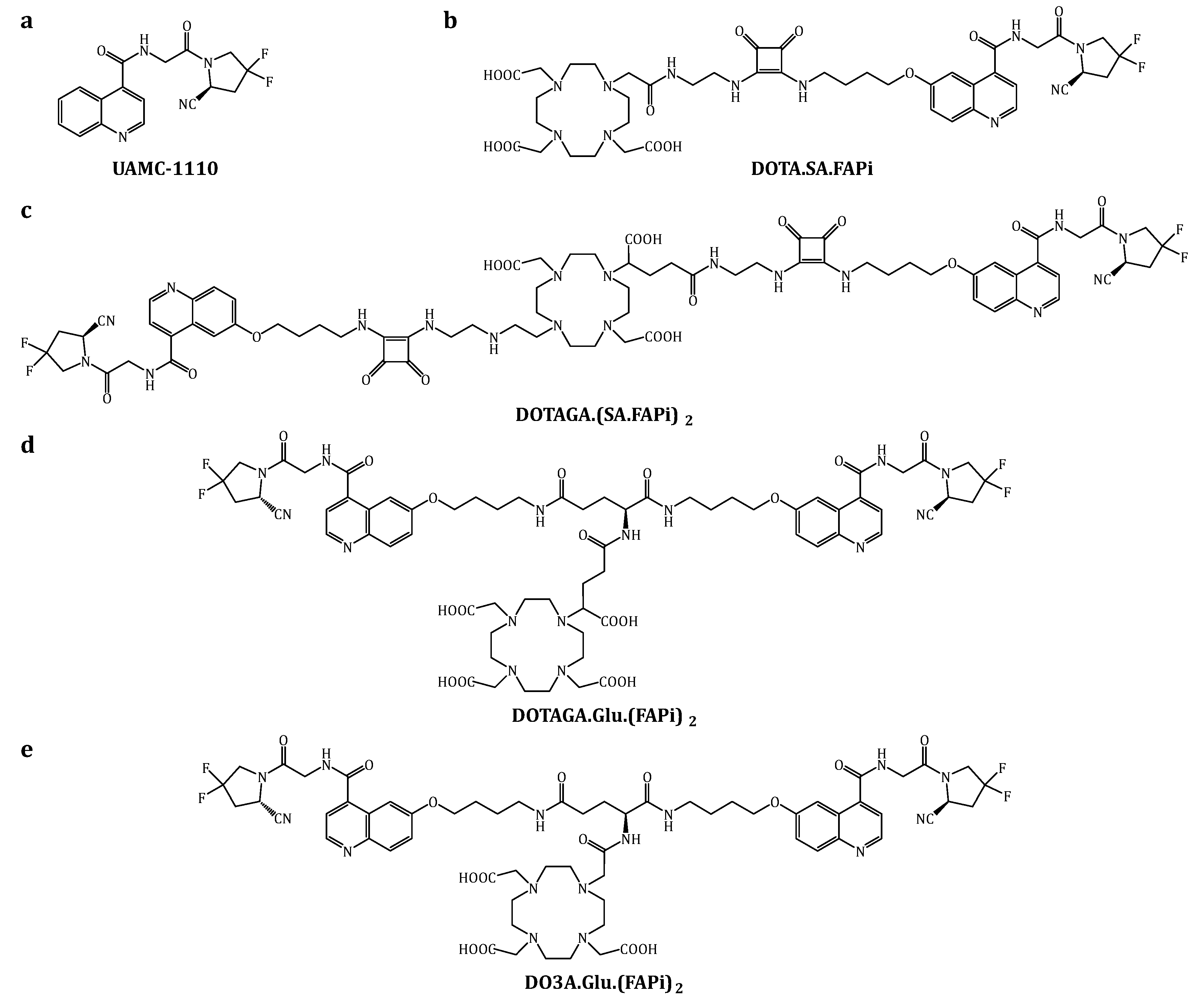
3.1. Organic Synthesis
3.2. Radiosynthesis
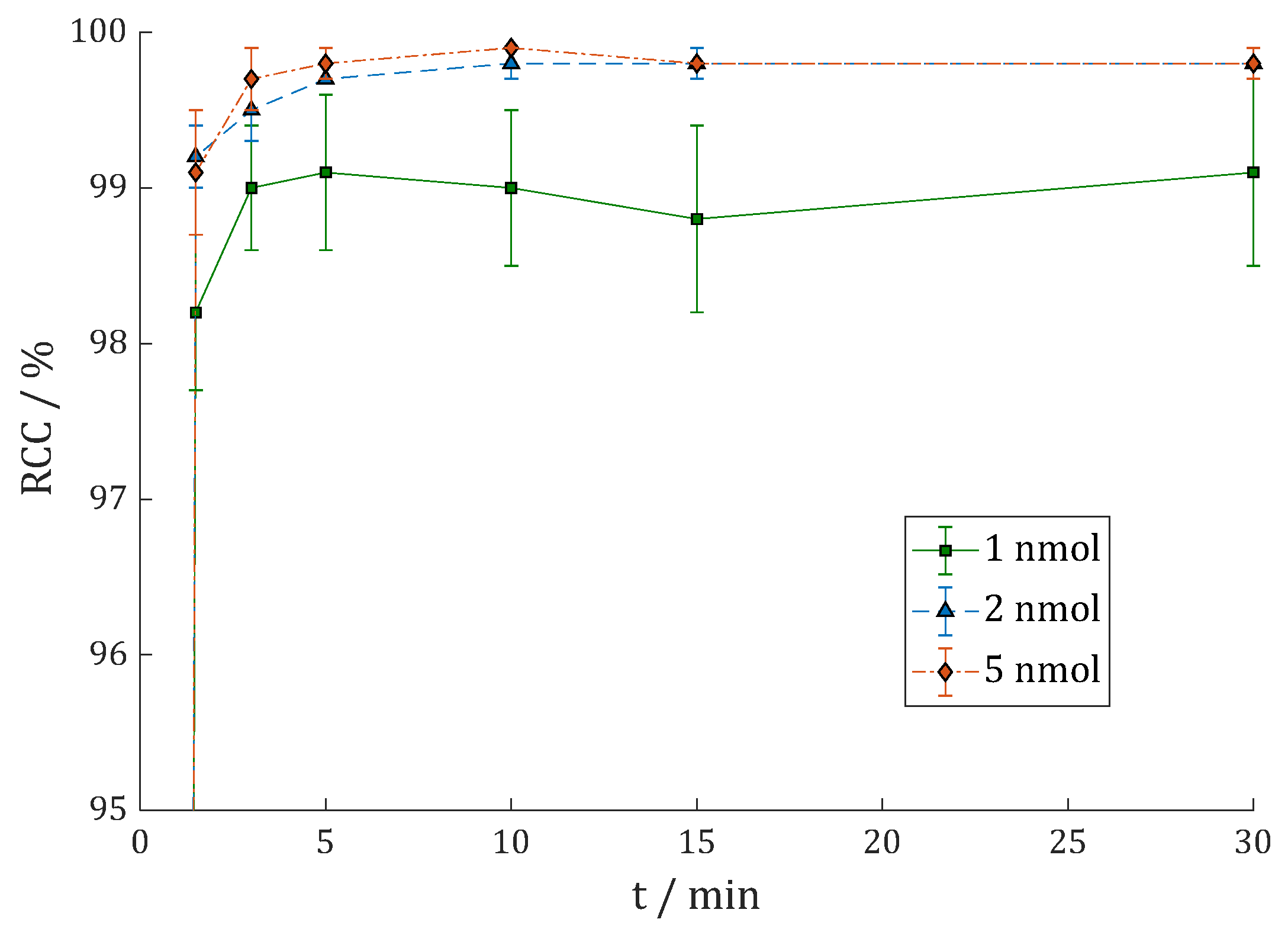
3.2.1. [68Ga]Ga-DOTAGA.Glu.(FAPi)2 (68Ga-7)
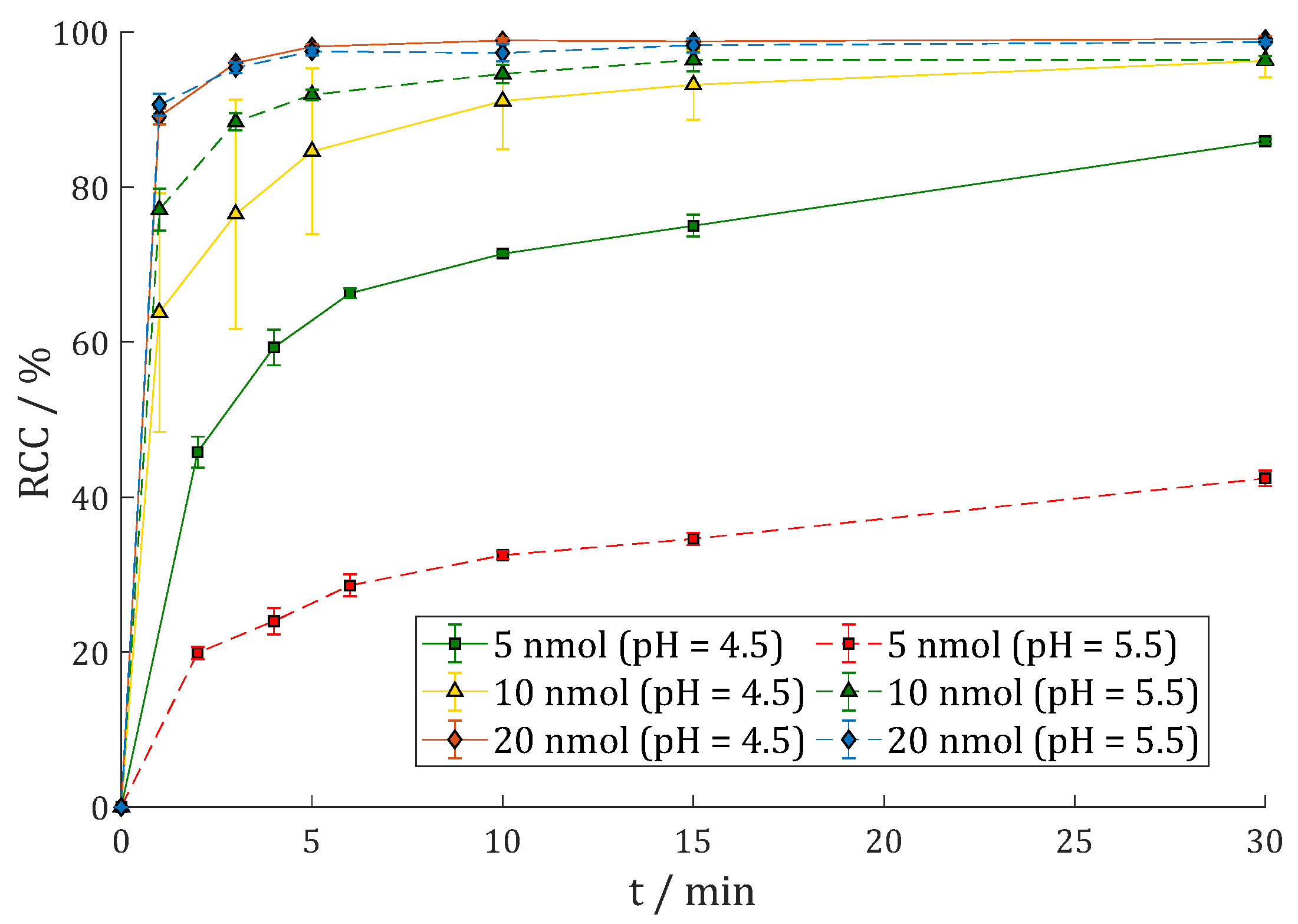
3.2.2. [177Lu]Lu-DOTAGA.Glu.(FAPi)2 (177Lu-7)
3.2.3. [225Ac]Ac-DOTAGA.Glu.(FAPi)2 (225Ac-7)
3.2.4. [68Ga]Ga-DO3A.Glu.(FAPi)2 (68Ga-11)
3.2.5. [177Lu]Lu-DO3A.Glu.(FAPi)2 (177Lu-11)
3.2.6. [90Y]Y-DO3A.Glu.(FAPi)2 (90Y-11)
3.2.7. Lipophilicity
3.2.8. Comparison with DOTAGA.(SA.FAPi)2
3.3. In Vitro Inhibition Assays
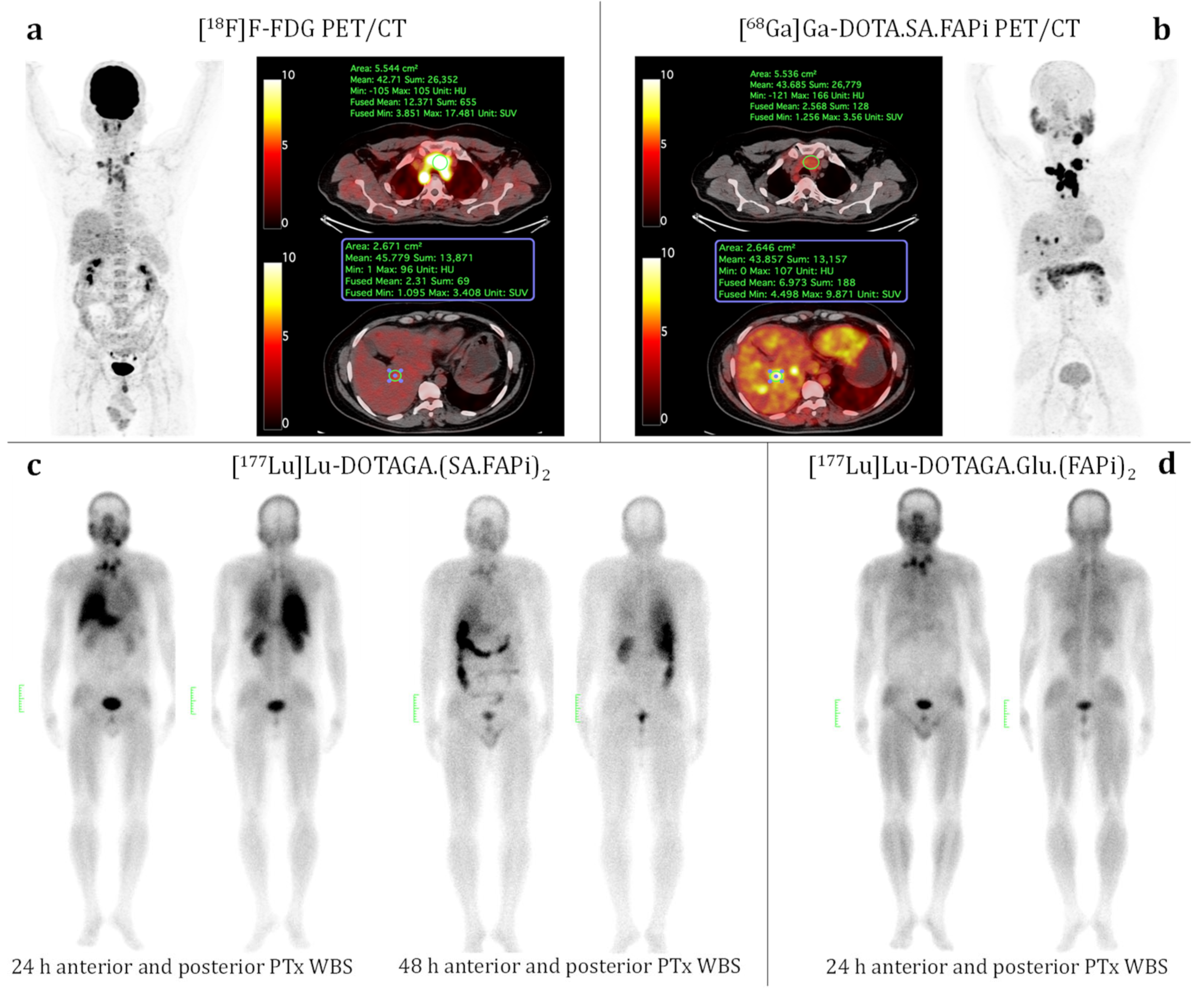
3.4. Patient Study (Medullary Thyroid Cancer)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, P.; O’Connor, B.F. Seprase: An Overview of an Important Matrix Serine Protease. Biochim. Biophys. Acta—Proteins Proteom. 2008, 1784, 1130–1145. [Google Scholar] [CrossRef] [PubMed]
- Rettig, W.J.; Garin-Chesa, P.; Beresford, H.R.; Oettgen, H.F.; Melamed, M.R.; Old, L.J. Cell-Surface Glycoproteins of Human Sarcomas: Differential Expression in Normal and Malignant Tissues and Cultured Cells. Proc. Natl. Acad. Sci. USA 1988, 85, 3110–3114. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.M.; Kevorkian, L.; Young, D.A.; Jones, D.; Wait, R.; Donell, S.T.; Barksby, E.; Patterson, A.M.; Middleton, J.; Cravatt, B.F.; et al. Fibroblast Activation Protein Alpha Is Expressed by Chondrocytes Following a Pro-Inflammatory Stimulus and Is Elevated in Osteoarthritis. Arthritis Res. Ther. 2006, 8, R23. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.T.; McCaughan, G.W.; Abbott, C.A.; Park, J.E.; Cunningham, A.M.; Müller, E.; Rettig, W.J.; Gorrell, M.D. Fibroblast Activation Protein: A Cell Surface Dipeptidyl Peptidase and Gelatinase Expressed by Stellate Cells at the Tissue Remodelling Interface in Human Cirrhosis. Hepatology 1999, 29, 1768–1778. [Google Scholar] [CrossRef]
- Ramamonjisoa, N.; Ackerstaff, E. Characterization of the Tumor Microenvironment and Tumor-Stroma Interaction by Non-Invasive Preclinical Imaging. Front. Oncol. 2017, 7, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Cell Surface Glycoprotein of Reactive Stromal Fibroblasts as a Potential Antibody Target in Human Epithelial Cancers. Proc. Natl. Acad. Sci. USA 1990, 87, 7235–7239. [Google Scholar] [CrossRef]
- Puré, E. The Road to Integrative Cancer Therapies: Emergence of a Tumor-Associated Fibroblast Protease as a Potential Therapeutic Target in Cancer. Expert Opin. Ther. Targets 2009, 13, 967–973. [Google Scholar] [CrossRef]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding Fibroblast Activation Protein (FAP): Substrates, Activities, Expression and Targeting for Cancer Therapy. Proteom. –Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.A.; Ghersi, G.; Piñeiro-Sánchez, M.L.; Salamone, M.; Yeh, Y.; Flessate, D.; Chen, W.-T. Molecular Cloning of Seprase: A Serine Integral Membrane Protease from Human Melanoma. Biochim. Biophys. Acta -Mol. Basis Dis. 1997, 1361, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Aertgeerts, K.; Levin, I.; Shi, L.; Snell, G.P.; Jennings, A.; Prasad, G.S.; Zhang, Y.; Kraus, M.L.; Salakian, S.; Sridhar, V.; et al. Structural and Kinetic Analysis of the Substrate Specificity of Human Fibroblast Activation Protein α. J. Biol. Chem. 2005, 280, 19441–19444. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.M.T.; Yao, T.-W.; Chowdhury, S.; Nadvi, N.A.; Osborne, B.; Church, W.B.; McCaughan, G.W.; Gorrell, M.D. The Dipeptidyl Peptidase IV Family in Cancer and Cell Biology. FEBS J. 2010, 277, 1126–1144. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, E.; Garnier, D.; De Meester, I.; Bauvois, B. Validating Cell Surface Proteases as Drug Targets for Cancer Therapy: What Do We Know, and Where Do We Go? Cancers 2022, 14, 624. [Google Scholar] [CrossRef]
- Keane, F.M.; Nadvi, N.A.; Yao, T.W.; Gorrell, M.D. Neuropeptide Y, B-Type Natriuretic Peptide, Substance P and Peptide YY Are Novel Substrates of Fibroblast Activation Protein-α. FEBS J. 2011, 278, 1316–1332. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Lenter, M.C.; Zimmermann, R.N.; Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Fibroblast Activation Protein, a Dual Specificity Serine Protease Expressed in Reactive Human Tumor Stromal Fibroblasts. J. Biol. Chem. 1999, 274, 36505–36512. [Google Scholar] [CrossRef]
- Lee, K.N.; Jackson, K.W.; Christiansen, V.J.; Chung, K.H.; McKee, P.A. A Novel Plasma Proteinase Potentiates Alpha2-Antiplasmin Inhibition of Fibrin Digestion. Blood 2004, 103, 3783–3788. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.N.; Jackson, K.W.; Christiansen, V.J.; Dolence, E.K.; Mckee, P.A. Enhancement of Fibrinolysis by Inhibiting Enzymatic Cleavage of Precursor A2-Antiplasmin. J. Thromb. Haemost. 2011, 9, 987–996. [Google Scholar] [CrossRef]
- Aimes, R.T.; Zijlstra, A.; Hooper, J.D.; Ogbourne, S.M.; Sit, M.-L.; Fuchs, S.; Gotley, D.C.; Quigley, J.P.; Antalis, T.M. Endothelial Cell Serine Proteases Expressed during Vascular Morphogenesis and Angiogenesis. Thromb. Haemost. 2003, 89, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wang, S.; Wang, H.; Tang, W. Fibroblast Activation Protein-α in Tumor Cells Promotes Colorectal Cancer Angiogenesis via the Akt and ERK Signaling Pathways. Mol. Med. Rep. 2018, 17, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Martin, T.A.; Ye, L.; Meng, L.; Xia, N.; Jiang, W.G.; Zhang, X. Fibroblast Activation Protein-α Promotes the Growth and Migration of Lung Cancer Cells via the PI3K and Sonic Hedgehog Pathways. Int. J. Mol. Med. 2018, 41, 275–283. [Google Scholar] [CrossRef]
- Huang, Y.; Simms, A.E.; Mazur, A.; Wang, S.; León, N.R.; Jones, B.; Aziz, N.; Kelly, T. Fibroblast Activation Protein-α Promotes Tumor Growth and Invasion of Breast Cancer Cells through Non-Enzymatic Functions. Clin. Exp. Metastasis 2011, 28, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, Q.; Wu, J.; Xu, B.; Wang, J.; Liang, L.; Guo, Y.; Peng, M.; Zhao, Y.; Liao, Q. Fibroblast Activation Protein α-Positive Pancreatic Stellate Cells Promote the Migration and Invasion of Pancreatic Cancer by CXCL1-Mediated Akt Phosphorylation. Ann. Transl. Med. 2019, 7, 532. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, C.; Peng, C.; Xu, F.; Li, Y.; Yutaka, Y.; Xiong, B.; Yang, X. Stromal Fibroblast Activation Protein Alpha Promotes Gastric Cancer Progression via Epithelial-Mesenchymal Transition through Wnt/ β-Catenin Pathway. BMC Cancer 2018, 18, 1009. [Google Scholar] [CrossRef] [PubMed]
- Calais, J. FAP: The Next Billion Dollar Nuclear Theranostics Target? J. Nucl. Med. 2020, 61, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Heirbaut, L.; Verkerk, R.; Cheng, J.D.; Joossens, J.; Cos, P.; Maes, L.; Lambeir, A.M.; De Meester, I.; Augustyns, K.; et al. Extended Structure-Activity Relationship and Pharmacokinetic Investigation of (4-Quinolinoyl)Glycyl-2-Cyanopyrrolidine Inhibitors of Fibroblast Activation Protein (FAP). J. Med. Chem. 2014, 57, 3053–3074. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Lindner, T.; Altmann, A.; Krämer, S.; Kleist, C.; Loktev, A.; Kratochwil, C.; Giesel, F.; Mier, W.; Marme, F.; Debus, J.; et al. Design and Development of 99mTc-Labeled FAPI Tracers for SPECT Imaging and 188Re Therapy. J. Nucl. Med. 2020, 61, 1507–1513. [Google Scholar] [CrossRef]
- Roy, J.; Hettiarachchi, S.U.; Kaake, M.; Mukkamala, R.; Low, P.S. Design and Validation of Fibroblast Activation Protein Alpha Targeted Imaging and Therapeutic Agents. Theranostics 2020, 10, 5778–5789. [Google Scholar] [CrossRef]
- Moon, E.S.; Elvas, F.; Vliegen, G.; De Lombaerde, S.; Vangestel, C.; De Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting Fibroblast Activation Protein (FAP): Next Generation PET Radiotracers Using Squaramide Coupled Bifunctional DOTA and DATA5m Chelators. EJNMMI Radiopharm. Chem. 2020, 5, 19. [Google Scholar] [CrossRef]
- Moon, E.S.; Van Rymenant, Y.; Battan, S.; De Loose, J.; Bracke, A.; Van der Veken, P.; De Messter, I.; Rosch, F. In Vitro Evaluation of the Squaramide-Conjugated Fibroblast Activation Protein Inhibitor-Based Agents AAZTA5.SA.FAPi and DOTA.SA.FAPi. Molecules 2021, 26, 3482. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Ballal, S.; Yadav, M.P.; Bal, C.; Rymenant, Y. Van Fibroblast Activation Protein (FAP) Targeting Homodimeric FAP Inhibitor Radiotheranostics: A Step to Improve Tumor Uptake and Retention Time. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 476–491. [Google Scholar] [PubMed]
- Toms, J.; Kogler, J.; Maschauer, S.; Daniel, C.; Schmidkonz, C.; Kuwert, T.; Prante, O. Targeting Fibroblast Activation Protein: Radiosynthesis and Preclinical Evaluation of an 18F-Labeled FAP Inhibitor. J. Nucl. Med. 2020, 61, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Altmann, A.; Giesel, F.; Kratochwil, C.; Kleist, C.; Krämer, S.; Mier, W.; Cardinale, J.; Kauczor, H.U.; Jäger, D.; et al. 18F-Labeled Tracers Targeting Fibroblast Activation Protein. EJNMMI Radiopharm. Chem. 2021, 6, 26. [Google Scholar] [CrossRef]
- Slania, S.L.; Das, D.; Lisok, A.; Du, Y.; Jiang, Z.; Mease, R.C.; Rowe, S.P.; Nimmagadda, S.; Yang, X.; Pomper, M.G. Imaging of Fibroblast Activation Protein in Cancer Xenografts Using Novel (4-Quinolinoyl)-Glycyl-2-Cyanopyrrolidine-Based Small Molecules. J. Med. Chem. 2021, 64, 4059–4070. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-Head Intra-Individual Comparison of Biodistribution and Tumor Uptake of 68Ga-FAPI and 18F-FDG PET/CT in Cancer Patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4377–4385. [Google Scholar] [CrossRef] [PubMed]
- Wegen, S.; van Heek, L.; Linde, P.; Claus, K.; Akuamoa-Boateng, D.; Baues, C.; Sharma, S.J.; Schomäcker, K.; Fischer, T.; Roth, K.S.; et al. Head-to-Head Comparison of [68Ga]Ga-FAPI-46-PET/CT and [18F]F-FDG-PET/CT for Radiotherapy Planning in Head and Neck Cancer. Mol. Imaging Biol. 2022, 24, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Bal, C. First-In-Human Results on the Biodistribution, Pharmacokinetics, and Dosimetry of [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)2. Pharmaceuticals 2021, 14, 1212. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A Theranostic Approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-Guided [177Lu]Lu-DOTA.SA.FAPi Radionuclide Therapy in an End-Stage Breast Cancer Patient: New Frontier in Targeted Radionuclide Therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 942–944. [Google Scholar] [CrossRef]
- Zboralski, D.; Hoehne, A.; Bredenbeck, A.; Schumann, A.; Nguyen, M.; Schneider, E.; Ungewiss, J.; Paschke, M.; Haase, C.; von Hacht, J.L.; et al. Preclinical Evaluation of FAP-2286 for Fibroblast Activation Protein Targeted Radionuclide Imaging and Therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3651–3667. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Schuchardt, C.; Singh, A.; Chantadisai, M.; Robiller, F.C.; Zhang, J.; Mueller, D.; Eismant, A.; Almaguel, F.; Zboralski, D.; et al. Feasibility, Biodistribution, and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy of Diverse Adenocarcinomas Using 177Lu-FAP-2286: First-in-Humans Results. J. Nucl. Med. 2022, 63, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Hermann, P.; Havlíčková, J.; Kotek, J.; Kubíček, V.; Plutnar, J.; Loktionova, N.; Riss, P.J.; Rösch, F.; Lukeš, I. A Triazacyclononane-Based Bifunctional Phosphinate Ligand for the Preparation of Multimeric 68Ga Tracers for Positron Emission Tomography. Chem.—A Eur. J. 2010, 16, 7174–7185. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Pohle, K.; Wester, H.J. Be Spoilt for Choice with Radiolabelled RGD Peptides: Preclinical Evaluation of 68Ga-TRAP(RGD)3. Nucl. Med. Biol. 2013, 40, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Šimeček, J.; Hermann, P.; Havlíčková, J.; Herdtweck, E.; Kapp, T.G.; Engelbogen, N.; Kessler, H.; Wester, H.-J.; Notni, J. A Cyclen-Based Tetraphosphinate Chelator for the Preparation of Radiolabeled Tetrameric Bioconjugates. Chemistry 2013, 19, 7748–7757. [Google Scholar] [CrossRef]
- Wurzer, A.; Vágner, A.; Horváth, D.; Fellegi, F.; Wester, H.J.; Kálmán, F.K.; Notni, J. Synthesis of Symmetrical Tetrameric Conjugates of the Radiolanthanide Chelator DOTPI for Application in Endoradiotherapy by Means of Click Chemistry. Front. Chem. 2018, 6, 107. [Google Scholar] [CrossRef]
- Zia, N.A.; Cullinane, C.; Van Zuylekom, J.K.; Waldeck, K.; McInnes, L.E.; Buncic, G.; Haskali, M.B.; Roselt, P.D.; Hicks, R.J.; Donnelly, P.S. A Bivalent Inhibitor of Prostate Specific Membrane Antigen Radiolabeled with Copper-64 with High Tumor Uptake and Retention. Angew. Chem. Int. Ed. 2019, 58, 14991–14994. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Niu, B.; Fang, J.; Pang, Y.; Li, S.; Xie, C.; Sun, L.; Zhang, X.; Guo, Z.; Lin, Q.; et al. Synthesis, Preclinical Evaluation, and a Pilot Clinical PET Imaging Study of 68Ga-Labeled FAPI Dimer. J. Nucl. Med. 2022, 63, 862–868. [Google Scholar] [CrossRef]
- Younis, M.H.; Lan, X.; Cai, W. PET with a 68Ga-Labeled FAPI Dimer: Moving towards Theranostics. J. Nucl. Med. 2022, 63, 860–861. [Google Scholar] [CrossRef]
- Galbiati, A.; Zana, A.; Bocci, M.; Millul, J.; Elsayed, A.; Mock, J.; Neri, D.; Cazzamalli, S. A Novel Dimeric FAP-Targeting Small Molecule-Radio Conjugate with High and Prolonged Tumour Uptake. J. Nucl. Med. 2022, 63, 1852–1858. [Google Scholar] [CrossRef]
- Li, H.; Ye, S.; Li, L.; Zhong, J.; Yan, Q.; Zhong, Y.; Feng, P.; Hu, K. 18F- or 177Lu-Labeled Bivalent Ligand of Fibroblast Activation Protein with High Tumor Uptake and Retention. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Roesch, F.; Kumari, S.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Mangu, B.S.; Tupalli, A.; et al. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid 2022, 32, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Eppard, E.; Wuttke, M.; Nicodemus, P.L.; Rösch, F. Ethanol-Based Post-Processing of Generator-Derived 68Ga towards Kit-Type Preparation of 68Ga-Radiopharmaceuticals. J. Nucl. Med. 2014, 55, 1023–1028. [Google Scholar] [CrossRef]
- De Decker, A.; Vliegen, G.; Van Rompaey, D.; Peeraer, A.; Bracke, A.; Verckist, L.; Jansen, K.; Geiss-Friedlander, R.; Augustyns, K.; De Winter, H.; et al. Novel Small Molecule-Derived, Highly Selective Substrates for Fibroblast Activation Protein (FAP). ACS Med. Chem. Lett. 2019, 10, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Van Rymenant, Y.; Tanc, M.; Van Elzen, R.; Bracke, A.; De Wever, O.; Augustyns, K.; Lambeir, A.M.; Kockx, M.; De Meester, I.; Van Der Veken, P. In Vitro and In Situ Activity-Based Labeling of Fibroblast Activation Protein with UAMC1110-Derived Probes. Front. Chem. 2021, 9, 640566. [Google Scholar] [CrossRef] [PubMed]
- De Meester, I.; Vanhoof, G.; Lambeir, A.-M.; Scharpé, S. Use of Immobilized Adenosine Deaminase (EC 3.5.4.4) for the Rapid Purification of Native Human CD26/Dipeptidyl Peptidase IV (EC 3.4.14.5). J. Immunol. Methods 1996, 189, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Benramdane, S.; De Loose, J.; Beyens, O.; Van Rymenant, Y.; Vliegen, G.; Augustyns, K.; De Winter, H.; De Meester, I.; der Veken, P. Vildagliptin-Derived Dipeptidyl Peptidase 9 (DPP9) Inhibitors: Identification of a DPP8/9-Specific Lead. ChemMedChem 2022, 17, e202200097. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Tripathi, M.; ArunRaj, S.T.; Sarswat, S.; Bal, C. Biodistribution, Pharmacokinetics, Dosimetry of [68Ga]Ga-DOTA.SA.FAPi, and the Head-to-Head Comparison with [18F]F-FDG PET/CT in Patients with Various Cancers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1915–1931. [Google Scholar] [CrossRef]
- Kelly, J.; Amor-Coarasa, A.; Sweeney, E.; Wilson, J.; Causey, P.; Babich, J. A Consensus Time for Performing Quality Control of 225Ac-Labeled Radiopharmaceuticals. 2021. Available online: https://assets.researchsquare.com/files/rs-39342/v2_covered.pdf?c=1631863217 (accessed on 21 December 2022).
- Aime, S.; Barge, A.; Botta, M.; Fasano, M.; Ayala, J.D.; Bombieri, G. Crystal Structure and Solution Dynamics of the Lutetium(III) Chelate of DOTA. Inorg. Chim. Acta 1996, 246, 423–429. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholomä, M.; Ezziddin, S. 225Ac-PSMA-617/177Lu-PSMA-617 Tandem Therapy of Metastatic Castration-Resistant Prostate Cancer: Pilot Experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, H.; Gosewisch, A.; Böning, G.; Völter, F.; Zacherl, M.; Unterrainer, M.; Bartenstein, P.; Todica, A.; Gildehaus, F.J. Response to 225Ac-PSMA-I&T after Failure of Long-Term 177Lu-PSMA RLT in MCRPC. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1262–1263. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening Horizons with 225Ac-DOTATATE Targeted Alpha Therapy for Gastroenteropancreatic Neuroendocrine Tumour Patients Stable or Refractory to 177Lu-DOTATATE PRRT: First Clinical Experience on the Efficacy and Safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: 64Cu- And 225Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef]
- Thiele, N.A.; Wilson, J.J. Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm. 2018, 33, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.K.H.; Ramogida, C.F.; Schaffer, P.; Radchenko, V. Development of 225Ac Radiopharmaceuticals: TRIUMF Perspectives and Experiences. Curr. Radiopharm. 2018, 11, 156–172. [Google Scholar] [CrossRef]
- Ansems, M.; Span, P.N. The Tumor Microenvironment and Radiotherapy Response; a Central Role for Cancer-Associated Fibroblasts. Clin. Transl. Radiat. Oncol. 2020, 22, 90–97. [Google Scholar] [CrossRef]
- Domogauer, J.D.; de Toledo, S.M.; Howell, R.W.; Azzam, E.I. Acquired Radioresistance in Cancer Associated Fibroblasts Is Concomitant with Enhanced Antioxidant Potential and DNA Repair Capacity. Cell Commun. Signal. 2021, 19, 30. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Tan, Y.; Wei, Q.; Yu, W. Cancer-Associated Fibroblasts in Radiotherapy: Challenges and New Opportunities. Cell Commun. Signal. 2019, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Cirri, P.; Chiarugi, P. Cancer Associated Fibroblasts: The Dark Side of the Coin. Am. J. Cancer Res. 2011, 1, 482–497. [Google Scholar] [PubMed]
- De Veirman, K.; Rao, L.; De Bruyne, E.; Menu, E.; Van Valckenborgh, E.; Van Riet, I.; Frassanito, M.A.; Di Marzo, L.; Vacca, A.; Vanderkerken, K. Cancer Associated Fibroblasts and Tumor Growth: Focus on Multiple Myeloma. Cancers 2014, 6, 1363–1381. [Google Scholar] [CrossRef] [PubMed]


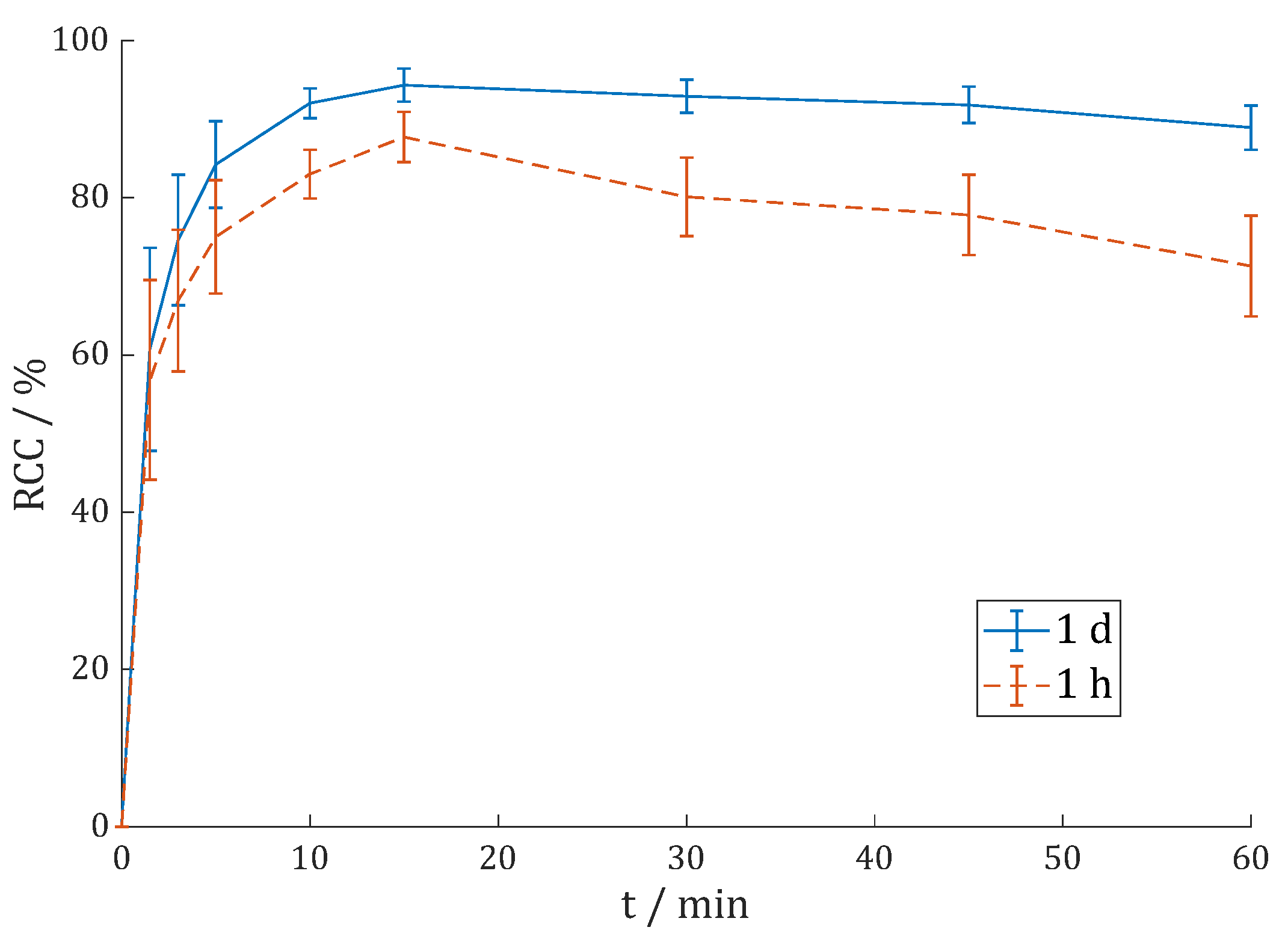
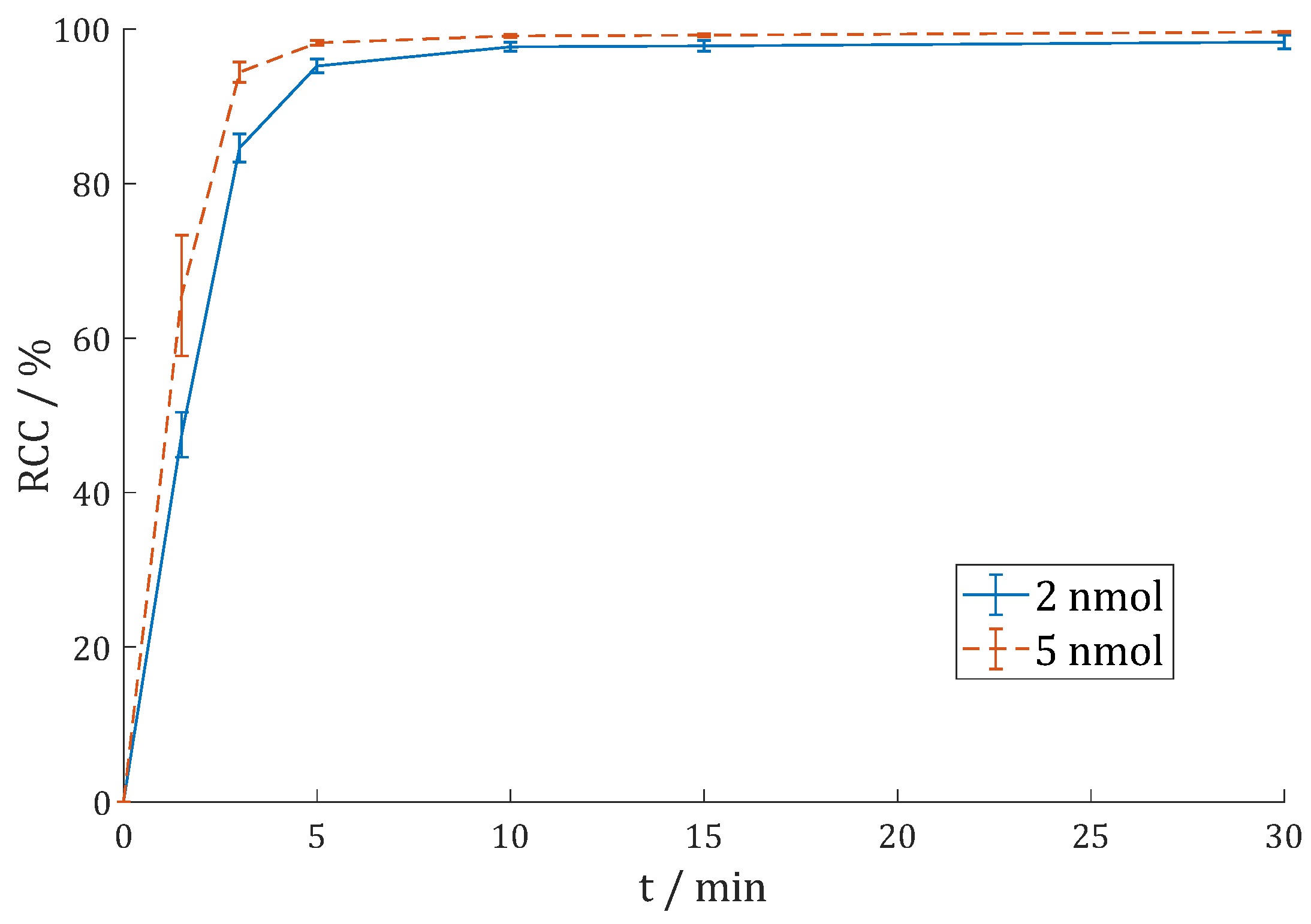

| RCC/% | 1 h (Citrate) | 1 d (Citrate) | 1 d (AmOAc/MeOH) | 1 h (γ) |
|---|---|---|---|---|
| after 15 min | 87.7 ± 3.2% | 94.3 ± 2.1% | - | - |
| after 60 min | 71.3 ± 6.4% | 88.9 ± 2.8% | 90.8 ± 2.3% | 94.8 ± 2.5% |
| after C18 | 93.9% | 98.1% | 97.9% | 100% |
| Complex | logD7.4 |
|---|---|
| [68Ga]Ga-DOTA.SA.FAPi [29] | −2.68 ± 0.06 |
| [68Ga]Ga-DOTAGA.(SA.FAPi)2 [31] | −2.02 ± 0.06 |
| [68Ga]Ga-DOTAGA.Glu.(FAPi)2 (68Ga-7) | −2.48 ± 0.05 |
| [177Lu]Lu-DOTAGA.Glu.(FAPi)2 (177Lu-7) | −2.77 ± 0.10 |
| [68Ga]Ga-DO3A.Glu.(FAPi)2 (68Ga-11) | −2.08 ± 0.07 |
| [177Lu]Lu-DO3A.Glu.(FAPi)2 (177Lu-11) | −1.77 ± 0.10 |
| Compound | DOTAGA.Glu.(FAPi)2 (7) | [natLu]Lu-DOTAGA.Glu.(FAPi)2 (natLu-7) | DO3A.Glu.(FAPi)2 (11) | DOTAGA.(SA.FAPi)2 [31] | [natLu]Lu- DOTAGA.(SA.FAPi)2 [31] | UAMC-1110 [24,31] |
|---|---|---|---|---|---|---|
| IC50(FAP)/nM | 0.26 ± 0.04 | 0.33 ± 0.02 | 0.60 ± 0.04 | 0.92 ± 0.06 | 1.54 ± 0.15 | 0.43 ± 0.02 |
| IC50(PREP)/µM | 0.59 ± 0.10 | 0.43 ± 0.16 | 1.00 ± 0.14 | 0.39 ± 0.02 | 0.56 ± 0.04 | 1.80 ± 0.01 |
| IC50(DPP4)/µM | 1.19 ± 0.08 | 0.65 ± 0.04 | 0.54 ± 0.06 | 0.40 ± 0.07 | 0.63 ± 0.07 | >10 |
| IC50(DPP8)/µM | 0.029 ± 0.004 | 0.22 ± 0.02 | 1.03 ± 0.18 | 0.42 ± 0.04 | 0.41 ± 0.03 | >10 |
| IC50(DPP9)/µM | 0.083 ± 0.0015 | 0.19 ± 0.01 | 0.95 ± 0.11 | 0.16 ± 0.02 | 0.18 ± 0.02 | 4.70 ± 0.40 |
| SI(PREP/FAP) | 2269 | 1292 | 1667 | 424 | 364 | 4186 |
| SI(DPP4/FAP) | 4577 | 1967 | 900 | 435 | 409 | 23,256 |
| SI(DPP8/FAP) | 112 | 661 | 1717 | 456 | 266 | 23,256 |
| SI(DPP9/FAP) | 319 | 557 | 1583 | 174 | 117 | 10,930 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, M.; Ballal, S.; Yadav, M.P.; Bal, C.; Van Rymenant, Y.; De Loose, J.; Verhulst, E.; De Meester, I.; Van Der Veken, P.; Roesch, F. Novel Generation of FAP Inhibitor-Based Homodimers for Improved Application in Radiotheranostics. Cancers 2023, 15, 1889. https://doi.org/10.3390/cancers15061889
Martin M, Ballal S, Yadav MP, Bal C, Van Rymenant Y, De Loose J, Verhulst E, De Meester I, Van Der Veken P, Roesch F. Novel Generation of FAP Inhibitor-Based Homodimers for Improved Application in Radiotheranostics. Cancers. 2023; 15(6):1889. https://doi.org/10.3390/cancers15061889
Chicago/Turabian StyleMartin, Marcel, Sanjana Ballal, Madhav Prasad Yadav, Chandrasekhar Bal, Yentl Van Rymenant, Joni De Loose, Emile Verhulst, Ingrid De Meester, Pieter Van Der Veken, and Frank Roesch. 2023. "Novel Generation of FAP Inhibitor-Based Homodimers for Improved Application in Radiotheranostics" Cancers 15, no. 6: 1889. https://doi.org/10.3390/cancers15061889
APA StyleMartin, M., Ballal, S., Yadav, M. P., Bal, C., Van Rymenant, Y., De Loose, J., Verhulst, E., De Meester, I., Van Der Veken, P., & Roesch, F. (2023). Novel Generation of FAP Inhibitor-Based Homodimers for Improved Application in Radiotheranostics. Cancers, 15(6), 1889. https://doi.org/10.3390/cancers15061889





