The Promise of Nanoparticles-Based Radiotherapy in Cancer Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Combinational Use of RT and Chemotherapy
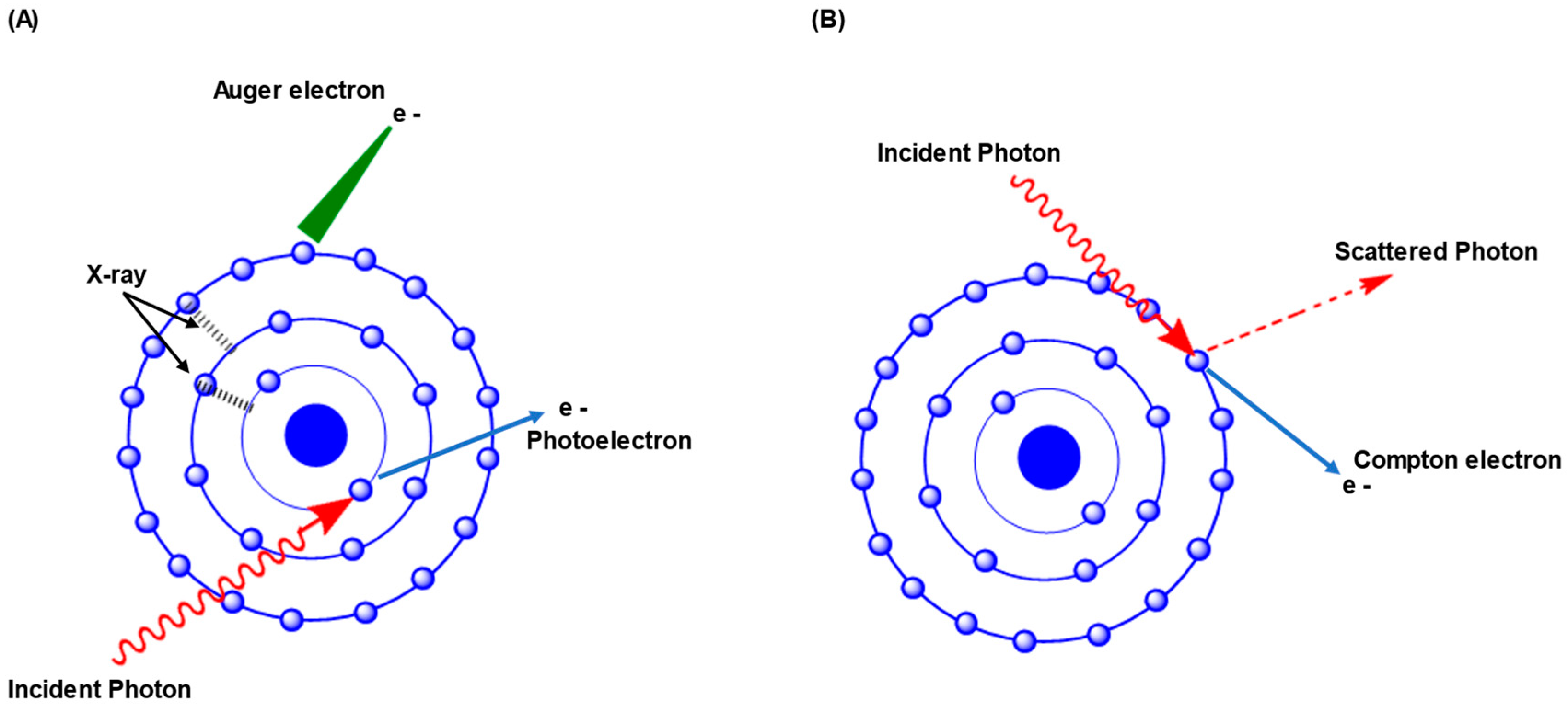
3. How Radiation Reacts with Radiosensitizers of High Z Materials
4. Biological Response of NPs-Based RT
4.1. DNA Damage
4.2. Reactive Oxygen Species (ROS)
4.3. Tumor Microenvironment (TME)
4.4. Targeting the Cell Cycle
| Nanoparticle Formulation | Test Animal Model | Animal Age | Cancer Type | Cell Line | Tumor Growth Delay Compared to Control Group | Concentration | Radiation Dose | Observations | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | Nanoparticle | Nanoparticle + RT | Statistical Significance Method | |||||||||
| KU55933 | Nu/Nu mice | 6–8 weeks | NSCL | H460 | Significant | Significant | Significant | Response features analysis method | 500 mg/kg | 15 Gy | Impedes the repair process of DNA double-stranded breaks | [82] |
| A549 | Significant | Significant | Significant | |||||||||
| Dbait@H1 NPs | Male nude mice | 4–6 weeks | PCa | PC-3 | Significant p = 0.032 | NR | Significant p = 0.004 | Two-sample t-tests and analysis of variance | 60 μg/kg | 9 Gy | Signaling pathways for repairing DNA damage are inhibited by Dbait@H1 NPs, which are competitive inhibitors of DSB. | [71] |
| 22Rv1 | Significant p = 0.001 | NR | Significant p < 0.001 | |||||||||
| Cat@PLGA_R837 | Balb/c mice | NR | CRC | CT-26 | No appreciable inhibition of tumor growth | NR | Significantly suppresses tumor growth | One-way ANOVA using the Tukey’s post-test | Cat = 0.5 mg/kg; R837 = 0.6 mg/kg | 8 Gy | Decomposes H2O2 to produce O2 in the tumor microenvironment. | [120] |
| IPI549@HMP | Balb/c mice | 6–8 weeks | CRC | Luc+ CT26 cells | Significant | Moderately significant | Significant | Student’s two-tailed unpaired t-test | MnO2 = 7.5 mg/kg; IPI549 = 1.5 mg/kg | 6 Gy | Myeloid cells are selectively targeted by IPI549@HMP and decompose endogenous H2O2 to O2 upon X-ray irradiation | [121] |
| AuNPs | Balb/C mice | NR | MC | EMT-6 | Non-significant | Significantly delayed tumor growth but did not reduce tumor volume significantly | Significantly decreased tumor growth and tumor volume | Wilcoxon’s non-parametric two-sample rank-sum | 1.35 g/kg | 30 Gy | AuNPs, being high-Z nanoparticles, preferentially absorb X-rays and subsequently reduce tumor. | [122] |
| NPs-lncAFAP1-AS1 siRNA | BALB/c normal mice and nude mice | 4–5 weeks | BC | MDA-MBA-231R | Significantly decreased tumor volume p < 0.001 | Significantly decreased tumor volume p < 0.01 | Significantly decreased tumor volume p < 0.001 | Student’s unpaired two-sided t-test and one-way ANOVA | 1 nmol siRNA | 10 Gy | Tumor growth is reduced due to blockage of the Wnt/β Catenin signaling pathway and scavenging intracellular GSH. | [123] |
| Au@Tat-R-EK NPs | BALB/c mice | 6–8 weeks | LC | LM3 | Moderately significant | Not Significant | Significant | One-way analysis of variance (ANOVA) in Origin software | 25 mg/kg | 6 Gy | Au@Tat-R-EK NPs respond to overexpressed cathepsin B in the tumor microenvironment that induces site-specific enhancement of tumor cell uptake and afterward damages DNA effectively upon X-ray irradiation. | [124] |
| AuNP@CRZ | Nude mice | 10–11 weeks | ACC of the salivary gland | ACC | Impeded tumor growth (p < 0.001) but did not significantly reduce tumor volume to less than its size at T0 (p > 0.05) | Significantly decreased tumor volume (p < 0.001) and caused a reduction in tumor volume (p < 0.001). | Decreased tumor volume compared to previously (p < 0.001), and it caused significant shrinkage of the tumor to near disappearance (p = 0.007). | Unpaired two-sided t-test | 2 mg/kg | 18 Gy | Tumor-cell repair mechanism is reduced | [125] |
| Lu–Au- NLS-RGD-anti-VEGF aptamer | Athymic male mice | 6–7 weeks | MG | U87MG | Tumor size progression was significantly slower (p < 0.05) | Tumor size progression was significantly slower (p < 0.05) | Tumor size progression was significantly slower (p < 0.05) | ANOVA | 75.19 | 80 Gy | Tumor development is inhibited by halting the formation of new blood vessels | [126] |
| Doc-NPs | BALB/c mice | 4–5 weeks | GC | BGC823 | Not significant in tumor doubling time | Not significant in tumor doubling time | Significant effect on tumor doubling time | t-test | 5 mg/kg | 15 Gy | Doc-NPs cause cell cycle arrest in G2-M phase whereas irradiation leads to ROS generation which induce DNA damage | [127] |
| AuNPs | C3H/HeJ mice | 8–10 weeks | SCC | SCCVII | Time for doubling of tumor volume and survival time increased significantly (p < 0.04) | Time for doubling of tumor volume and survival time increased by 23 days and 37%, respectively | Time for doubling of tumor volume and survival time increased most significantly (p < 0.04) | 2-sided Z-statistics test | 1.9 g/kg | 42 Gy | AuNPs boost the radiation treatment in a radioresistant squamous cell carcinoma | [128] |
| AuNPs | Syngeneic black B6C3f1 mice | NR | MBT | Tu-2449 | No abatement of tumor growth | 18% long-term survival | 56% long-term survival | Log-rank (Mantel–Cox) survival analysis test with 95% CIs using GraphPad Prism® software | 4 g/kg | 35 Gy | AuNPs combined with radiation cause about 53% (>1 year) tumor-free survival | [129] |
| NP@PVP + B + Pt | BALB/c mice | 5–6 weeks | BC | EMT-6 | Tumor growth was inhibited by 45.4% | Tumor growth was inhibited by 51.9% | Tumor growth was inhibited by 91.2% | Student’s t-test | 4 mg/kg | 5 Gy | The bismuth in NP@PVP + B + Pt functions as a radiosensitizer and increases the production of ROS, which damage DNA under X-ray irradiation | [92] |
5. Uptake and Excretion of NPs during RT
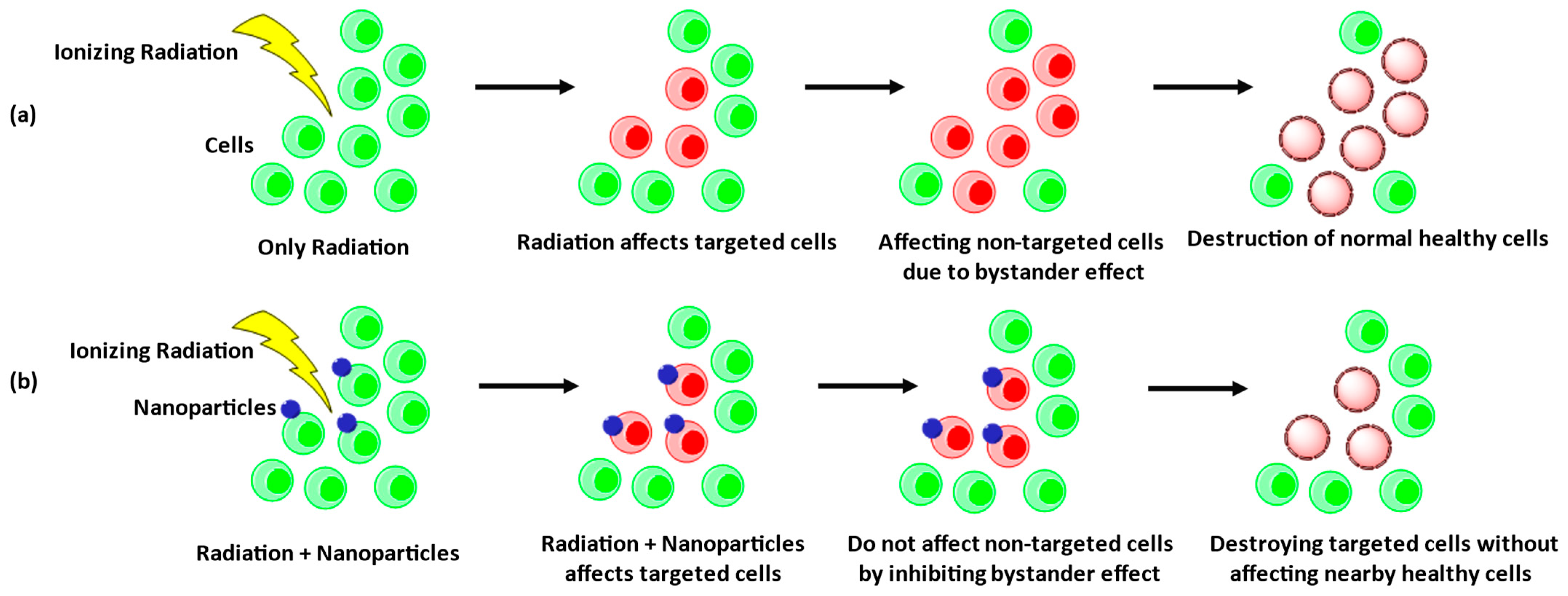
6. Radiation-Induced Bystander Effects
7. The Effects of NPs-Based RT in Cancer Stem Cells
8. NPs-Based RT Improving Phototherapy
9. NPs-Based RT to Overcome Radioresistance and Drug Resistance
10. Clinical Trials
11. Limitations and Future Perspective of NPs-Based RT
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gaidai, O.; Yan, P.; Xing, Y. Future world cancer death rate prediction. Sci. Rep. 2023, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; DeLancey, J.O.; Center, M.M.; Jemal, A.; Ward, E.M. The global burden of cancer: Priorities for prevention. Carcinogenesis 2010, 31, 100–110. [Google Scholar] [CrossRef]
- Shakil, M.S.; Mahmud, K.M.; Sayem, M.; Niloy, M.S.; Halder, S.K.; Hossen, M.S.; Uddin, M.F.; Hasan, M.A. Using Chitosan or Chitosan Derivatives in Cancer Therapy. Polysaccharides 2021, 2, 795–816. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Reviews Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Leroi, N.; Lallemand, F.; Coucke, P.; Noel, A.; Martinive, P. Impacts of Ionizing Radiation on the Different Compartments of the Tumor Microenvironment. Front. Pharmacol. 2016, 7, 78. [Google Scholar] [CrossRef]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Adv. Mater. 2017, 29, 1700996. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Li, F.; Jin, X.; Li, J.M.; Chen, W.; Li, Q.J.T. Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and Synergistic Effects on Tumor Cells. Theranostics 2018, 8, 1824–1849. [Google Scholar] [CrossRef]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.W.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.B.; Jacob, S.; Shafiq, J.; Wong, K.; Thompson, S.R.; Hanna, T.P.; Delaney, G.P. Estimating the demand for radiotherapy from the evidence: A review of changes from 2003 to 2012. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 112, 140–144. [Google Scholar] [CrossRef]
- Morrison, R.; Schleicher, S.M.; Sun, Y.; Niermann, K.J.; Kim, S.; Spratt, D.E.; Chung, C.H.; Lu, B. Targeting the Mechanisms of Resistance to Chemotherapy and Radiotherapy with the Cancer Stem Cell Hypothesis. J. Oncol. 2011, 2011, 941876. [Google Scholar] [CrossRef]
- Nagi, N.M.S.; Khair, Y.A.M.; Abdalla, A.M.E. Capacity of gold nanoparticles in cancer radiotherapy. Jpn. J. Radiol. 2017, 35, 555–561. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef]
- Dörr, W.; Hamilton, C.S.; Boyd, T.; Reed, B.; Denham, J.W. Radiation-induced changes in cellularity and proliferation in human oral mucosa. Int. J. Radiat. Oncol. 2002, 52, 911–917. [Google Scholar] [CrossRef]
- Adams, M.J.; Hardenbergh, P.H.; Constine, L.S.; Lipshultz, S.E. Radiation-associated cardiovascular disease. Rev. Oncol./Hematol. 2003, 45, 55–75. [Google Scholar] [CrossRef]
- Nakajima, T.; Ninomiya, Y.; Nenoi, M. Radiation-Induced Reactions in the Liver—Modulation of Radiation Effects by Lifestyle-Related Factors. Int. J. Mol. Sci. 2018, 19, 3855. [Google Scholar] [CrossRef] [PubMed]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-induced kidney toxicity: Molecular and cellular pathogenesis. Radiat. Oncol. 2021, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Boerma, M.; Sridharan, V.; Mao, X.-W.; Nelson, G.A.; Cheema, A.K.; Koturbash, I.; Singh, S.P.; Tackett, A.J.; Hauer-Jensen, M. Effects of ionizing radiation on the heart. Mutat. Res./Rev. Mutat. Res. 2016, 770, 319–327. [Google Scholar] [CrossRef]
- Xie, D.; Wang, M.P.; Qi, W.H. A simplified model to calculate the surface-to-volume atomic ratio dependent cohesive energy of nanocrystals. J. Phys. Condens. Matter 2004, 16, L401. [Google Scholar] [CrossRef]
- Mahmud, K.M.; Hossain, M.M.; Polash, S.A.; Takikawa, M.; Shakil, M.S.; Uddin, M.F.; Alam, M.; Ali Khan Shawan, M.M.; Saha, T.; Takeoka, S.; et al. Investigation of Antimicrobial Activity and Biocompatibility of Biogenic Silver Nanoparticles Synthesized using Syzigyum cymosum Extract. ACS Omega 2022, 7, 27216–27229. [Google Scholar] [CrossRef]
- Pallares, R.M.; Choo, P.; Cole, L.E.; Mirkin, C.A.; Lee, A.; Odom, T.W. Manipulating Immune Activation of Macrophages by Tuning the Oligonucleotide Composition of Gold Nanoparticles. Bioconjugate Chem. 2019, 30, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.; Westlake, M.; Li, N.; Vogel, S.; Gobert, Q.; Thorpe, N.; Rosenfeld, A.; Lerch, M.; Corde, S.; Tehei, M. Thulium Oxide Nanoparticles: A new candidate for image-guided radiotherapy. Biomed. Phys. Eng. Express 2018, 4, 044001. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Sedlmeier, A.; Gorris, H.H. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev. 2015, 44, 1526–1560. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2010, 60, 977–985. [Google Scholar] [CrossRef]
- Roeske, J.C.; Nunez, L.; Hoggarth, M.; Labay, E.; Weichselbaum, R.R. Characterization of the theorectical radiation dose enhancement from nanoparticles. Technol. Cancer Res. Treat. 2007, 6, 395–401. [Google Scholar] [CrossRef]
- Wu, P.H.; Onodera, Y.; Ichikawa, Y.; Rankin, E.B.; Giaccia, A.J.; Watanabe, Y.; Qian, W.; Hashimoto, T.; Shirato, H.; Nam, J.M. Targeting integrins with RGD-conjugated gold nanoparticles in radiotherapy decreases the invasive activity of breast cancer cells. Int. J. Nanomed. 2017, 12, 5069–5085. [Google Scholar] [CrossRef]
- Du, F.; Lou, J.; Jiang, R.; Fang, Z.; Zhao, X.; Niu, Y.; Zou, S.; Zhang, M.; Gong, A.; Wu, C. Hyaluronic acid-functionalized bismuth oxide nanoparticles for computed tomography imaging-guided radiotherapy of tumor. Int. J. Nanomed. 2017, 12, 5973–5992. [Google Scholar] [CrossRef]
- Sykes, E.A.; Dai, Q.; Sarsons, C.D.; Chen, J.; Rocheleau, J.V.; Hwang, D.M.; Zheng, G.; Cramb, D.T.; Rinker, K.D.; Chan, W.C.W. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc. Natl. Acad. Sci. USA 2016, 113, E1142–E1151. [Google Scholar] [CrossRef]
- Bennie, L.A.; McCarthy, H.O.; Coulter, J.A. Enhanced nanoparticle delivery exploiting tumour-responsive formulations. Cancer Nanotechnol. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Hong, C.R.; Wong, W.W.; Liew, L.P.; Shome, A.; Wang, J.; Gu, Y.; Stevenson, R.J.; Qi, W.; Anderson, R.F.; et al. Next-Generation Hypoxic Cell Radiosensitizers: Nitroimidazole Alkylsulfonamides. J. Med. Chem. 2018, 61, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Gal, O.; Betzer, O.; Rousso-Noori, L.; Sadan, T.; Motiei, M.; Nikitin, M.; Friedmann-Morvinski, D.; Popovtzer, R.; Popovtzer, A. Antibody Delivery into the Brain by Radiosensitizer Nanoparticles for Targeted Glioblastoma Therapy. J. Nanotheranostics 2022, 3, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.W.; Tsvankin, V.; Shrock, Z.; Meng, B.; Zhang, X.; Dewhirst, M.; Fecci, P.; Adamson, J.; Oldham, M. Enhancing Radiation Therapy Through Cherenkov Light-Activated Phototherapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 794–801. [Google Scholar] [CrossRef]
- Cao, W.; Gu, Y.; Meineck, M.; Xu, H. The combination of chemotherapy and radiotherapy towards more efficient drug delivery. Chem.–Asian J. 2014, 9, 48–57. [Google Scholar] [CrossRef]
- Laprise-Pelletier, M.; Simão, T.; Fortin, M.-A. Gold Nanoparticles in Radiotherapy and Recent Progress in Nanobrachytherapy. Adv. Healthc. Mater. 2018, 7, 1701460. [Google Scholar] [CrossRef]
- McMahon, S.J.; Paganetti, H.; Prise, K.M. Optimising element choice for nanoparticle radiosensitisers. Nanoscale 2016, 8, 581–589. [Google Scholar] [CrossRef]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy-a review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02TR01. [Google Scholar] [CrossRef] [PubMed]
- Rancoule, C.; Magné, N.; Vallard, A.; Guy, J.-B.; Rodriguez-Lafrasse, C.; Deutsch, E.; Chargari, C. Nanoparticles in radiation oncology: From bench-side to bedside. Cancer Lett. 2016, 375, 256–262. [Google Scholar] [CrossRef]
- Choi, J.; Kim, G.; Cho, S.B.; Im, H.-J. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. J. Nanobiotechnology 2020, 18, 122. [Google Scholar] [CrossRef]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for Radiation Therapy Enhancement: The Key Parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef]
- Cordelli, E.; Eleuteri, P.; Grollino, M.G.; Benassi, B.; Blandino, G.; Bartoleschi, C.; Pardini, M.C.; Di Caprio, E.V.; Spanò, M.; Pacchierotti, F.; et al. Direct and delayed X-ray-induced DNA damage in male mouse germ cells. Environ. Mol. Mutagen. 2012, 53, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Kotb, O.M.; Brozhik, D.S.; Verbenko, V.N.; Gulevich, E.P.; Ezhov, V.F.; Karlin, D.L.; Pak, F.A.; Paston, S.V.; Polyanichko, A.M.; Khalikov, A.I.; et al. Investigation of DNA Damage Induced by Proton and Gamma Radiation. Biophysics 2021, 66, 202–208. [Google Scholar] [CrossRef]
- Carter, R.J.; Nickson, C.M.; Thompson, J.M.; Kacperek, A.; Hill, M.A.; Parsons, J.L. Complex DNA Damage Induced by High Linear Energy Transfer Alpha-Particles and Protons Triggers a Specific Cellular DNA Damage Response. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 776–784. [Google Scholar] [CrossRef]
- Ui, A.; Chiba, N.; Yasui, A. Relationship among DNA double-strand break (DSB), DSB repair, and transcription prevents genome instability and cancer. Cancer Sci. 2020, 111, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, D. Repair pathway choice for double-strand breaks. Essays Biochem. 2020, 64, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. Genome maintenance mechanisms for preventing cancer. Nature 2001, 411, 366–374. [Google Scholar] [CrossRef]
- Menolfi, D.; Zha, S. ATM, ATR and DNA-PKcs kinases—The lessons from the mouse models: Inhibition ≠ deletion. Cell Biosci. 2020, 10, 8. [Google Scholar] [CrossRef]
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef]
- Iliakis, G.; Wang, H.; Perrault, A.R.; Boecker, W.; Rosidi, B.; Windhofer, F.; Wu, W.; Guan, J.; Terzoudi, G.; Pantelias, G. Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet. Genome Res. 2004, 104, 14–20. [Google Scholar] [CrossRef]
- Elbakry, A.; Löbrich, M. Homologous Recombination Subpathways: A Tangle to Resolve. Front. Genet. 2021, 12, 723847. [Google Scholar] [CrossRef]
- Khanna, K.K.; Jackson, S.P. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Li, L.-Y.; Guan, Y.-D.; Chen, X.-S.; Yang, J.-M.; Cheng, Y. DNA Repair Pathways in Cancer Therapy and Resistance. Front. Pharmacol. 2021, 11, 629266. [Google Scholar] [CrossRef]
- Alhmoud, J.F.; Woolley, J.F.; Al Moustafa, A.-E.; Malki, M.I. DNA Damage/Repair Management in Cancers. Cancers 2020, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Andrs, M.; Korabecny, J.; Jun, D.; Hodny, Z.; Bartek, J.; Kuca, K. Phosphatidylinositol 3-Kinase (PI3K) and Phosphatidylinositol 3-Kinase-Related Kinase (PIKK) Inhibitors: Importance of the Morpholine Ring. J. Med. Chem. 2015, 58, 41–71. [Google Scholar] [CrossRef]
- Curtin, N.J. Inhibiting the DNA damage response as a therapeutic manoeuvre in cancer. Br. J. Pharmacol. 2013, 169, 1745–1765. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Asaithamby, A. Repurposing DNA repair factors to eradicate tumor cells upon radiotherapy. Transl. Cancer Res. 2017, 6, S822–S839. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Reviews Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Mahmud, K.M.; Niloy, M.S.; Shakil, M.S.; Islam, M.A. Ruthenium Complexes: An Alternative to Platinum Drugs in Colorectal Cancer Treatment. Pharmaceutics 2021, 13, 1295. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, N.; Shao, Z.; Qiu, H.; Yao, H.; Ji, J.; Wang, J.; Lu, W.; Chen, R.C.; Zhang, L. Folate-targeted nanoparticle delivery of androgen receptor shRNA enhances the sensitivity of hormone-independent prostate cancer to radiotherapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1309–1321. [Google Scholar] [CrossRef]
- Löbrich, M.; Shibata, A.; Beucher, A.; Fisher, A.; Ensminger, M.; Goodarzi, A.A.; Barton, O.; Jeggo, P.A. gammaH2AX foci analysis for monitoring DNA double-strand break repair: Strengths, limitations and optimization. Cell Cycle 2010, 9, 662–669. [Google Scholar] [CrossRef]
- Olive, P.L. Detection of DNA damage in individual cells by analysis of histone H2AX phosphorylation. Methods Cell Biol. 2004, 75, 355–373. [Google Scholar]
- Yao, H.; Qiu, H.; Shao, Z.; Wang, G.; Wang, J.; Yao, Y.; Xin, Y.; Zhou, M.; Wang, A.Z.; Zhang, L. Nanoparticle formulation of small DNA molecules, Dbait, improves the sensitivity of hormone-independent prostate cancer to radiotherapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2261–2271. [Google Scholar] [CrossRef]
- Xia, W.; Low, P.S. Folate-targeted therapies for cancer. J. Med. Chem. 2010, 53, 6811–6824. [Google Scholar] [CrossRef]
- Quanz, M.; Berthault, N.; Roulin, C.; Roy, M.; Herbette, A.; Agrario, C.; Alberti, C.; Josserand, V.; Coll, J.L.; Sastre-Garau, X.; et al. Small-molecule drugs mimicking DNA damage: A new strategy for sensitizing tumors to radiotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 1308–1316. [Google Scholar] [CrossRef]
- Quanz, M.; Chassoux, D.; Berthault, N.; Agrario, C.; Sun, J.S.; Dutreix, M. Hyperactivation of DNA-PK by double-strand break mimicking molecules disorganizes DNA damage response. PLoS ONE 2009, 4, e6298. [Google Scholar] [CrossRef] [PubMed]
- Biau, J.; Devun, F.; Jdey, W.; Kotula, E.; Quanz, M.; Chautard, E.; Sayarath, M.; Sun, J.S.; Verrelle, P.; Dutreix, M. A preclinical study combining the DNA repair inhibitor Dbait with radiotherapy for the treatment of melanoma. Neoplasia 2014, 16, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Devun, F.; Biau, J.; Huerre, M.; Croset, A.; Sun, J.S.; Denys, A.; Dutreix, M. Colorectal cancer metastasis: The DNA repair inhibitor Dbait increases sensitivity to hyperthermia and improves efficacy of radiofrequency ablation. Radiology 2014, 270, 736–746. [Google Scholar] [CrossRef]
- Herath, N.I.; Devun, F.; Lienafa, M.C.; Herbette, A.; Denys, A.; Sun, J.S.; Dutreix, M. The DNA Repair Inhibitor DT01 as a Novel Therapeutic Strategy for Chemosensitization of Colorectal Liver Metastasis. Mol. Cancer Ther. 2016, 15, 15–22. [Google Scholar] [CrossRef]
- Chithrani, D.B.; Jelveh, S.; Jalali, F.; van Prooijen, M.; Allen, C.; Bristow, R.G.; Hill, R.P.; Jaffray, D.A. Gold Nanoparticles as Radiation Sensitizers in Cancer Therapy. J. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Joh, D.Y.; Sun, L.; Stangl, M.; Al Zaki, A.; Murty, S.; Santoiemma, P.P.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Bhang, D.; et al. Selective Targeting of Brain Tumors with Gold Nanoparticle-Induced Radiosensitization. PLoS ONE 2013, 8, e62425. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Yang, H.; Wei, J.; Tong, J.-l.; Shu, Y.-q. The role and mechanisms of nanoparticles to enhance radiosensitivity in hepatocellular cell. Biomed. Pharmacother. 2013, 67, 569–575. [Google Scholar] [CrossRef]
- Chen, N.; Yang, W.; Bao, Y.; Xu, H.; Qin, S.; Tu, Y. BSA capped Au nanoparticle as an efficient sensitizer for glioblastoma tumor radiation therapy. RSC Adv. 2015, 5, 40514–40520. [Google Scholar] [CrossRef]
- Tian, X.; Lara, H.; Wagner, K.T.; Saripalli, S.; Hyder, S.N.; Foote, M.; Sethi, M.; Wang, E.; Caster, J.M.; Zhang, L.; et al. Improving DNA double-strand repair inhibitor KU55933 therapeutic index in cancer radiotherapy using nanoparticle drug delivery. Nanoscale 2015, 7, 20211–20219. [Google Scholar] [CrossRef]
- Porosnicu, I.; Butnaru, C.M.; Tiseanu, I.; Stancu, E.; Munteanu, C.V.A.; Bita, B.I.; Duliu, O.G.; Sima, F. Y2O3 Nanoparticles and X-ray Radiation-Induced Effects in Melanoma Cells. Molecules 2021, 26, 3403. [Google Scholar] [CrossRef]
- Hasegawa, T.; Takahashi, J.; Nagasawa, S.; Doi, M.; Moriyama, A.; Iwahashi, H. DNA Strand Break Properties of Protoporphyrin IX by X-Ray Irradiation against Melanoma. Int. J. Mol. Sci. 2020, 21, 2302. [Google Scholar] [CrossRef]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Ni, D.; Cai, W. Harnessing the Power of Nanotechnology for Enhanced Radiation Therapy. ACS Nano 2017, 11, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, J.; Sun, X. Reactive Oxygen Species-Based Nanomaterials for Cancer Therapy. Front. Chem. 2021, 9, 650587. [Google Scholar] [CrossRef]
- Xie, A.; Li, H.; Hao, Y.; Zhang, Y. Tuning the Toxicity of Reactive Oxygen Species into Advanced Tumor Therapy. Nanoscale Res. Lett. 2021, 16, 142. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G.J.B. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Manna, P.P. Reactive oxygen species in cancer progression and its role in therapeutics. Explor. Med. 2022, 3, 43–57. [Google Scholar] [CrossRef]
- Yong, Y.; Zhang, C.; Gu, Z.; Du, J.; Guo, Z.; Dong, X.; Xie, J.; Zhang, G.; Liu, X.; Zhao, Y. Polyoxometalate-Based Radiosensitization Platform for Treating Hypoxic Tumors by Attenuating Radioresistance and Enhancing Radiation Response. ACS Nano 2017, 11, 7164–7176. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-C.; Tang, X.-F.; Xu, Y.-C.; Jiang, W.; Xin, Y.-J.; Zhao, W.; He, X.; Lu, L.-G.; Zhan, M.-X. Nano-enabled coordination platform of bismuth nitrate and cisplatin prodrug potentiates cancer chemoradiotherapy via DNA damage enhancement. Biomater. Sci. 2021, 9, 3401–3409. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.J.; Jung, K.O.; Graves, E.E.; Pratx, G. A gold nanoparticle system for the enhancement of radiotherapy and simultaneous monitoring of reactive-oxygen-species formation. Nanotechnology 2018, 29, 504001. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.; Li, N.; Wang, J.; Zhang, X.; Pan, W.; Yu, L.; Tang, B. Enhancement of mitochondrial ROS accumulation and radiotherapeutic efficacy using a Gd-doped titania nanosensitizer. Theranostics 2019, 9, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New horizons in tumor microenvironment biology: Challenges and opportunities. BMC Med. 2015, 13, 45. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D.J.C. Reactive oxygen species in the tumor microenvironment: An overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Zhu, S.; Gu, Z.; Zhao, Y. Harnessing Tumor Microenvironment for Nanoparticle-Mediated Radiotherapy. Adv. Ther. 2018, 1, 1800050. [Google Scholar] [CrossRef]
- Gu, H.; Huang, T.; Shen, Y.; Liu, Y.; Zhou, F.; Jin, Y.; Sattar, H.; Wei, Y. Reactive Oxygen Species-Mediated Tumor Microenvironment Transformation: The Mechanism of Radioresistant Gastric Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 5801209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shang, W.; Niu, M.; Tian, J.; Xu, K. Hypoxia-active nanoparticles used in tumor theranostic. Int. J. Nanomed. 2019, 14, 3705–3722. [Google Scholar] [CrossRef]
- Horsman, M.R.; Overgaard, J. The impact of hypoxia and its modification of the outcome of radiotherapy. J. Radiat. Res. 2016, 57, i90–i98. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Patiar, S.; Harris, A.L. Role of hypoxia-inducible factor-1alpha as a cancer therapy target. Endocr.-Relat. Cancer 2006, 13 (Suppl. 1), S61–S75. [Google Scholar] [CrossRef]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-Membrane-Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv. Mater. 2017, 29, 1701429. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, E.-J.; Kim, J.-W.; Chung, U.S.; Koh, W.-G.; Keum, K.C.; Koom, W.S. Gold nanoparticles enhance anti-tumor effect of radiotherapy to hypoxic tumor. Radiat. Oncol. J. 2016, 34, 230–238. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Song, K.; Xing, J.Z.; Yuan, C.; Yan, S.; Yang, Q.; Chen, J.; Kong, B. Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology 2011, 22, 285101. [Google Scholar] [CrossRef] [PubMed]
- Mackey, M.A.; Saira, F.; Mahmoud, M.A.; El-Sayed, M.A. Inducing Cancer Cell Death by Targeting Its Nucleus: Solid Gold Nanospheres versus Hollow Gold Nanocages. Bioconjugate Chem. 2013, 24, 897–906. [Google Scholar] [CrossRef]
- Turner, J.; Koumenis, C.; Kute, T.E.; Planalp, R.P.; Brechbiel, M.W.; Beardsley, D.; Cody, B.; Brown, K.D.; Torti, F.M.; Torti, S.V. Tachpyridine, a metal chelator, induces G2 cell-cycle arrest, activates checkpoint kinases, and sensitizes cells to ionizing radiation. Blood 2005, 106, 3191–3199. [Google Scholar] [CrossRef] [PubMed]
- Roa, W.; Zhang, X.; Guo, L.; Shaw, A.; Hu, X.; Xiong, Y.; Gulavita, S.; Patel, S.; Sun, X.; Chen, J.; et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology 2009, 20, 375101. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, X.H.; Hu, X.D.; Liu, P.D.; Zhang, H.Q. The effects of combined selenium nanoparticles and radiation therapy on breast cancer cells in vitro. Artif. Cells Nanomed. Biotechnol. 2018, 46, 937–948. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with se-methylselenocysteine in mice. Toxicol. Sci. Off. J. Soc. Toxicol. 2008, 101, 22–31. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Ghosh, P.; Biswas, J.; Bhattacharya, S. Protective effect of Selenium nanoparticle against cyclophosphamide induced hepatotoxicity and genotoxicity in Swiss albino mice. J. Biomater. Appl. 2014, 29, 303–317. [Google Scholar] [CrossRef]
- Xu, W.; Luo, T.; Li, P.; Zhou, C.; Cui, D.; Pang, B.; Ren, Q.; Fu, S. RGD-conjugated gold nanorods induce radiosensitization in melanoma cancer cells by downregulating α(v)β₃ expression. Int. J. Nanomed. 2012, 7, 915–924. [Google Scholar]
- Chen, Q.; Chen, J.; Yang, Z.; Xu, J.; Xu, L.; Liang, C.; Han, X.; Liu, Z. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv. Mater. 2019, 31, 1802228. [Google Scholar] [CrossRef]
- Guan, X.; Sun, L.; Shen, Y.; Jin, F.; Bo, X.; Zhu, C.; Han, X.; Li, X.; Chen, Y.; Xu, H.; et al. Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis. Nat. Commun. 2022, 13, 2834. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef]
- Bi, Z.; Li, Q.; Dinglin, X.; Xu, Y.; You, K.; Hong, H.; Hu, Q.; Zhang, W.; Li, C.; Tan, Y.; et al. Nanoparticles (NPs)-Meditated LncRNA AFAP1-AS1 Silencing to Block Wnt/β-Catenin Signaling Pathway for Synergistic Reversal of Radioresistance and Effective Cancer Radiotherapy. Adv. Sci. 2020, 7, 2000915. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, Z.; Tong, Z.; Zhang, S.; Min, J.; Xu, Q.; Zhou, L.; Mao, Z.; Xia, H.; Wang, W. Tumor microenvironment-responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy. Theranostics 2020, 10, 5195–5208. [Google Scholar] [CrossRef]
- Hazkani, I.; Motiei, M.; Betzer, O.; Sadan, T.; Bragilovski, D.; Lubimov, L.; Mizrachi, A.; Hadar, T.; Levi, M.; Ben-Aharon, I.; et al. Can molecular profiling enhance radiotherapy? Impact of personalized targeted gold nanoparticles on radiosensitivity and imaging of adenoid cystic carcinoma. Theranostics 2017, 7, 3962–3971. [Google Scholar] [CrossRef]
- González-Ruíz, A.; Ferro-Flores, G.; Jiménez-Mancilla, N.; Escudero-Castellanos, A.; Ocampo-García, B.; Luna-Gutiérrez, M.; Santos-Cuevas, C.; Morales-Avila, E.; Isaac-Olivé, K. In vitro and in vivo synergistic effect of radiotherapy and plasmonic photothermal therapy on the viability of cancer cells using 177Lu–Au-NLS-RGD-Aptamer nanoparticles under laser irradiation. J. Radioanal. Nucl. Chem. 2018, 318, 1913–1921. [Google Scholar] [CrossRef]
- Cui, F.-B.; Li, R.-T.; Liu, Q.; Wu, P.-Y.; Hu, W.-J.; Yue, G.-F.; Ding, H.; Yu, L.-X.; Qian, X.-P.; Liu, B.-R. Enhancement of radiotherapy efficacy by docetaxel-loaded gelatinase-stimuli PEG-Pep-PCL nanoparticles in gastric cancer. Cancer Lett. 2014, 346, 53–62. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Smilowitz, H.M.; O’Connor, M.J.; Dilmanian, F.A.; Slatkin, D.N. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine 2013, 8, 1601–1609. [Google Scholar] [CrossRef]
- Yi, X.; Chen, L.; Chen, J.; Maiti, D.; Chai, Z.; Liu, Z.; Yang, K. Biomimetic Copper Sulfide for Chemo-Radiotherapy: Enhanced Uptake and Reduced Efflux of Nanoparticles for Tumor Cells under Ionizing Radiation. Adv. Funct. Mater. 2018, 28, 1705161. [Google Scholar] [CrossRef]
- Kim, J.A.; Åberg, C.; Salvati, A.; Dawson, K.A. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat. Nanotechnol. 2012, 7, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, W.; Zhang, P.; Jin, X.; Liu, X.; Li, P.; Li, F.; Zhang, H.; Zou, G.; Li, Q. Dynamically-enhanced retention of gold nanoclusters in HeLa cells following X-rays exposure: A cell cycle phase-dependent targeting approach. Radiother. Oncol. 2016, 119, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Davies Cde, L.; Lundstrøm, L.M.; Frengen, J.; Eikenes, L.; Bruland, S.Ø.; Kaalhus, O.; Hjelstuen, M.H.; Brekken, C. Radiation improves the distribution and uptake of liposomal doxorubicin (caelyx) in human osteosarcoma xenografts. Cancer Res. 2004, 64, 547–553. [Google Scholar] [CrossRef]
- Sheikh-Hamad, D.; Timmins, K.; Jalali, Z. Cisplatin-induced renal toxicity: Possible reversal by N-acetylcysteine treatment. J. Am. Soc. Nephrol. JASN 1997, 8, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, B.; Zheng, H.; Xing, Y.; Chen, G.; Zhou, P.; Qian, L.; Min, Y. Advances of Nanomedicine in Radiotherapy. Pharmaceutics 2021, 13, 1757. [Google Scholar] [CrossRef]
- Tan, G.-R.; Feng, S.-S.; Leong, D.T. The reduction of anti-cancer drug antagonism by the spatial protection of drugs with PLA–TPGS nanoparticles. Biomaterials 2014, 35, 3044–3051. [Google Scholar] [CrossRef]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Zhang, X.D.; Luo, Z.; Chen, J.; Shen, X.; Song, S.; Sun, Y.; Fan, S.; Fan, F.; Leong, D.T.; Xie, J. Ultrasmall Au(10-12)(SG)(10-12) nanomolecules for high tumor specificity and cancer radiotherapy. Adv. Mater. 2014, 26, 4565–4568. [Google Scholar] [CrossRef]
- Marín, A.; Martín, M.; Liñán, O.; Alvarenga, F.; López, M.; Fernández, L.; Büchser, D.; Cerezo, L. Bystander effects and radiotherapy. Rep. Pract. Oncol. Radiother. 2015, 20, 12–21. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Uncomfortable issues in radiation protection posed by low-dose radiobiology. Radiat. Environ. Biophys. 2013, 52, 293–298. [Google Scholar] [CrossRef]
- Mancuso, M.; Pasquali, E.; Giardullo, P.; Leonardi, S.; Tanori, M.; Di Majo, V.; Pazzaglia, S.; Saran, A. The radiation bystander effect and its potential implications for human health. Curr. Mol. Med. 2012, 12, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Fardid, R.; Hadadi, G.; Fardid, M. The mechanisms of radiation-induced bystander effect. J. Biomed. Phys. Eng. 2014, 4, 163–172. [Google Scholar] [PubMed]
- Heuskin, A.-C.; Wéra, A.-C.; Riquier, H.; Michiels, C.; Lucas, S. Low-Dose Hypersensitivity and Bystander Effect are Not Mutually Exclusive in A549 Lung Carcinoma Cells after Irradiation with Charged Particles. J. Radiat. Res. 2013, 180, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Toossi, M.T.B.; Sazgarnia, A.; Soleymanifard, S. The effect of glucose-coated gold nanoparticles on radiation bystander effect induced in MCF-7 and QUDB cell lines. Radiat. Environ. Biophys. 2016, 55, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, Y.; Lu, Z.; He, J.; Wang, L.; Zhang, L.; Zhang, Y.; Liu, Y. Astragalus Polysaccharide Inhibits Ionizing Radiation-Induced Bystander Effects by Regulating MAPK/NF-kB Signaling Pathway in Bone Mesenchymal Stem Cells (BMSCs). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 4649–4658. [Google Scholar] [CrossRef]
- Azzam, I.E.; de Toledo, M.S.; Little, B.J. Stress Signaling from Irradiated to Non-Irradiated Cells. Curr. Cancer Drug Targets 2004, 4, 53–64. [Google Scholar] [CrossRef]
- Barber, G.N. Cytoplasmic DNA innate immune pathways. Immunol. Rev. 2011, 243, 99–108. [Google Scholar] [CrossRef]
- Mittra, I.; Samant, U.; Sharma, S.; Raghuram, G.V.; Saha, T.; Tidke, P.; Pancholi, N.; Gupta, D.; Prasannan, P.; Gaikwad, A.; et al. Cell-free chromatin from dying cancer cells integrate into genomes of bystander healthy cells to induce DNA damage and inflammation. Cell Death Discov. 2017, 3, 17015. [Google Scholar] [CrossRef]
- Kirolikar, S.; Prasannan, P.; Raghuram, G.V.; Pancholi, N.; Saha, T.; Tidke, P.; Chaudhari, P.; Shaikh, A.; Rane, B.; Pandey, R.; et al. Prevention of radiation-induced bystander effects by agents that inactivate cell-free chromatin released from irradiated dying cells. Cell Death Discov. 2018, 9, 1142. [Google Scholar] [CrossRef]
- Mohd Zainudin, N.H.; Talik Sisin, N.N.; Rashid, R.A.; Jamil, A.; Khairil Anuar, M.A.; Razak, K.A.; Abdullah, R.; Rahman, W.N. Cellular analysis on the radiation induced bystander effects due to bismuth oxide nanoparticles with 6 MV photon beam radiotherapy. J. Radiat. Res. Appl. Sci. 2022, 15, 318–325. [Google Scholar] [CrossRef]
- Sinha, N.; Mukhopadhyay, S.; Das, D.N.; Panda, P.K.; Bhutia, S.K. Relevance of cancer initiating/stem cells in carcinogenesis and therapy resistance in oral cancer. Oral Oncol. 2013, 49, 854–862. [Google Scholar] [CrossRef]
- Aponte, P.M.; Caicedo, A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int. 2017, 2017, 5619472. [Google Scholar] [CrossRef]
- Qin, W.; Huang, G.; Chen, Z.; Zhang, Y. Nanomaterials in Targeting Cancer Stem Cells for Cancer Therapy. Front. Pharmacol. 2017, 8, 1. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef]
- Eyler, C.E.; Rich, J.N. Survival of the Fittest: Cancer Stem Cells in Therapeutic Resistance and Angiogenesis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2839–2845. [Google Scholar] [CrossRef]
- Fiorillo, M.; Verre, A.F.; Iliut, M.; Peiris-Pagés, M.; Ozsvari, B.; Gandara, R.; Cappello, A.R.; Sotgia, F.; Vijayaraghavan, A.; Lisanti, M.P. Graphene oxide selectively targets cancer stem cells, across multiple tumor types: Implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”. Oncotarget 2015, 6, 3553–3562. [Google Scholar] [CrossRef]
- Yao, H.-J.; Zhang, Y.-G.; Sun, L.; Liu, Y. The effect of hyaluronic acid functionalized carbon nanotubes loaded with salinomycin on gastric cancer stem cells. Biomaterials 2014, 35, 9208–9223. [Google Scholar] [CrossRef]
- Faraj, A.A.; Shaik, A.S.; Sayed, B.A.; Halwani, R.; Jammaz, I.A. Specific targeting and noninvasive imaging of breast cancer stem cells using single-walled carbon nanotubes as novel multimodality nanoprobes. Nanomedicine 2016, 11, 31–46. [Google Scholar] [CrossRef]
- Shi, H.; Sadler, P.J. How promising is phototherapy for cancer? Br. J. Cancer 2020, 123, 871–873. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Ban, Q.; Bai, T.; Duan, X.; Kong, J. Noninvasive photothermal cancer therapy nanoplatforms via integrating nanomaterials and functional polymers. Biomater. Sci. 2017, 5, 190–210. [Google Scholar] [CrossRef]
- Gai, S.; Yang, G.; Yang, P.; He, F.; Lin, J.; Jin, D.; Xing, B. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Suo, X.; Zhang, J.; Zhang, Y.; Liang, X.-J.; Zhang, J.; Liu, D. A nano-based thermotherapy for cancer stem cell-targeted therapy. J. Mater. Chem. B 2020, 8, 3985–4001. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, D.; Wang, L.; Sun, Y.; Li, J.J.; Hill, B.; Yang, F.; Li, Y.; Lam, K.S. Unique Photochemo-Immuno-Nanoplatform against Orthotopic Xenograft Oral Cancer and Metastatic Syngeneic Breast Cancer. Nano Lett. 2018, 18, 7092–7103. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Semiconducting Polymer Nanomaterials as Near-Infrared Photoactivatable Protherapeutics for Cancer. Acc. Chem. Res. 2020, 53, 752–762. [Google Scholar] [CrossRef]
- Dąbrowski, J.M. Chapter Nine-Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. In Advances in Inorganic Chemistry; van Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 70, pp. 343–394. [Google Scholar]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 11522–11531. [Google Scholar] [CrossRef]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-Ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Shen, P.; Wang, J.; Shen, Y.; Shen, Y.; Webster, T.J.; Deng, J. Applications of Inorganic Nanomaterials in Photothermal Therapy Based on Combinational Cancer Treatment. Int. J. Nanomed. 2020, 15, 1903–1914. [Google Scholar] [CrossRef]
- Hadi, F.; Tavakkol, S.; Laurent, S.; Pirhajati, V.; Mahdavi, S.R.; Neshastehriz, A.; Shakeri-Zadeh, A. Combinatorial effects of radiofrequency hyperthermia and radiotherapy in the presence of magneto-plasmonic nanoparticles on MCF-7 breast cancer cells. J. Cell. Physiol. 2019, 234, 20028–20035. [Google Scholar] [CrossRef]
- Hosseini, V.; Mirrahimi, M.; Shakeri-Zadeh, A.; Koosha, F.; Ghalandari, B.; Maleki, S.; Komeili, A.; Kamrava, S.K. Multimodal cancer cell therapy using Au@Fe2O3 core–shell nanoparticles in combination with photo-thermo-radiotherapy. Photodiagnosis Photodyn. Ther. 2018, 24, 129–135. [Google Scholar] [CrossRef]
- Movahedi, M.M.; Alamzadeh, Z.; Hosseini-Nami, S.; Shakeri-Zadeh, A.; Taheripak, G.; Ahmadi, A.; Zare-Sadeghi, A.; Ghaznavi, H.; Mehdizadeh, A. Investigating the mechanisms behind extensive death in human cancer cells following nanoparticle assisted photo-thermo-radiotherapy. Photodiagnosis Photodyn. Ther. 2020, 29, 101600. [Google Scholar] [CrossRef]
- Ghasemi, A.; Khanzadeh, T.; Zadi Heydarabad, M.; Khorrami, A.; Jahanban Esfahlan, A.; Ghavipanjeh, S.; Gholipour Belverdi, M.; Darvishani Fikouhi, S.; Darbin, A.; Najafpour, M.; et al. Evaluation of BAX and BCL-2 Gene Expression and Apoptosis Induction in Acute Lymphoblastic Leukemia Cell Line CCRFCEM after High-Dose Prednisolone Treatment. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 2319–2323. [Google Scholar] [PubMed]
- Schildkopf, P.; Frey, B.; Ott, O.J.; Rubner, Y.; Multhoff, G.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2011, 101, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.M.; Mehdizadeh, A.; Koosha, F.; Eslahi, N.; Mahabadi, V.P.; Ghaznavi, H.; Shakeri-Zadeh, A. Investigating the photo-thermo-radiosensitization effects of folate-conjugated gold nanorods on KB nasopharyngeal carcinoma cells. Photodiagnosis Photodyn. Ther. 2018, 24, 324–331. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, Y.; Adachi, M.; Wen, X.; Erwin, B.; Mawlawi, O.; Lai, S.Y.; Li, C. Single agent nanoparticle for radiotherapy and radio-photothermal therapy in anaplastic thyroid cancer. Biomaterials 2015, 57, 41–49. [Google Scholar] [CrossRef]
- Liu, T.; Song, Y.; Huang, Z.; Pu, X.; Wang, Y.; Yin, G.; Gou, L.; Weng, J.; Meng, X. Photothermal photodynamic therapy and enhanced radiotherapy of targeting copolymer-coated liquid metal nanoparticles on liver cancer. Colloids Surf. B Biointerfaces 2021, 207, 112023. [Google Scholar] [CrossRef]
- Yong, Y.; Cheng, X.; Bao, T.; Zu, M.; Yan, L.; Yin, W.; Ge, C.; Wang, D.; Gu, Z.; Zhao, Y. Tungsten Sulfide Quantum Dots as Multifunctional Nanotheranostics for In Vivo Dual-Modal Image-Guided Photothermal/Radiotherapy Synergistic Therapy. ACS Nano 2015, 9, 12451–12463. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Yang, K.; Liang, C.; Zhong, X.; Ning, P.; Song, G.; Wang, D.; Ge, C.; Chen, C.; Chai, Z.; et al. Imaging-Guided Combined Photothermal and Radiotherapy to Treat Subcutaneous and Metastatic Tumors Using Iodine-131-Doped Copper Sulfide Nanoparticles. Adv. Funct. Mater. 2015, 25, 4689–4699. [Google Scholar] [CrossRef]
- Safari, A.; Sarikhani, A.; Shahbazi-Gahrouei, D.; Alamzadeh, Z.; Beik, J.; Dezfuli, A.S.; Mahabadi, V.P.; Tohfeh, M.; Shakeri-Zadeh, A. Optimal scheduling of the nanoparticle-mediated cancer photo-thermo-radiotherapy. Photodiagnosis Photodyn. Ther. 2020, 32, 102061. [Google Scholar] [CrossRef]
- Galeaz, C.; Totis, C.; Bisio, A. Radiation Resistance: A Matter of Transcription Factors. Front. Oncol. 2021, 11, 662840. [Google Scholar] [CrossRef]
- Al-Dimassi, S.; Abou-Antoun, T.; El-Sibai, M. Cancer cell resistance mechanisms: A mini review. Clin. Transl. Oncol. 2014, 16, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Reviews Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Reviews Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Du, B.; Li, X.; Liu, S.; Yang, X.-Y.; Ding, H.; Yang, W.; Pan, F.; Wu, X.; et al. Gold Nanoparticle–Mediated Targeted Delivery of Recombinant Human Endostatin Normalizes Tumour Vasculature and Improves Cancer Therapy. Sci. Rep. 2016, 6, 30619. [Google Scholar] [CrossRef]
- Jayne, D.G.; Perry, S.L.; Morrison, E.; Farmery, S.M.; Guillou, P.J. Activated mesothelial cells produce heparin-binding growth factors: Implications for tumour metastases. Br. J. Cancer 2000, 82, 1233–1238. [Google Scholar] [CrossRef]
- Child, H.W.; Hernandez, Y.; Conde, J.; Mullin, M.; Baptista, P.; de la Fuente, J.M.; Berry, C.C. Gold nanoparticle-siRNA mediated oncogene knockdown at RNA and protein level, with associated gene effects. Nanomedicine 2015, 10, 2513–2525. [Google Scholar] [CrossRef]
- Lee, K.J.; Ko, E.J.; Park, Y.-Y.; Park, S.S.; Ju, E.J.; Park, J.; Shin, S.H.; Suh, Y.-A.; Hong, S.-M.; Park, I.J.; et al. A novel nanoparticle-based theranostic agent targeting LRP-1 enhances the efficacy of neoadjuvant radiotherapy in colorectal cancer. Biomaterials 2020, 255, 120151. [Google Scholar] [CrossRef]
- Roy, A.; Coum, A.; Marinescu, V.D.; Põlajeva, J.; Smits, A.; Nelander, S.; Uhrbom, L.; Westermark, B.; Forsberg-Nilsson, K.; Pontén, F.; et al. Glioma-derived plasminogen activator inhibitor-1 (PAI-1) regulates the recruitment of LRP1 positive mast cells. Oncotarget 2015, 6, 23647–23661. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, J.; Lee, H.J.; Kwon, B.M.; Lee, D.K.; Hong, S.H. LRP1-dependent pepsin clearance induced by 2’-hydroxycinnamaldehyde attenuates breast cancer cell invasion. Int. J. Biochem. Cell Biol. 2014, 53, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Porcel, E.; Remita, H.; Marco, S.; Réfrégiers, M.; Dutertre, M.; Confalonieri, F.; Lacombe, S. Platinum nanoparticles: An exquisite tool to overcome radioresistance. Cancer Nanotechnol. 2017, 8, 4. [Google Scholar] [CrossRef]
- Luqmani, Y.A. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2005, 14 (Suppl. 1), 35–48. [Google Scholar] [CrossRef]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updates 2011, 14, 191–201. [Google Scholar] [CrossRef]
- McAleese, C.E.; Choudhury, C.; Butcher, N.J.; Minchin, R.F. Hypoxia-mediated drug resistance in breast cancers. Cancer Lett. 2021, 502, 189–199. [Google Scholar] [CrossRef]
- Wei, G.; Yang, G.; Wei, B.; Wang, Y.; Zhou, S. Near-infrared light switching nitric oxide nanoemitter for triple-combination therapy of multidrug resistant cancer. Acta Biomater. 2019, 100, 365–377. [Google Scholar] [CrossRef]
- Zhao, Y.; Ouyang, X.; Peng, Y.; Peng, S. Stimuli Responsive Nitric Oxide-Based Nanomedicine for Synergistic Therapy. Pharmaceutics 2021, 13, 1917. [Google Scholar] [CrossRef]
- Huang, X.; Xu, F.; Hou, H.; Hou, J.; Wang, Y.; Zhou, S. Stimuli-responsive nitric oxide generator for light-triggered synergistic cancer photothermal/gas therapy. Nano Res. 2019, 12, 1361–1370. [Google Scholar] [CrossRef]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, S.; Zhang, N.; Kuang, Y.; Li, W.; Gai, S.; He, F.; Gulzar, A.; Yang, P. X-ray-triggered NO-released Bi–SNO nanoparticles: All-in-one nano-radiosensitizer with photothermal/gas therapy for enhanced radiotherapy. Nanoscale 2020, 12, 19293–19307. [Google Scholar] [CrossRef]
- Akhondzadeh, S. The Importance of Clinical Trials in Drug Development. Avicenna J. Med. Biotechnol. 2016, 8, 151. [Google Scholar]
- Bonvalot, S.; Le Pechoux, C.; De Baere, T.; Kantor, G.; Buy, X.; Stoeckle, E.; Terrier, P.; Sargos, P.; Coindre, J.M.; Lassau, N.; et al. First-in-Human Study Testing a New Radioenhancer Using Nanoparticles (NBTXR3) Activated by Radiation Therapy in Patients with Locally Advanced Soft Tissue Sarcomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 908–917. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): A multicentre, phase 2-3, randomised, controlled trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, Q. Engineering nanoparticles to reprogram radiotherapy and immunotherapy: Recent advances and future challenges. J. Nanobiotechnol. 2020, 18, 75. [Google Scholar] [CrossRef]
- Ngwa, W.; Boateng, F.; Kumar, R.; Irvine, D.J.; Formenti, S.; Ngoma, T.; Herskind, C.; Veldwijk, M.R.; Hildenbrand, G.L.; Hausmann, M.; et al. Smart Radiation Therapy Biomaterials. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 624–637. [Google Scholar] [CrossRef]
- Ngwa, W.; Kumar, R.; Moreau, M.; Dabney, R.; Herman, A. Nanoparticle Drones to Target Lung Cancer with Radiosensitizers and Cannabinoids. Front. Oncol. 2017, 7, 208. [Google Scholar] [CrossRef]
- Ngwa, W.; Kumar, R.; Sridhar, S.; Korideck, H.; Zygmanski, P.; Cormack, R.A.; Berbeco, R.; Makrigiorgos, G.M. Targeted radiotherapy with gold nanoparticles: Current status and future perspectives. Nanomedicine 2014, 9, 1063–1082. [Google Scholar] [CrossRef]
- Pottier, A.; Borghi, E.; Levy, L. The future of nanosized radiation enhancers. Br. J. Radiol. 2015, 88, 20150171. [Google Scholar] [CrossRef]
- Boateng, F.; Ngwa, W. Delivery of Nanoparticle-Based Radiosensitizers for Radiotherapy Applications. Int. J. Mol. Sci. 2020, 21, 273. [Google Scholar] [CrossRef]
- Sinha, N.; Cifter, G.; Sajo, E.; Kumar, R.; Sridhar, S.; Nguyen, P.L.; Cormack, R.A.; Makrigiorgos, G.M.; Ngwa, W. Brachytherapy Application with In Situ Dose Painting Administered by Gold Nanoparticle Eluters. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 385–392. [Google Scholar] [CrossRef]
- Boateng, F.; Ngwa, W. Novel bioerodable eluting-spacers for radiotherapy applications with in situ dose painting. Br. J. Radiol. 2019, 92, 20180745. [Google Scholar] [CrossRef]
- Moreau, M.; Yasmin-Karim, S.; Kunjachan, S.; Sinha, N.; Gremse, F.; Kumar, R.; Chow, K.F.; Ngwa, W. Priming the Abscopal Effect Using Multifunctional Smart Radiotherapy Biomaterials Loaded with Immunoadjuvants. Front. Oncol. 2018, 8, 56. [Google Scholar] [CrossRef]
- Miller, S.M.; Wang, A.Z. Nanomedicine in chemoradiation. Ther. Deliv. 2013, 4, 239–250. [Google Scholar] [CrossRef]
- Kvols, L.K. Radiation sensitizers: A selective review of molecules targeting DNA and non-DNA targets. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2005, 46 (Suppl. 1), 187s–190s. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, M.; Shakil, M.S.; Mahmud, K.M. The Promise of Nanoparticles-Based Radiotherapy in Cancer Treatment. Cancers 2023, 15, 1892. https://doi.org/10.3390/cancers15061892
Haque M, Shakil MS, Mahmud KM. The Promise of Nanoparticles-Based Radiotherapy in Cancer Treatment. Cancers. 2023; 15(6):1892. https://doi.org/10.3390/cancers15061892
Chicago/Turabian StyleHaque, Munima, Md Salman Shakil, and Kazi Mustafa Mahmud. 2023. "The Promise of Nanoparticles-Based Radiotherapy in Cancer Treatment" Cancers 15, no. 6: 1892. https://doi.org/10.3390/cancers15061892
APA StyleHaque, M., Shakil, M. S., & Mahmud, K. M. (2023). The Promise of Nanoparticles-Based Radiotherapy in Cancer Treatment. Cancers, 15(6), 1892. https://doi.org/10.3390/cancers15061892







