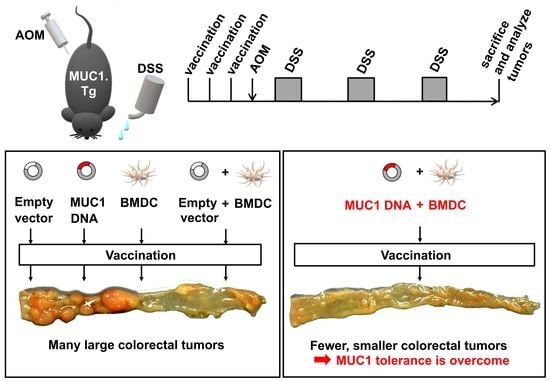
Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antibodies
2.3. Construction of MUC1 cDNA
2.4. Preparation of BMDCs
2.5. Vaccination
2.6. Experimental Colitis-Associated Colorectal Carcinogenesis
2.7. Gross and Histopathological Examination
2.8. Immunohistochemical Analysis
2.9. Effect of MUC1 DNA Vaccination on Azoxymethane-Dextran Sulfate Sodium (AOM-DSS)-Induced Colorectal Inflammation
2.10. Statistical Analysis
3. Results
3.1. MUC1 Expression in Tumors Developed by Colitis-Associated Colorectal Carcinogenesis in MUC1.Tg Mice

3.2. Preventive Efficacy of MUC1 DNA Vaccine on Experimental Colitis-Associated Colorectal Carcinogenesis
3.3. Effect of MUC1 DNA Vaccination on AOM-DSS Induced Colorectal Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e2105. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.F. DNA vaccine for cancer immunotherapy. Hum. Vaccines Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, J.J.; Ulmer, J.B.; Shiver, J.W.; Liu, M.A. DNA vaccines. Annu. Rev. Immunol. 1997, 15, 617–648. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Klinman, D.M.; Seder, R.A. DNA vaccines: Immunology, application, and optimization. Annu. Rev. Immunol. 2000, 18, 927–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.L.; Chang, M.C.; Chiang, Y.C.; Lin, H.W.; Sun, N.Y.; Chen, C.A.; Sun, W.Z.; Cheng, W.F. Immuno-modulators enhance antigen-specific immunity and anti-tumor effects of mesothelin-specific chimeric DNA vaccine through promoting DC maturation. Cancer Lett. 2018, 425, 152–163. [Google Scholar] [CrossRef]
- Iwasaki, A.; Torres, C.A.; Ohashi, P.S.; Robinson, H.L.; Barber, B.H. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J. Immunol. 1997, 159, 11–14. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, D.; Eling, D.J.; Robbins, J.; Kipps, T.J. DNA vaccines encoding full-length or truncated Neu induce protective immunity against Neu-expressing mammary tumors. Cancer Res. 1998, 58, 1965–1971. [Google Scholar]
- Chen, C.H.; Wang, T.L.; Hung, C.F.; Yang, Y.; Young, R.A.; Pardoll, D.M.; Wu, T.C. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000, 60, 1035–1042. [Google Scholar]
- Hanisch, F.G.; Muller, S. MUC1: The polymorphic appearance of a human mucin. Glycobiology 2000, 10, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Shao, Z.; Gao, J. Antitumor Effect of a DNA Vaccine Harboring Prostate Cancer-Specific Antigen with IL-12 as an Intramolecular Adjuvant. J. Mol. Microbiol. Biotechnol. 2017, 27, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Baghban Rahimi, S.; Mohebbi, A.; Vakilzadeh, G.; Biglari, P.; Razeghi Jahromi, S.; Mohebi, S.R.; Shirian, S.; Gorji, A.; Ghaemi, A. Enhancement of therapeutic DNA vaccine potency by melatonin through inhibiting VEGF expression and induction of antitumor immunity mediated by CD8+ T cells. Arch. Virol. 2018, 163, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Patel, A.; Tursi, N.J.; Zhu, X.; Muthumani, K.; Kulp, D.W.; Weiner, D.B. Harnessing Recent Advances in Synthetic DNA and Electroporation Technologies for Rapid Vaccine Development Against COVID-19 and Other Emerging Infectious Diseases. Front. Med. Technol. 2020, 2, 571030. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Nakamori, S.; Ota, D.M.; Cleary, K.R.; Shirotani, K.; Irimura, T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology 1994, 106, 353–361. [Google Scholar] [CrossRef]
- Ajioka, Y.; Allison, L.J.; Jass, J.R. Significance of MUC1 and MUC2 mucin expression in colorectal cancer. J. Clin. Pathol. 1996, 49, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Vlad, A.M.; Kettel, J.C.; Alajez, N.M.; Carlos, C.A.; Finn, O.J. MUC1 immunobiology: From discovery to clinical applications. Adv. Immunol. 2004, 82, 249–293. [Google Scholar] [CrossRef]
- Adsay, N.V.; Merati, K.; Andea, A.; Sarkar, F.; Hruban, R.H.; Wilentz, R.E.; Goggins, M.; Iocobuzio-Donahue, C.; Longnecker, D.S.; Klimstra, D.S. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: Differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod. Pathol. 2002, 15, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Furr, A.E.; Ranganathan, S.; Finn, O.J. Aberrant expression of MUC1 mucin in pediatric inflammatory bowel disease. Pediatr. Dev. Pathol. 2010, 13, 24–31. [Google Scholar] [CrossRef]
- Ryan, S.O.; Vlad, A.M.; Islam, K.; Gariepy, J.; Finn, O.J. Tumor-associated MUC1 glycopeptide epitopes are not subject to self-tolerance and improve responses to MUC1 peptide epitopes in MUC1 transgenic mice. Biol. Chem. 2009, 390, 611–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlad, A.M.; Muller, S.; Cudic, M.; Paulsen, H.; Otvos, L., Jr.; Hanisch, F.G.; Finn, O.J. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: Processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J. Exp. Med. 2002, 196, 1435–1446. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beatty, P.L.; Narayanan, S.; Gariepy, J.; Ranganathan, S.; Finn, O.J. Vaccine against MUC1 antigen expressed in inflammatory bowel disease and cancer lessens colonic inflammation and prevents progression to colitis-associated colon cancer. Cancer Prev. Res. 2010, 3, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Basu, G.D.; Tinder, T.L.; Subramani, D.B.; Bradley, J.M.; Arefayene, M.; Skaar, T.; De Petris, G. Progression of pancreatic adenocarcinoma is significantly impeded with a combination of vaccine and COX-2 inhibition. J. Immunol. 2009, 182, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor-Papadimitriou, J.; Burchell, J.M.; Graham, R.; Beatson, R. Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 2018, 46, 659–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; McKolanis, J.R.; Dzubinski, L.A.; Islam, K.; Potter, D.M.; Salazar, A.M.; Schoen, R.E.; Finn, O.J. MUC1 vaccine for individuals with advanced adenoma of the colon: A cancer immunoprevention feasibility study. Cancer Prev. Res. 2013, 6, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Graeber, L.A.; Helling, F.; Ragupathi, G.; Adluri, S.; Lloyd, K.O.; Livingston, P.O. Augmenting the immunogenicity of synthetic MUC1 peptide vaccines in mice. Cancer Res. 1996, 56, 3315–3319. [Google Scholar] [PubMed]
- Gong, J.; Chen, L.; Chen, D.; Kashiwaba, M.; Manome, Y.; Tanaka, T.; Kufe, D. Induction of antigen-specific antitumor immunity with adenovirus-transduced dendritic cells. Gene Ther. 1997, 4, 1023–1028. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Cao, W.; Yang, Z.G.; Zhao, G.F. DC targeting DNA vaccines induce protective and therapeutic antitumor immunity in mice. Int. J. Clin. Exp. Med. 2015, 8, 17565–17577. [Google Scholar]
- Glaffig, M.; Stergiou, N.; Hartmann, S.; Schmitt, E.; Kunz, H. A Synthetic MUC1 Anticancer Vaccine Containing Mannose Ligands for Targeting Macrophages and Dendritic Cells. ChemMedChem 2018, 13, 25–29. [Google Scholar] [CrossRef]
- Lees, C.J.; Apostolopoulos, V.; Acres, B.; Ong, C.S.; Popovski, V.; McKenzie, I.F. The effect of T1 and T2 cytokines on the cytotoxic T cell response to mannan-MUC1. Cancer Immunol. Immunother. 2000, 48, 644–652. [Google Scholar] [CrossRef]
- Graham, R.A.; Burchell, J.M.; Beverley, P.; Taylor-Papadimitriou, J. Intramuscular immunisation with MUC1 cDNA can protect C57 mice challenged with MUC1-expressing syngeneic mouse tumour cells. Int. J. Cancer 1996, 65, 664–670. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Shi, H.; Yu, X.; Kong, W.; Li, W. Induction of immune response and anti-tumor activities in mice with a DNA vaccine encoding human mucin 1 variable-number tandem repeats. Hum. Immunol. 2008, 69, 250–258. [Google Scholar] [CrossRef]
- Mukherjee, P.; Pathangey, L.B.; Bradley, J.B.; Tinder, T.L.; Basu, G.D.; Akporiaye, E.T.; Gendler, S.J. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine 2007, 25, 1607–1618. [Google Scholar] [CrossRef] [Green Version]
- Stergiou, N.; Glaffig, M.; Jonuleit, H.; Schmitt, E.; Kunz, H. Immunization with a Synthetic Human MUC1 Glycopeptide Vaccine against Tumor-Associated MUC1 Breaks Tolerance in Human MUC1 Transgenic Mice. ChemMedChem 2017, 12, 1424–1428. [Google Scholar] [CrossRef]
- Shi, F.F.; Gunn, G.R.; Snyder, L.A.; Goletz, T.J. Intradermal vaccination of MUC1 transgenic mice with MUC1/IL-18 plasmid DNA suppresses experimental pulmonary metastases. Vaccine 2007, 25, 3338–3346. [Google Scholar] [CrossRef]
- Snyder, L.A.; Goletz, T.J.; Gunn, G.R.; Shi, F.F.; Harris, M.C.; Cochlin, K.; McCauley, C.; McCarthy, S.G.; Branigan, P.J.; Knight, D.M. A MUC1/IL-18 DNA vaccine induces anti-tumor immunity and increased survival in MUC1 transgenic mice. Vaccine 2006, 24, 3340–3352. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Tsibanis, A.; Tsikkinis, A.; Drakaki, H.; Loveland, B.E.; Piddlesden, S.J.; Plebanski, M.; Pouniotis, D.S.; Alexis, M.N.; et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835]. Breast Cancer Res. 2006, 8, R27. [Google Scholar] [CrossRef]
- Vassilaros, S.; Tsibanis, A.; Tsikkinis, A.; Pietersz, G.A.; McKenzie, I.F.; Apostolopoulos, V. Up to 15-year clinical follow-up of a pilot Phase III immunotherapy study in stage II breast cancer patients using oxidized mannan-MUC1. Immunotherapy 2013, 5, 1177–1182. [Google Scholar] [CrossRef] [Green Version]
- Kamata, M.; Denda-Nagai, K.; Kubota, N.; Aida, S.; Takeda, K.; Irimura, T. Vaccination of mice with MUC1 cDNA suppresses the development of lung metastases. Clin. Exp. Metastasis 2002, 19, 689–696. [Google Scholar] [CrossRef]
- Sugiura, D.; Aida, S.; Denda-Nagai, K.; Takeda, K.; Kamata-Sakurai, M.; Yagita, H.; Irimura, T. Differential effector mechanisms induced by vaccination with MUC1 DNA in the rejection of colon carcinoma growth at orthotopic sites and metastases. Cancer Sci. 2008, 99, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Rowse, G.J.; Tempero, R.M.; VanLith, M.L.; Hollingsworth, M.A.; Gendler, S.J. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998, 58, 315–321. [Google Scholar] [PubMed]
- Neufert, C.; Becker, C.; Neurath, M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007, 2, 1998. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003, 94, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Tsibanis, A.; Tsikkinis, A.; Stojanovska, L.; McKenzie, I.F.; Vassilaros, S. Dendritic cell immunotherapy: Clinical outcomes. Clin. Transl. Immunol. 2014, 3, e21. [Google Scholar] [CrossRef] [Green Version]
- Denda-Nagai, K.; Fujita, K.; Fujime, M.; Nakatsugawa, S.; Ishigaki, T.; Irimura, T. Absence of correlation of MUC1 expression to malignant behavior of renal cell carcinoma in experimental systems. Clin. Exp. Metastasis 2000, 18, 77–81. [Google Scholar] [CrossRef]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef]
- Boivin, G.P.; Washington, K.; Yang, K.; Ward, J.M.; Pretlow, T.P.; Russell, R.; Besselsen, D.G.; Godfrey, V.L.; Doetschman, T.; Dove, W.F.; et al. Pathology of mouse models of intestinal cancer: Consensus report and recommendations. Gastroenterology 2003, 124, 762–777. [Google Scholar] [CrossRef] [Green Version]
- Reeve, D.R. Squamous metaplasia in the healing of chronic colonic ulcers of the rat. J. Pathol. 1975, 117, 15–22. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 25 January 2023).
- GraphPad Software. Available online: www.graphpad.com (accessed on 25 January 2023).
- Cao, Y.; Schlag, P.M.; Karsten, U. Immunodetection of epithelial mucin (MUC1, MUC3) and mucin-associated glycotopes (TF, Tn, and sialosyl-Tn) in benign and malignant lesions of colonic epithelium: Apolar localization corresponds to malignant transformation. Virchows Arch. 1997, 431, 159–166. [Google Scholar] [CrossRef]
- Higuchi, T.; Xin, P.; Buckley, M.S.; Erickson, D.R.; Bhavanandan, V.P. Characterization of the rabbit homolog of human MUC1 glycoprotein isolated from bladder by affinity chromatography on immobilized jacalin. Glycobiology 2000, 10, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Kato, K.; Denda-Nagai, K.; Hanisch, F.G.; Clausen, H.; Irimura, T. The epitope recognized by the unique anti-MUC1 monoclonal antibody MY.1E12 involves sialyl alpha 2-3galactosyl beta 1-3N-acetylgalactosaminide linked to a distinct threonine residue in the MUC1 tandem repeat. J. Immunol. Methods 2002, 270, 199–209. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Denda-Nagai, K.; Takahashi, Y.; Nagashima, I.; Shimizu, H.; Kishimoto, T.; Noji, M.; Shichino, S.; Chiba, Y.; Irimura, T. Products of Chemoenzymatic Synthesis Representing MUC1 Tandem Repeat Unit with T-, ST- or STn-antigen Revealed Distinct Specificities of Anti-MUC1 Antibodies. Sci. Rep. 2019, 9, 16641. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, D.; Denda-Nagai, K.; Takashima, M.; Murakami, R.; Nagai, S.; Takeda, K.; Irimura, T. Local effects of regulatory T cells in MUC1 transgenic mice potentiate growth of MUC1 expressing tumor cells in vivo. PLoS ONE 2012, 7, e44770. [Google Scholar] [CrossRef] [Green Version]
- Maecker, H.T.; Umetsu, D.T.; DeKruyff, R.H.; Levy, S. Cytotoxic T cell responses to DNA vaccination: Dependence on antigen presentation via class II MHC. J. Immunol. 1998, 161, 6532–6536. [Google Scholar] [CrossRef]
- Liu, M.A. DNA vaccines: A review. J. Intern. Med. 2003, 253, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Kontani, K.; Taguchi, O.; Ozaki, Y.; Hanaoka, J.; Tezuka, N.; Sawai, S.; Inoue, S.; Fujino, S.; Maeda, T.; Itoh, Y.; et al. Novel vaccination protocol consisting of injecting MUC1 DNA and nonprimed dendritic cells at the same region greatly enhanced MUC1-specific antitumor immunity in a murine model. Cancer Gene Ther. 2002, 9, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jager, E.; Ringhoffer, M.; Altmannsberger, M.; Arand, M.; Karbach, J.; Jager, D.; Oesch, F.; Knuth, A. Immunoselection in vivo: Independent loss of MHC class I and melanocyte differentiation antigen expression in metastatic melanoma. Int. J. Cancer 1997, 71, 142–147. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murwanti, R.; Denda-Nagai, K.; Sugiura, D.; Mogushi, K.; Gendler, S.J.; Irimura, T. Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells. Cancers 2023, 15, 1920. https://doi.org/10.3390/cancers15061920
Murwanti R, Denda-Nagai K, Sugiura D, Mogushi K, Gendler SJ, Irimura T. Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells. Cancers. 2023; 15(6):1920. https://doi.org/10.3390/cancers15061920
Chicago/Turabian StyleMurwanti, Retno, Kaori Denda-Nagai, Daisuke Sugiura, Kaoru Mogushi, Sandra J. Gendler, and Tatsuro Irimura. 2023. "Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells" Cancers 15, no. 6: 1920. https://doi.org/10.3390/cancers15061920
APA StyleMurwanti, R., Denda-Nagai, K., Sugiura, D., Mogushi, K., Gendler, S. J., & Irimura, T. (2023). Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells. Cancers, 15(6), 1920. https://doi.org/10.3390/cancers15061920