Reticulocalbin 3 Is a Novel Mediator of Glioblastoma Progression
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Expression Analysis
2.2. Diagnostic and Prognostic Value of RCN3 in Glioblastoma
2.3. Cell Culture, Reagents, and Generation RCN3 Knockdown and Knockout Cells
2.4. Cell Viability, Neurosphere Formation, and Extreme Limiting Dilution Assays
2.5. RNA-seq and RT-qPCR
2.6. Western Blotting
2.7. In Vivo Orthotopic Tumor Model
2.8. Immunohistochemistry
2.9. Statistical Analyses
3. Results
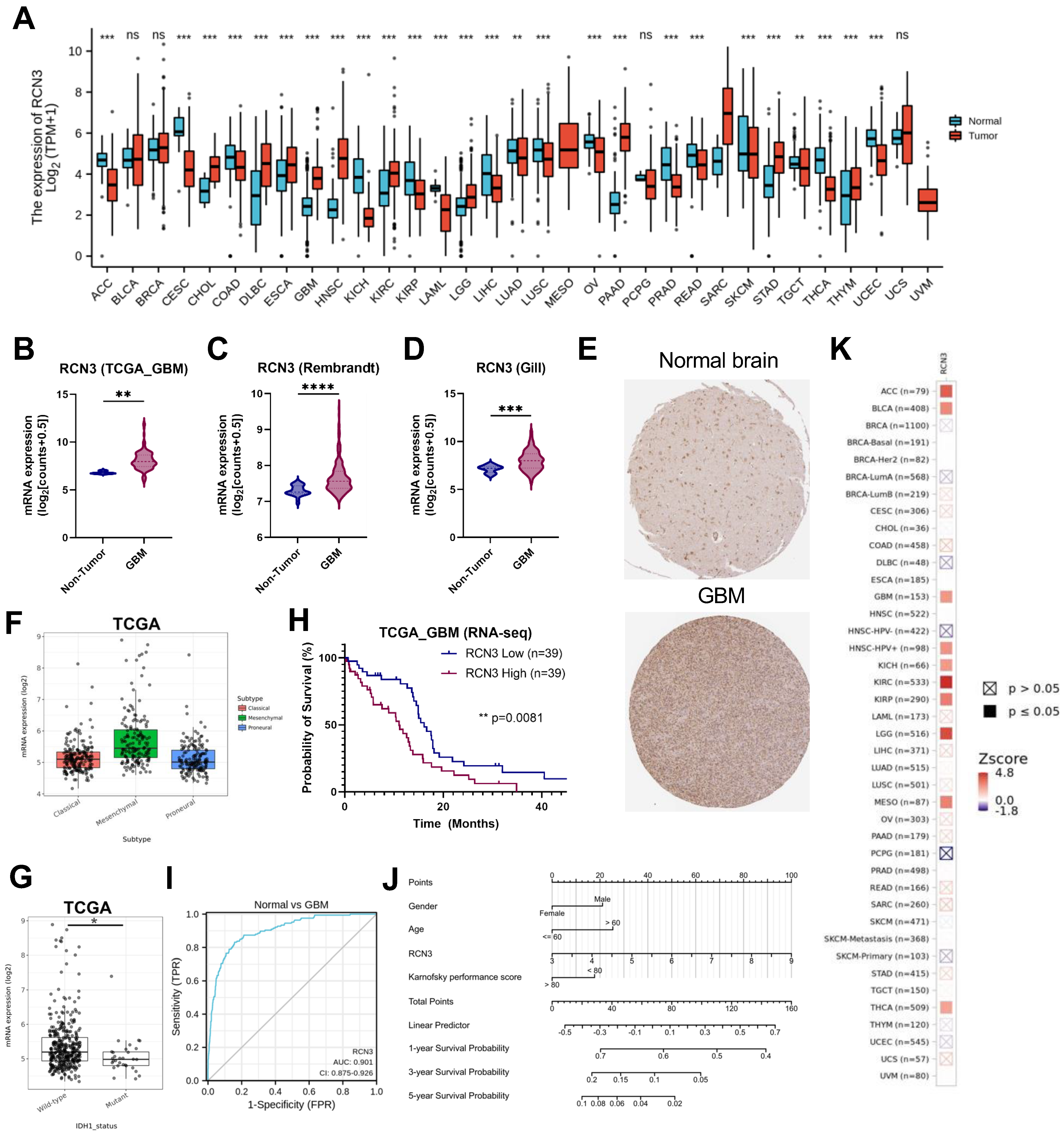
3.1. RCN3 Is an Upregulated Prognostic Marker of Poor Survival in Glioblastoma
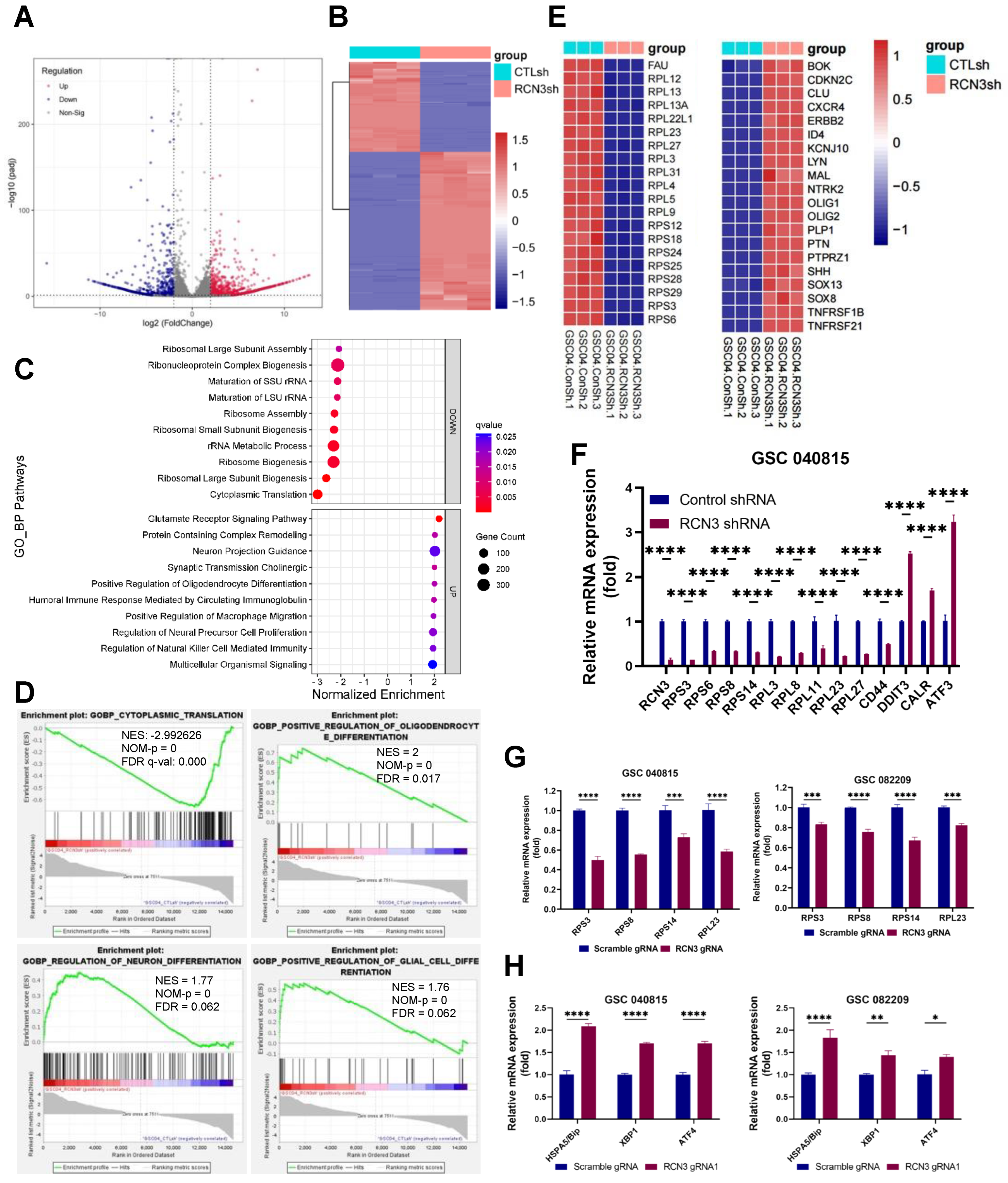
3.2. RCN3 Knockout or Knockdown Inhibited Proliferation, Neurosphere Formation, and Self-Renewal of GSCs
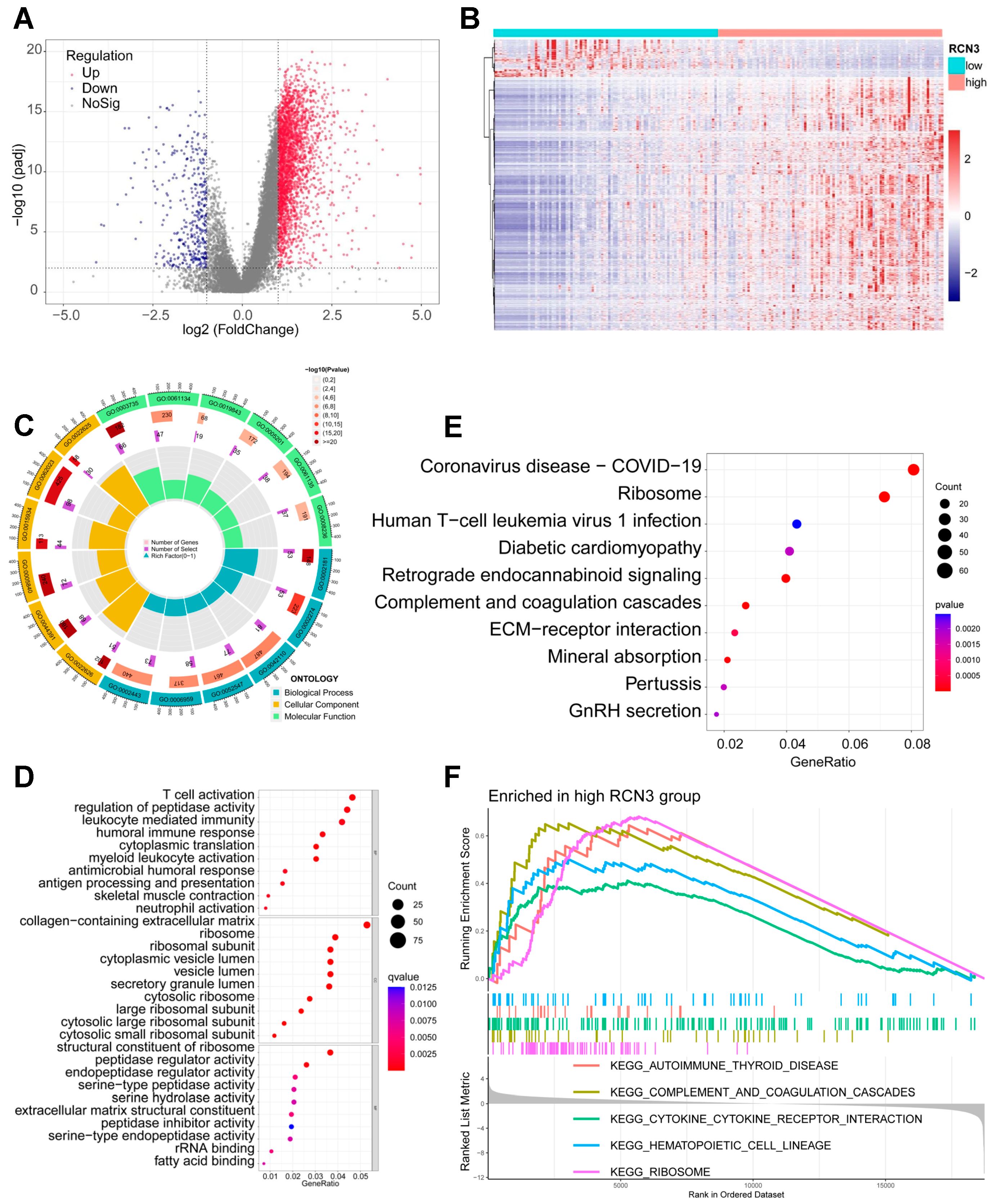
3.3. Identification of Global Transcriptional Changes Altered by RCN3 Knockdown
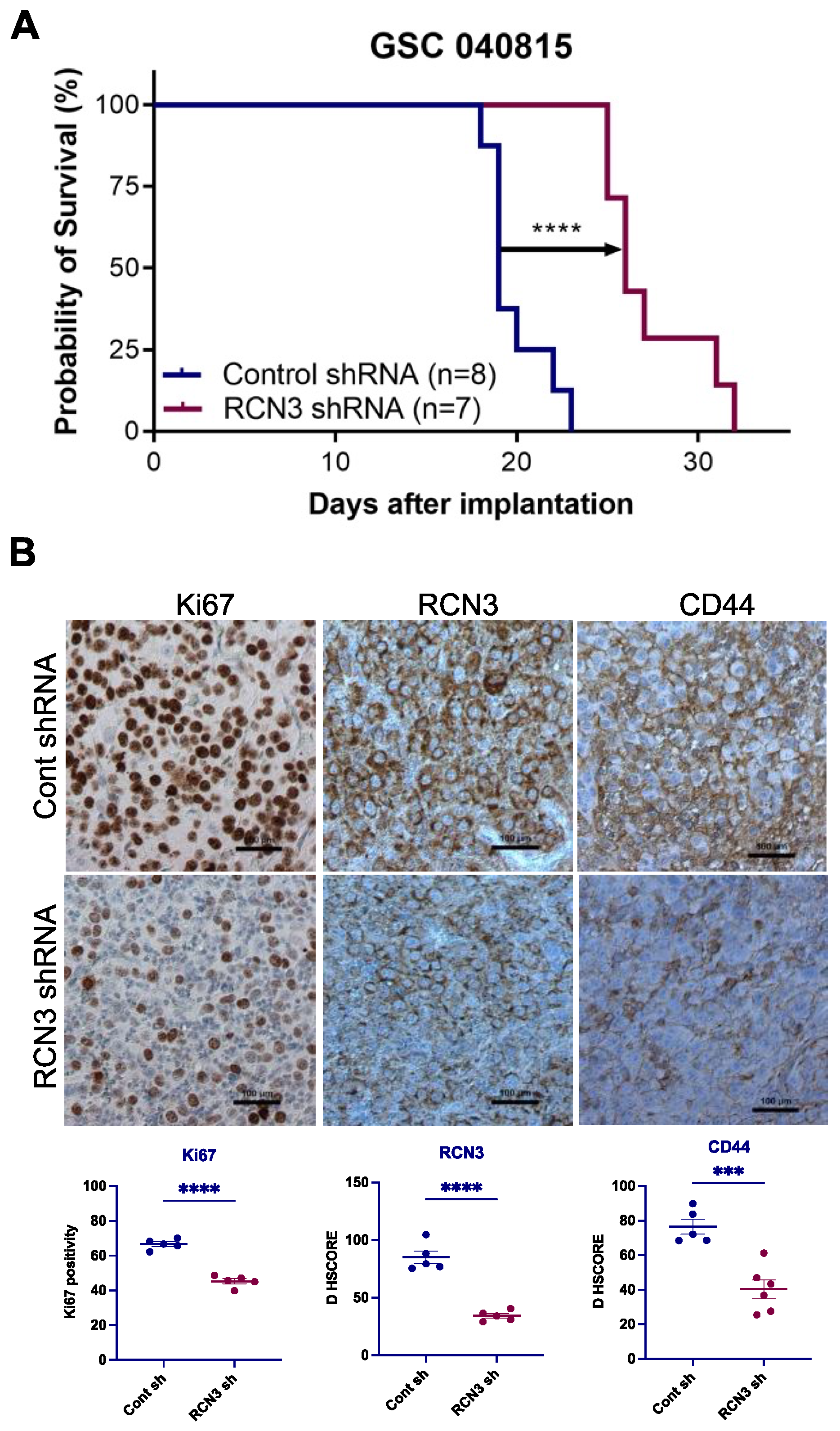
3.4. RCN3 Knockdown Reduced Tumor Progression in Orthotopic Xenograft Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; AWaite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncology 2022, 24, v1–v95. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Crespo, I.; Vital, A.L.; Gonzalez-Tablas, M.; Patino, M.D.C.; Otero, A.; Lopes, M.C.; de Oliveira, C.; Domingues, P.; Orfao, A.; Tabernero, M.D. Molecular and Genomic Alterations in Glioblastoma Multiforme. Am. J. Pathol. 2015, 185, 1820–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filbin, M.G.; Suvà, M.L. Gliomas Genomics and Epigenomics: Arriving at the Start and Knowing It for the First Time. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 497–521. [Google Scholar] [CrossRef]
- Bonavia, R.; Inda, M.-d.-M.; Cavenee, W.K.; Furnari, F.B. Heterogeneity Maintenance in Glioblastoma: A Social Network. Cancer Res. 2011, 71, 4055–4060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broekman, M.L.; Maas, S.L.N.; Abels, E.R.; Mempel, T.R.; Krichevsky, A.M.; Breakefield, X.O. Multidimensional communication in the microenvirons of glioblastoma. Nat. Rev. Neurol. 2018, 14, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.R.P.; Scheyltjens, I.; Duerinck, J.; Neyns, B.; Movahedi, K.; Van Ginderachter, J.A. Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. eLife 2020, 9, e52176. [Google Scholar] [CrossRef]
- Zhu, Z.; Khan, M.A.; Weiler, M.; Blaes, J.; Jestaedt, L.; Geibert, M.; Zou, P.; Gronych, J.; Bernhardt, O.; Korshunov, A.; et al. Targeting Self-Renewal in High-Grade Brain Tumors Leads to Loss of Brain Tumor Stem Cells and Prolonged Survival. Cell Stem Cell 2014, 15, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Yu, T.-S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [Green Version]
- Honoré, B.; Vorum, H. The CREC family, a novel family of multiple EF-hand, low-affinity Ca(2+)-binding proteins localised to the secretory pathway of mammalian cells. FEBS Lett. 2000, 466, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honoré, B. The rapidly expanding CREC protein family: Members, localization, function, and role in disease. Bioessays 2009, 31, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Ibarrola, J.; Fernández-Celis, A.; Santamaria, E.; Fernández-Irigoyen, J.; Rossignol, P.; Jaisser, F.; López-Andrés, N. Differential Proteomics Identifies Reticulocalbin-3 as a Novel Negative Mediator of Collagen Production in Human Cardiac Fibroblasts. Sci. Rep. 2017, 7, 12192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, A.; Kikuchi, Y.; Sato, Y.; Koide, S.; Yuasa, K.; Nagahama, M.; Matsuda, Y. A proteomic approach reveals transient association of reticulocalbin-3, a novel member of the CREC family, with the precursor of subtilisin-like proprotein convertase, PACE4. Biochem. J. 2006, 396, 51–59. [Google Scholar] [CrossRef]
- Xu, M.; Che, L.; Yang, Z.; Zhang, P.; Shi, J.; Li, J.; Lin, Y.; Fang, Z.; Che, L.; Feng, B.; et al. Proteomic Analysis of Fetal Ovaries Reveals That Primordial Follicle Formation and Transition Are Differentially Regulated. BioMed Res. Int. 2017, 2017, 6972030. [Google Scholar] [CrossRef] [Green Version]
- Park, N.R.; Shetye, S.S.; Bogush, I.; Keene, D.R.; Tufa, S.; Hudson, D.M.; Archer, M.; Qin, L.; Soslowsky, L.J.; Dyment, N.A.; et al. Reticulocalbin 3 is involved in postnatal tendon development by regulating collagen fibrillogenesis and cellular maturation. Sci. Rep. 2021, 11, 10868. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Ren, J.; Lam, S.M.; Zhang, Y.; Hou, Y.; Zhang, X.; Xu, R.; Shui, G.; Ma, R.Z. Neonatal Respiratory Failure with Retarded Perinatal Lung Maturation in Mice Caused by Reticulocalbin 3 Disruption. Am. J. Respir. Cell Mol. Biol. 2016, 54, 410–423. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, S.; Zhou, X.; Cui, Y.; Wang, W.; Wen, L.; Guo, L.; Fu, W.; Tang, F. Single-Cell Multiomics Sequencing Reveals Prevalent Genomic Alterations in Tumor Stromal Cells of Human Colorectal Cancer. Cancer Cell 2020, 38, 818–828.e5. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; Siegfried, G.; Hooks, K.B.; Khatib, A.-M. Integration of zebrafish fin regeneration genes with expression data of human tumors in silico uncovers potential novel melanoma markers. Oncotarget 2016, 7, 71567–71579. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.; Wang, P.; Zhao, X.; Lu, X.; Deng, R.; Wang, X.; Chang, H.; Lin, J. Reticulocalbin3: A Ca(2+) homeostasis regulator that promotes esophageal squamous cell carcinoma progression and cisplatin resistance. Cancer Sci. 2022, 113, 3593–3607. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, Y.; Gong, F.; Jin, J.; Huang, A.; Fang, Q.; Ma, R.Z. A Preliminary Study on RCN3 Protein Expression in Non-small Cell Lung Cancer. Clin. Lab. 2016, 62, 293–300. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Q.; Wen, Y.-Q.; Chen, L.-L.; Wang, L.-T.; Liu, Y.-L.; Luo, C.-Q.; Liang, H.-Z.; Li, M.-T.; Li, Z. Comparative proteomics analysis of human osteosarcomas and benign tumor of bone. Cancer Genet. Cytogenet. 2010, 198, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Alejo, S.; Palacios, B.E.; Venkata, P.P.; He, Y.; Li, W.; Johnson, J.D.; Yihong, C.; Jayamohan, S.; Pratap, U.P.; Clarke, K.; et al. Lysine-specific histone demethylase 1A (KDM1A/LSD1) inhibition attenuates DNA double strand break repair and augments efficacy of temozolomide in glioblastoma. Neuro-Oncology 2023. [Google Scholar] [CrossRef]
- Sareddy, G.R.; Viswanadhapalli, S.; Surapaneni, P.; Suzuki, T.; Brenner, A.; Vadlamudi, R.K. Novel KDM1A inhibitors induce differentiation and apoptosis of glioma stem cells via unfolded protein response pathway. Oncogene 2016, 36, 2423–2434. [Google Scholar] [CrossRef] [Green Version]
- Wakimoto, H.; Kesari, S.; Farrell, C.J.; Curry, W.T.; Zaupa, C.; Aghi, M.; Kuroda, T.; Stemmer-Rachamimov, A.; Shah, K.; Liu, T.-C.; et al. Human Glioblastoma–Derived Cancer Stem Cells: Establishment of Invasive Glioma Models and Treatment with Oncolytic Herpes Simplex Virus Vectors. Cancer Res. 2009, 69, 3472–3481. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer 2006, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, A.L.; Galli, R.; Reynolds, B.A. Brain tumour stem cells. Nat. Rev. Cancer 2006, 6, 425–436. [Google Scholar] [CrossRef]
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015, 528, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; An, X.; Yang, L.; Wu, Z.; Zan, D.; Li, Z.; Pang, B.; Chen, Y.; Li, J.; Tan, P.; et al. Reticulocalbin 3 deficiency in alveolar epithelium attenuated LPS-induced ALI via NF-κB signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L627–L639. [Google Scholar] [PubMed]
- Jin, J.; Shi, X.; Li, Y.; Zhang, Q.; Guo, Y.; Li, C.; Tan, P.; Fang, Q.; Ma, Y.; Ma, R.Z. Reticulocalbin 3 Deficiency in Alveolar Epithelium Exacerbated Bleomycin-induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 59, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, T.; Jin, J.; Shi, X.; Huang, A.; Ma, Z.; Li, J.; Wang, S.; ZMa, R.; Fang, Q. Rcn3 Suppression Was Responsible for Partial Relief of Emphysema as Shown by Specific Type II Alveolar Epithelial Cell Rcn3 CKO Mouse Model. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 147–158. [Google Scholar]
- El Khoury, W.; Nasr, Z. Deregulation of ribosomal proteins in human cancers. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, W.-J.; Liao, J.-M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Ebright, R.Y.; Lee, S.; Wittner, B.S.; Niederhoffer, K.L.; Nicholson, B.T.; Bardia, A.; Truesdell, S.; Wiley, D.F.; Wesley, B.; Li, S.; et al. Deregulation of ribosomal protein expression and translation promotes breast cancer metastasis. Science 2020, 367, 1468–1473. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Alejo, S.; Johnson, J.D.; Jayamohan, S.; Sareddy, G.R. Reticulocalbin 3 Is a Novel Mediator of Glioblastoma Progression. Cancers 2023, 15, 2008. https://doi.org/10.3390/cancers15072008
He Y, Alejo S, Johnson JD, Jayamohan S, Sareddy GR. Reticulocalbin 3 Is a Novel Mediator of Glioblastoma Progression. Cancers. 2023; 15(7):2008. https://doi.org/10.3390/cancers15072008
Chicago/Turabian StyleHe, Yi, Salvador Alejo, Jessica D. Johnson, Sridharan Jayamohan, and Gangadhara R. Sareddy. 2023. "Reticulocalbin 3 Is a Novel Mediator of Glioblastoma Progression" Cancers 15, no. 7: 2008. https://doi.org/10.3390/cancers15072008
APA StyleHe, Y., Alejo, S., Johnson, J. D., Jayamohan, S., & Sareddy, G. R. (2023). Reticulocalbin 3 Is a Novel Mediator of Glioblastoma Progression. Cancers, 15(7), 2008. https://doi.org/10.3390/cancers15072008





