Immune Environment and Immunotherapy in Endometrial Carcinoma and Cervical Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Endometrial Cancers
| FIGO Stage | TNM Category | Description |
|---|---|---|
| I | T1N0M0 | Tumor confined to the uterus, including endocervical glandular involvement |
| IA | T1aN0M0 | Tumor confined to the endometrium or invades <50% of the myometrial wall |
| IB | T1bN0M0 | Invasion ≥ 50% of the myometrium |
| II | T2N0M0 | Tumor invades cervical stroma, but does not extend beyond the uterus |
| III | T3N0-1M0 | Local and/or regional spread of the tumor |
| IIIA | T3aN0M0 | Tumor involves serosa or adnexa (direct extension or metastasis) |
| IIIB | T3bN0M0 | Tumor involves vagina or parametrial (direct extension or metastasis) |
| IIIC | T1-3N1M0 | Tumor with pelvic and/or para-aortic lymph node metastasis |
| IIIC1 | T1-3N1M0 | Tumor with positive pelvic nodes |
| IIIC2 | T1-3N1M0 | Tumor with positive para-aortic nodes with or without positive pelvic lymph nodes |
| IV | Tumor invades bladder and/or bowel mucosa and/or involves distant organs | |
| IVA | T4NxM0 | Tumor invades bladder mucosa and/or bowel mucosa |
| IVB | TxNxM1 | Distant metastasis, including intra-abdominal metastasis and/or inguinal nodes |
1.2. Cervical Cancers
2. The Immune Microenvironment of Endometrial Cancers and Immunotherapy-Based Clinical Trials
2.1. Immune Environment of Endometrial Cancers
2.2. Immunotherapy in Endometrial Cancers: Recent and Ongoing Trials (Table 5)
| Clinical Trial | Phase | Condition or Disease | Number of Patients | Drugs Combination | Mechanism of Action | Primary Endpoint (Time Frame) | Status |
|---|---|---|---|---|---|---|---|
| First-line treatment | |||||||
| DOMENICA (NCT05201547) [74] | III | dMMR (IIIC2/IV or first recurrent EC without curative treatment by RT, CT or surgery) | 142 | Dostarlimab vs. Chemotherapy | Anti-PD-1 (dostarlimab) | PFS (5 years) | Recruiting |
| AtTEND (NCT03603184) [75] | III | EC with residual disease after surgery or inoperable FIGO III–IV | 550 | Atezolizumab or placebo + taxane platinum-based CT | Anti-PD-L1 (atezolizumab) | OS and PFS (2 years) | Active, not recruiting |
| RUBY (NCT03981796) [76] | III | FIGO III-IV or first recurrent EC | 785 | Dostarlimab (or placebo) + taxane platinum-based CT followed by dostarlimab or niraparib (or placebo) | Anti-PD-1 (dostarlimab) and PARPi (niraparib) | Investigator assessed PFS and OS (6 years) | Active, not recruiting |
| EnGOT-en9 (NCT03884101) [77] | III | First-line treatment of FIGO stage III, IVA, IVB or recurrent EC | 875 | Pembrolizumab+ lenvatinib vs. taxane platinum-based CT | Anti-PD-1 (pembrolizumab) and protein kinase inhibitor (lenvatinib) | PFS (31 months) and OS (45 months) | Active, not recruiting |
| Advanced and recurrent EC | |||||||
| NRG-GY018 (NCT03914612) [78] | III | FIGO stage III, IVA, IVB or recurrent EC | 810 | Pembrolizumab or placebo + taxane platinum-based CT | Anti-PD-1 (pembrolizumab) | PFS (5 years) | Active, not recruiting |
| GYNET (NCT04652076) [79] | I/II | Pretreated advanced EC | 240 | Netrin-1 mAbs (NP137) + Carboplatin Plus Paclitaxel and/or Pembrolizumab | Anti-PD-1 (pembrolizumab) and anti-netrin-1 (NP137) | Dose limiting toxicity occurrence, ORR (12 weeks up to 2 years) | Recruiting |
| NCT04885413 [80] | II | Recurrent/advanced EC | 37 | Niraparib + sintilimab | PARPi (niraparib) and anti-PD-1 (sintilimab) | ORR (6 months) | Recruiting |
| NCT02912572 [81] | II | Recurrent or persistent EC MSI-H and/or POLEmut, MSS | 105 | Avelumab or Avelumab and Talazoparib or Avelumab and Axitinib | Anti-PD-L1 (avelumab) and anti-HER2 (talazoparib) and tyrosine kinase inhibitor (axitinib) | Activity of avelumab + talazoparib in EC, activity of avelumab + axitinib in MSS patients assessed by frequency of patients with PFS > 6 months or with objective tumor response (2 years) | Recruiting |
| NCT05036681 [82] | II | Metastatic EC not amenable to surgery or RT MSS | 30 | Futibatinib and Pembrolizumab | FGFR1-4 (futibatinib) and anti-PD-1 (pembrolizumab) | ORR, safety, and tolerability (1 year) | Recruiting |
| POD1UM-204 (NCT04463771) [83] | II | Advanced or metastatic EC with CT progression | 300 | Retifanlimab +/− epacadostat or pemigatinib or anti-LAG3 and anti-TIM3 | Anti-PD-1 (Retifanlimab) and anti-IDO1 (epacadostat) | ORR, PFS (2.5 years) | Recruiting |
| NCT05156268 [84] | II | Persistent/recurrent EC (including CS) | 25 | Pembrolizumab + olaparib | Anti-PD-1 (pembrolizumab) and PARPi (olaparib) | ORR (24 weeks) | Recruiting |
| NCT03526432 [85] | II | Pretreated advanced, recurrent, or persistent EC | 55 | Atezolizumab + bevacizumab | Anti-PD-L1 (atezolizumab) and anti-angiogenic (bevacizumab) | ORR (3 years) | Active, not recruiting |
| NCT03016338 [86] | II | Pretreated advanced/recurrent EC | 51 | Dostarlimab + niraparib | Anti-PD-1 (dostarlimab) and PARPi (niraparib) | Clinical benefit rate (16 weeks) | Active, not recruiting |
| DOMEC (NCT03951415) [87] | II | Recurrent or persistent EC | 55 | Olaparib + Durvalumab | Anti-PD-L1 (durvalumab) and PARPi (olaparib) | PFS (6 months) | Active, not recruiting |
| DUO-E (NCT04269200) [88] | III | After first-line treatment of advanced/recurrent EC | 699 | Taxan platinum-based CT and durvalumab followed by placebo vs. durvalumab vs. durvalumab and olaparib | Anti-PD-L1 (durvalumab) and PARPi (olaparib) | PFS (4 years) | Recruiting |
| Recurrent/relapsed/advanced EC dMMR and/or MSI-H only | |||||||
| CAN-RESPOND (NCT05550558) [89] | II | Recurrent sporadic dMMR EC | 43 | Camrelizumab + anlotinib | Anti-PD-1 (camrelizumab) and multitarget tyrosine kinase inhibitor (anlotinib) | ORR (24 months) | Not yet recruiting |
| NCT05419817 [90] | II | Recurrent EC and other solid tumors dMMR post PD1 exposure | 30 | Pembrolizumab + Sitravatinib | Anti-PD-1 (pembrolizumab) and receptor tyrosine kinases inhibitor (Sitravatinib) | ORR (12 weeks) | Recruiting |
| NCT05112601 [91] | II | Recurrent dMMR | 12 | Ipilimumab + Nivolumab vs. nivolumab | Anti-CTLA-4 (ipilimumab) and anti-PD-1 (nivolumab) | PFS (5 years) | Recruiting |
| Recurrent/relapsed/advanced EC MSS only | |||||||
| IMGN853 (NCT03835819) [92] | II | Advanced or recurrent serous EC, MSS, FRαpos | 35 | Mirvetuximab Soravtansine (IMGN853) and Pembrolizumab | Anti-FRα (Mirvetuximab soravtansine) and anti-PD-1 (pembrolizumab) | ORR, PFS (6 months) | Recruiting |
2.2.1. Anti-PD1/PD-L1
2.2.2. ICI and Anti-Angiogenic/Tyrosine Kinase Inhibitors
2.2.3. ICI and Polymerase Inhibitors
2.2.4. ICI and Other Targeted Therapies
3. The Immune Microenvironment of Cervical Cancers and Immunotherapy-Based Clinical Trials
3.1. Immune Environment of Cervical Cancers
3.2. Immunotherapy in Cervical Cancers: Recent and Ongoing Trials (Table 6)
| Clinical Trial | Phase | Condition or Disease | Number of Patients | Drugs Combination | Mechanism of Tion | Primary Endpoint (Time Frame) | Status |
|---|---|---|---|---|---|---|---|
| First-line treatment | |||||||
| NCT05311566 [116] | II | FIGO IB2-IIIB | 92 | Camrelizumab plus concurrent CRT | Anti-PD-1 (camrelizumab) | OS (3 years) | Recruiting |
| NCT04974944 [117] | II | FIGO IVB, Recurrent or Persistent | 172 | Camrelizumab and apatinib vs. paclitaxel and cisplatin and carboplatin and bevacizumab | Anti-PD-1 (camrelizumab) and anti-VEGFR2 (apatinib) | PFS (36 months) | Recruiting |
| NCT05511623 [118] | II | FIGO IIIC2 | 112 | Tislelizumab and concurrent CRT | Anti-PD-1 (tislelizumab) | PFS and side effects (3 years) | Not yet recruiting |
| FERMATA (NCT03912415) [119] | III | FIGO IVB | 316 | BCD-100 + CT +/− bevacizumab | Anti-PD-1 (BCD-100) | OS (3 years) | Recruiting |
| BEATcc (NCT03556839) [120] | III | FIGO IVB | 404 | Atezolizumab + CT+ bevacizumab | Anti-PD-L1 (atezolizumab) | PFS and OS (48 months) | Active, not recruiting |
| NCT04982237 [121] | III | FIGO IVB | 440 | Cadonilimab + CT +/− bevacizumab | Anti-PD-1/CTLA-4 bispecific antibody (Cadonilimab, AK104) | PFS and OS (2 years) | Recruiting |
| NCT04973904 [122] | II | Recurrent, Refractory and Metastatic CC | 35 | Toripalimab + paclitaxel + cisplatin + bevacizumab | Anti-PD-1 (toripalimab) | ORR (1 year) | Not yet recruiting |
| Recurrent/persistent/metastatic CC | |||||||
| ITTACC (NCT05614453) [123] | II | After Platinum-Based CT | 57 | Sitravatinib Tislelizumab | Tyrosine kinase inhibitor (sitravatinib) and anti-PD-1 (tislelizumab) | ORR (2 years) | Not yet recruiting |
| NCT03108495 [124] | II | Recurrent, metastatic, or persistent CC | 189 | LN-145 and LN-145 + Pembrolizumab | Autologous tumor infiltrating lymphocytes (TIL) infusion (LN-145) followed by IL-2 | ORR and AE (60 months) | Recruiting |
| NCT04646005 [125] | II | Recurrent/Metastatic HPV16 CC | 105 | Cemiplimab + ISA101b vaccine | Anti-PD-1 (cemipilimab) | ORR (36 months) | Recruiting |
| NCT05247619 [126] | II | Recurrent, metastatic, or persistent CC | 49 | Tislelizumab/Bevacizumab/Paclitaxel/Cisplatin/Carboplatin | Anti-PD-1 (tislelizumab) | Median PFS (24 months) | Recruiting |
| NCT05033132 [127] | II | Advanced CC | 177 | Balstilimab or balstilimab + Zalifrelimab | Anti-PD-L1 (balstilimab) and anti-CTLA-4 (zalifrelimab) | ORR (36 months) | Recruiting |
| ALARICE (NCT03826589) [128] | NA | Persistent or recurrent CC after platinum-based CT | 23 | Avelumab and Axitinib | Anti-PD-L1 (avelumab) and tyrosine kinase inhibitor (axitinib) | ORR (2years) | Recruiting |
| NCT03808857 [129] | II | Recurrent or metastatic, PD-L1 positive who failed in platinum-based CT | 80 | GB226 | Anti-PD-1 inhibitor (GB226) | ORR (2 years) | Recruiting |
| STAR (NCT04068753) [130] | II | Recurrent or progressive CC | 66 | Dostarlimab and niraparib | Anti-PD-1 (dostarlimab) and PARPi (niraparib) | Proportion of patients with response to treatment (1 year) | Recruiting |
| NCT05446883 [131] | III | First-Line treatment of persistent, recurrent or metastatic CC | 498 | QL1706 and placebo and paclitaxel and cisplatin/carboplatin with or without bevacizumab | Anti-PD-1 and –CTLA-4 (QL1706) | PFS and OS (2 years) | Recruiting |
| ATOMICC (NCT03833479) [132] | II | Maintenance therapy for patients with high-risk locally advanced CC | 132 | TSR-042 | Anti-PD-1 (TSR-042) | PFS (30 months) | Recruiting |
| ATEZOLACC (NCT03612791) [133] | II | Locally advanced CC | 189 | Atezolizumab and RCT | Anti-PD-L1 (atezolizumab) | PFS (24 months) | Recruiting |
3.2.1. PD-1 and PD-L1 Inhibitors
3.2.2. CTLA-4 Inhibitors
3.2.3. ICI and Tyrosine Kinase Inhibitors
3.2.4. ICI Combination
3.2.5. ICI, Anti-Angiogenics and Other Targets
3.2.6. Therapeutic Vaccines
4. Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, B.A.; Maiorano, M.F.P.; Lorusso, D.; Maiello, E. Ovarian Cancer in the Era of Immune Checkpoint Inhibitors: State of the Art and Future Perspectives. Cancers 2021, 13, 4438. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results Program. SEER. 2022. Available online: https://seer.cancer.gov/index.html (accessed on 7 January 2023).
- Kim, S.M.; Choi, H.S.; Byun, J.S. Overall 5-Year Survival Rate and Prognostic Factors in Patients with Stage IB and IIA Cervical Cancer Treated by Radical Hysterectomy and Pelvic Lymph Node Dissection. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2000, 10, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Endometrial Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Survival Rates for Vaginal Cancer. Available online: https://www.cancer.org/cancer/vaginal-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 7 January 2023).
- Adams, T.S.; Rogers, L.J.; Cuello, M.A. Cancer of the Vagina: 2021 Update. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 19–27. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial Cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Bendifallah, S.; Ouldamer, L.; Lavoue, V.; Canlorbe, G.; Raimond, E.; Coutant, C.; Graesslin, O.; Touboul, C.; Collinet, P.; Daraï, E.; et al. Patterns of Recurrence and Outcomes in Surgically Treated Women with Endometrial Cancer According to ESMO-ESGO-ESTRO Consensus Conference Risk Groups: Results from the FRANCOGYN Study Group. Gynecol. Oncol. 2017, 144, 107–112. [Google Scholar] [CrossRef]
- Friberg, E.; Orsini, N.; Mantzoros, C.S.; Wolk, A. Diabetes Mellitus and Risk of Endometrial Cancer: A Meta-Analysis. Diabetologia 2007, 50, 1365–1374. [Google Scholar] [CrossRef]
- Secord, A.A.; Hasselblad, V.; Von Gruenigen, V.E.; Gehrig, P.A.; Modesitt, S.C.; Bae-Jump, V.; Havrilesky, L.J. Body Mass Index and Mortality in Endometrial Cancer: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2016, 140, 184–190. [Google Scholar] [CrossRef]
- McVicker, L.; Cardwell, C.R.; Edge, L.; McCluggage, W.G.; Quinn, D.; Wylie, J.; McMenamin, Ú.C. Survival Outcomes in Endometrial Cancer Patients According to Diabetes: A Systematic Review and Meta-Analysis. BMC Cancer 2022, 22, 427. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Kuo, S.-J.; Liaw, Y.-P.; Avital, I.; Stojadinovic, A.; Man, Y.; Mannion, C.; Wang, J.; Chou, M.-C.; Tsai, H.-D.; et al. Endometrial Cancer Incidence in Breast Cancer Patients Correlating with Age and Duration of Tamoxifen Use: A Population Based Study. J. Cancer 2014, 5, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jiang, W.; Mao, K.; An, Y.; Su, F.; Kim, B.Y.S.; Liu, Q.; Jacobs, L.K. Elevated Risks of Subsequent Endometrial Cancer Development among Breast Cancer Survivors with Different Hormone Receptor Status: A SEER Analysis. Breast Cancer Res. Treat. 2015, 150, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, S.; Balasubramaniam, S.; Zhang, W.; Zhang, L.; Sridhara, R.; Spillman, D.; Mathai, J.P.; Scott, B.; Golding, S.J.; Coory, M.; et al. FDA Approval Summary: Pembrolizumab plus Lenvatinib for Endometrial Carcinoma, a Collaborative International Review under Project Orbis. Clin. Cancer Res. 2020, 26, 5062–5067. [Google Scholar] [CrossRef] [Green Version]
- Makker, V.; Colombo, N.; Casado Herráez, A.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Lenvatinib Plus Pembrolizumab in Patients with Either Treatment-Naive or Previously Treated Metastatic Renal Cell Carcinoma (Study 111/KEYNOTE-146): A Phase 1b/2 Study–The Lancet Oncology. Available online: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(21)00241-2/fulltext (accessed on 7 January 2023).
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouëlian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.-P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical Activity and Safety of the Anti–Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients with Recurrent or Advanced Mismatch Repair–Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020, 6, 1766. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Grants Accelerated Approval to Dostarlimab-Gxly for DMMR Endometrial Cancer; FDA: Silver Spring, MD, USA, 2021. [Google Scholar]
- EMA. Jemperli; European Medicines Agency: Amsterdam, The Netherlands, 2021; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/jemperli (accessed on 7 January 2023).
- Koskas, M.; Amant, F.; Mirza, M.R.; Creutzberg, C.L. Cancer of the Corpus Uteri: 2021 Update. Int. J. Gynecol. Obstet. 2021, 155, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical Cancer. Lancet Lond. Engl. 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Bosch, F.X.; Lorincz, A.; Muñoz, N.; Meijer, C.J.L.M.; Shah, K.V. The Causal Relation between Human Papillomavirus and Cervical Cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [Green Version]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Ibrahim Khalil, A.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the Global Burden of Cervical Cancer Associated with HIV. Lancet Glob. Health 2021, 9, e161–e169. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Moutinho, J.A. Smoking and Cervical Cancer. ISRN Obstet. Gynecol. 2011, 2011, 847684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervical Cancer–Risk Factors. Cancer.Net. Available online: https://www.cancer.net/cancer-types/cervical-cancer/risk-factors (accessed on 5 January 2023).
- Li, S.; Wen, X. Seropositivity to Herpes Simplex Virus Type 2, but Not Type 1 Is Associated with Cervical Cancer: NHANES (1999–2014). BMC Cancer 2017, 17, 726. [Google Scholar] [CrossRef] [Green Version]
- Yim, E.-K.; Park, J.-S. The Role of HPV E6 and E7 Oncoproteins in HPV-Associated Cervical Carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyer, B.A.; Zamarin, D.; Eskandar, R.N.; Mayadev, J.M. Role of Immunotherapy in the Management of Locally Advanced and Recurrent/Metastatic Cervical Cancer. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Otter, S.J.; Chatterjee, J.; Stewart, A.J.; Michael, A. The Role of Biomarkers for the Prediction of Response to Checkpoint Immunotherapy and the Rationale for the Use of Checkpoint Immunotherapy in Cervical Cancer. Clin. Oncol. R. Coll. Radiol. G. B. 2019, 31, 834–843. [Google Scholar] [CrossRef]
- Meng, Y.; Chu, T.; Lin, S.; Wu, P.; Zhi, W.; Peng, T.; Ding, W.; Luo, D.; Wu, P. Clinicopathological Characteristics and Prognosis of Cervical Cancer with Different Histological Types: A Population-Based Cohort Study. Gynecol. Oncol. 2021, 163, 545–551. [Google Scholar] [CrossRef]
- Watson, M.; Saraiya, M.; Benard, V.; Coughlin, S.S.; Flowers, L.; Cokkinides, V.; Schwenn, M.; Huang, Y.; Giuliano, A. Burden of Cervical Cancer in the United States, 1998-2003. Cancer 2008, 113 (Suppl. 10), 2855–2864. [Google Scholar] [CrossRef]
- Cervical Cancer Prognosis and Survival Rates–NCI. Available online: https://www.cancer.gov/types/cervical/survival (accessed on 5 January 2023).
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Meder, C.H.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 919–936. [Google Scholar] [CrossRef]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent Cisplatin-Based Radiotherapy and Chemotherapy for Locally Advanced Cervical Cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef]
- Monk, B.J.; Sill, M.W.; McMeekin, D.S.; Cohn, D.E.; Ramondetta, L.M.; Boardman, C.H.; Benda, J.; Cella, D. Phase III Trial of Four Cisplatin-Containing Doublet Combinations in Stage IVB, Recurrent, or Persistent Cervical Carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4649–4655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewari, K.S.; Sill, M.W.; Long, H.J.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Improved Survival with Bevacizumab in Advanced Cervical Cancer. N. Engl. J. Med. 2014, 370, 734–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, R.; Katsumata, N.; Shibata, T.; Kamura, T.; Kasamatsu, T.; Nakanishi, T.; Nishimura, S.; Ushijima, K.; Takano, M.; Satoh, T.; et al. Paclitaxel Plus Carboplatin versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.; Colombo, N.; McCormack, M.; Dreosti, L.; Nogueira-Rodrigues, A.; Scambia, G.; Lorusso, D.; Joly, F.; Schenker, M.; Ruff, P.; et al. Primary Results from CECILIA, a Global Single-Arm Phase II Study Evaluating Bevacizumab, Carboplatin and Paclitaxel for Advanced Cervical Cancer. Gynecol. Oncol. 2020, 159, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Sill, M.W.; McMeekin, D.S.; Leitao, M.M.; Brown, J.; Sutton, G.P.; Van Le, L.; Griffin, P.; Boardman, C.H. Phase II Trial of Cetuximab in the Treatment of Persistent or Recurrent Squamous or Non-Squamous Cell Carcinoma of the Cervix: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2011, 122, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.-S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Approves Pembrolizumab Combination for the First-Line Treatment of Cervical Cancer; FDA: Silver Spring, MD, USA, 2022. [Google Scholar]
- EMA. Libtayo: Pending EC Decision; European Medicines Agency: Amsterdam, The Netherlands, 2022; Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/libtayo-0 (accessed on 7 January 2023).
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. CB 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Emens, L.A.; Ascierto, P.A.; Darcy, P.K.; Demaria, S.; Eggermont, A.M.M.; Redmond, W.L.; Seliger, B.; Marincola, F.M. Cancer Immunotherapy: Opportunities and Challenges in the Rapidly Evolving Clinical Landscape. Eur. J. Cancer 2017, 81, 116–129. [Google Scholar] [CrossRef]
- Low, J.L.; Walsh, R.J.; Ang, Y.; Chan, G.; Soo, R.A. The Evolving Immuno-Oncology Landscape in Advanced Lung Cancer: First-Line Treatment of Non-Small Cell Lung Cancer. Ther. Adv. Med. Oncol. 2019, 11, 175883591987036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, L.C.; Desai, K.; Zhang, T.; Ornstein, M.C. The Immunotherapy Landscape in Renal Cell Carcinoma. BioDrugs 2020, 34, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Vanderstraeten, A.; Tuyaerts, S.; Amant, F. The Immune System in the Normal Endometrium and Implications for Endometrial Cancer Development. J. Reprod. Immunol. 2015, 109, 7–16. [Google Scholar] [CrossRef]
- León-Castillo, A.; Britton, H.; McConechy, M.K.; McAlpine, J.N.; Nout, R.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; Rau, T.T.; et al. Interpretation of Somatic POLE Mutations in Endometrial Carcinoma. J. Pathol. 2020, 250, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.-Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 2017, PO.17.00073. [Google Scholar] [CrossRef] [PubMed]
- Pakish, J.B.; Zhang, Q.; Jazaeri, A.A.; Celestino, J.; Kwan, S.Y.; Mok, S.; Yates, M.; Lu, K.H. Endometrial Cancer Subtypes and Immunotherapy: Is the Immune Microenvironment Different in Microsatellite Instable Endometrial Cancer? Gynecol. Oncol. 2016, 141, 6. [Google Scholar] [CrossRef] [Green Version]
- Pakish, J.B.; Zhang, Q.; Chen, Z.; Liang, H.; Chisholm, G.B.; Yuan, Y.; Mok, S.C.; Broaddus, R.R.; Lu, K.H.; Yates, M.S. Immune Microenvironment in Microsatellite-Instable Endometrial Cancers: Hereditary or Sporadic Origin Matters. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4473–4481. [Google Scholar] [CrossRef] [Green Version]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D’Andrea, A.D.; Wu, C.J.; et al. Association of Polymerase E-Mutated and Microsatellite-Instable Endometrial Cancers with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef] [Green Version]
- van Gool, I.C.; Eggink, F.A.; Freeman-Mills, L.; Stelloo, E.; Marchi, E.; de Bruyn, M.; Palles, C.; Nout, R.A.; de Kroon, C.D.; Osse, E.M.; et al. POLE Proofreading Mutations Elicit an Antitumor Immune Response in Endometrial Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3347–3355. [Google Scholar] [CrossRef] [Green Version]
- Kondratiev, S.; Sabo, E.; Yakirevich, E.; Lavie, O.; Resnick, M.B. Intratumoral CD8+ T Lymphocytes as a Prognostic Factor of Survival in Endometrial Carcinoma. Clin. Cancer Res. 2004, 10, 4450–4456. [Google Scholar] [CrossRef] [Green Version]
- de Jong, R.A.; Leffers, N.; Boezen, H.M.; ten Hoor, K.A.; van der Zee, A.G.J.; Hollema, H.; Nijman, H.W. Presence of Tumor-Infiltrating Lymphocytes Is an Independent Prognostic Factor in Type I and II Endometrial Cancer. Gynecol. Oncol. 2009, 114, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Kübler, K.; Ayub, T.H.; Weber, S.K.; Zivanovic, O.; Abramian, A.; Keyver-Paik, M.-D.; Mallmann, M.R.; Kaiser, C.; Serçe, N.B.; Kuhn, W.; et al. Prognostic Significance of Tumor-Associated Macrophages in Endometrial Adenocarcinoma. Gynecol. Oncol. 2014, 135, 176–183. [Google Scholar] [CrossRef]
- Halla, K. Emerging Treatment Options for Advanced or Recurrent Endometrial Cancer. J. Adv. Pract. Oncol. 2022, 13, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Mamat @ Yusof, M.N.; Chew, K.T.; Kampan, N.; Abd Aziz, N.H.; Md Zin, R.R.; Tan, G.C.; Shafiee, M.N. PD-L1 Expression in Endometrial Cancer and Its Association with Clinicopathological Features: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3911. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ino, K.; Yoshida, N.; Kajiyama, H.; Shibata, K.; Yamamoto, E.; Kidokoro, K.; Takahashi, N.; Terauchi, M.; Nawa, A.; Nomura, S.; et al. Indoleamine 2,3-Dioxygenase Is a Novel Prognostic Indicator for Endometrial Cancer. Br. J. Cancer 2006, 95, 1555–1561. [Google Scholar] [CrossRef] [Green Version]
- De Jong, R.A.; Kema, I.P.; Boerma, A.; Boezen, H.M.; der Want, J.J.L.v.; Gooden, M.J.M.; Hollema, H.; Nijman, H.W. Prognostic Role of Indoleamine 2,3-Dioxygenase in Endometrial Carcinoma. Gynecol. Oncol. 2012, 126, 474–480. [Google Scholar] [CrossRef]
- Ino, K.; Yamamoto, E.; Shibata, K.; Kajiyama, H.; Yoshida, N.; Terauchi, M.; Nawa, A.; Nagasaka, T.; Takikawa, O.; Kikkawa, F. Inverse Correlation between Tumoral Indoleamine 2,3-Dioxygenase Expression and Tumor-Infiltrating Lymphocytes in Endometrial Cancer: Its Association with Disease Progression and Survival. Clin. Cancer Res. 2008, 14, 2310–2317. [Google Scholar] [CrossRef] [Green Version]
- Mills, A.; Zadeh, S.; Sloan, E.; Chinn, Z.; Modesitt, S.C.; Ring, K.L. Indoleamine 2,3-Dioxygenase in Endometrial Cancer: A Targetable Mechanism of Immune Resistance in Mismatch Repair-Deficient and Intact Endometrial Carcinomas. Mod. Pathol. 2018, 31, 1282–1290. [Google Scholar] [CrossRef] [Green Version]
- Kozłowski, M.; Borzyszkowska, D.; Cymbaluk-Płoska, A. The Role of TIM-3 and LAG-3 in the Microenvironment and Immunotherapy of Ovarian Cancer. Biomedicines 2022, 10, 2826. [Google Scholar] [CrossRef]
- Friedman, L.A.; Ring, K.L.; Mills, A.M. LAG-3 and GAL-3 in Endometrial Carcinoma: Emerging Candidates for Immunotherapy. Int. J. Gynecol. Pathol. 2020, 39, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, R.; Xu, C.; Zhang, Y.; Deng, M.; Wu, D.; Tang, F.; Liu, X.; Han, Y.; Zhan, Y.; et al. Analysis of the Immune Checkpoint Lymphocyte Activation Gene-3 (LAG-3) in Endometrial Cancer: An Emerging Target for Immunotherapy. Pathol. Res. Pract. 2022, 236, 153990. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- ARCAGY/GINECO GROUP. Randomized Phase III Trial in MMR Deficient Endometrial Cancer Patients Comparing Chemotherapy Alone versus Dostarlimab in First Line Advanced/Metastatic Setting; Clinical Trial Registration NCT05201547; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05201547 (accessed on 28 December 2022).
- Mario Negri Institute for Pharmacological Research. Phase III Double-Blind Randomized Placebo Controlled Trial of Atezolizumab in Combination With Paclitaxel and Carboplatin in Women With Advanced/Recurrent Endometrial Cancer; Clinical Trial Registration NCT03603184; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03603184 (accessed on 29 December 2022).
- Tesaro, Inc. A Phase 3, Randomized, Double-Blind, Multicenter Study of Dostarlimab (TSR-042) Plus Carboplatin-Paclitaxel versus Placebo Plus Carboplatin-Paclitaxel in Patients with Recurrent or Primary Advanced Endometrial Cancer (RUBY); Clinical Trial Registration NCT03981796; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03981796 (accessed on 29 December 2022).
- Merck Sharp & Dohme LLC. A Phase 3 Randomized, Open-Label, Study of Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) versus Chemotherapy for First-Line Treatment of Advanced or Recurrent Endometrial Carcinoma (LEAP-001); Clinical Trial Registration NCT03884101; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03884101 (accessed on 29 December 2022).
- National Cancer Institute (NCI). A Phase III Randomized, Placebo-Controlled Study of Pembrolizumab (MK-3475, NSC #776864) in Addition to Paclitaxel and Carboplatin for Measurable Stage III or IVA, Stage IVB or Recurrent Endometrial Cancer; Clinical Trial Registration NCT03914612; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03914612 (accessed on 29 December 2022).
- NETRIS Pharma. A Randomized, Multicenter, Open Label, Phase I/II Study to Evaluate the Safety, Clinical and Biological Activity of a Humanized Monoclonal Antibody Targeting Netrin-1 (NP137) in Combination With Carboplatin Plus Paclitaxel and/or Pembrolizumab in Patients With Locally Advanced/Metastatic Endometrial Carcinoma or Cervix Carcinoma Progressing/Relapsing after at Least One Prior Systemic Chemotherapy.; Clinical Trial Registration NCT04652076; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04652076 (accessed on 28 December 2022).
- Li, J. An Open-Label, Single Arm, Phase II Trial of Niraparib in Combination with Anti-PD1 Antibody in Recurrent/Advanced Stage Endometrial Cancer Patients; Clinical Trial Registration NCT04885413; clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04885413 (accessed on 28 December 2022).
- Konstantinopoulos, P.A.; Gockley, A.A.; Xiong, N.; Krasner, C.; Horowitz, N.; Campos, S.; Wright, A.A.; Liu, J.F.; Shea, M.; Yeku, O.; et al. Evaluation of Treatment with Talazoparib and Avelumab in Patients with Recurrent Mismatch Repair Proficient Endometrial Cancer. JAMA Oncol. 2022, 8, 1317–1322. [Google Scholar] [CrossRef]
- M.D. Anderson Cancer Center. A Phase II Study of Futibatinib and Pembrolizumab in Metastatic Microsatellite Stable Endometrial Carcinoma; Clinical Trial Registration NCT05036681; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05036681 (accessed on 28 December 2022).
- Incyte Corporation. An Umbrella Study of INCMGA00012 Alone and in Combination with Other Therapies in Participants with Advanced or Metastatic Endometrial Cancer Who Have Progressed on or after Platinum-Based Chemotherapy (POD1UM-204); Clinical Trial Registration NCT04463771; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04463771 (accessed on 28 December 2022).
- Memorial Sloan Kettering Cancer Center. A Phase II Trial sof Pembrolizumab Plus Olaparib for the Treatment of Patients with Persistent/Recurrent Endometrial Cancers; Clinical Trial Registration NCT05156268; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05156268 (accessed on 28 December 2022).
- University of Oklahoma. A Phase II, Single Arm Study of Atezolizumab + Bevacizumab in Women with Advanced, Recurrent or Persistent Endometrial Cancer; Clinical Trial Registration NCT03526432; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03526432 (accessed on 29 December 2022).
- University Health Network, Toronto. A Phase II, Open Label Study of the Poly(ADP-Ribose) Polymerase Inhibitor Niraparib in Monotherapy or in Combination With Anti-PD1 Inhibitor TSR-042 in Recurrent Endometrial Cancer; Clinical Trial Registration NCT03016338; clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03016338 (accessed on 29 December 2022).
- Kroep, J.R. Durvalumab and Olaparib in Metastatic or Recurrent Endometrial Cancer; Clinical Trial Registration NCT03951415; clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03951415 (accessed on 28 December 2022).
- AstraZeneca. A Randomised, Multicentre, Double-Blind, Placebo-Controlled, Phase III Study of First-Line Carboplatin and Paclitaxel in Combination with Durvalumab, Followed by Maintenance Durvalumab with or without Olaparib in Patients with Newly Diagnosed Advanced or Recurrent Endometrial Cancer (DUO-E); Clinical Trial Registration NCT04269200; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04269200 (accessed on 28 December 2022).
- Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University. Camrelizumab Plus Anlotinib in Patients with Recurrent Sporadic Mismatch Repair Deficient Endometrial Cancer (CAN-RESPOND): A Single-Arm, Multicentre, Phase 2 Study; Clinical Trial Registration NCT05550558; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05550558 (accessed on 28 December 2022).
- Mahdi, H. Pembrolizumab with Sitravatinib in Recurrent Endometrial Cancer and Other Solid Tumors with Deficient Mismatch Repair System Post PD1 Exposure: Phase II Trial; Clinical Trial Registration NCT05419817; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05419817 (accessed on 28 December 2022).
- National Cancer Institute (NCI). A Randomized Phase II Trial of Nivolumab and Ipilimumab Compared to Nivolumab Monotherapy in Patients with Deficient Mismatch Repair System Recurrent Endometrial Carcinoma; Clinical Trial Registration NCT05112601; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05112601 (accessed on 28 December 2022).
- Konstantinopoulo, P. A Phase 2, Two-Stage, Study of Mirvetuximab Soravtansine (IMGN853) in Combination with Pembrolizumab in Patients with Microsatellite Stable (MSS) Recurrent or Persistent Endometrial Cancer (EC); Clinical Trial Registration NCT03835819; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03835819 (accessed on 28 December 2022).
- Katsumata, N.; Tamura, K.; Hasegawa, K.; Matsumoto, K.; Mukai, H.; Takahashi, S.; Nomura, H.; Minami, H. Efficacy and Safety of Nivolumab in Patients with Uterine Cervical Cancer, Uterine Corpus Cancer, or Soft-Tissue Sarcoma. Ann. Oncol. 2018, 29, vii60. [Google Scholar] [CrossRef]
- Liu, J.F.; Gordon, M.; Veneris, J.; Braiteh, F.; Balmanoukian, A.; Eder, J.P.; Oaknin, A.; Hamilton, E.; Wang, Y.; Sarkar, I.; et al. Safety, Clinical Activity and Biomarker Assessments of Atezolizumab from a Phase I Study in Advanced/Recurrent Ovarian and Uterine Cancers. Gynecol. Oncol. 2019, 154, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Luo, W.; Liu, J.F.; Gulhan, D.C.; Krasner, C.; Ishizuka, J.J.; Gockley, A.A.; Buss, M.; Growdon, W.B.; Crowe, H.; et al. Phase II Study of Avelumab in Patients with Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2786–2794. [Google Scholar] [CrossRef]
- Antill, Y.; Kok, P.S.; Stockler, M.R.; Robledo, K.; Yip, S.; Parry, M.; Smith, D.; Spurdle, A.; Barnes, E.; Friedlander, M.L.; et al. Updated Results of Activity of Durvalumab in Advanced Endometrial Cancer (AEC) According to Mismatch Repair (MMR) Status: The Phase II PHAEDRA Trial (ANZGOG1601). Ann. Oncol. 2019, 30, ix192. [Google Scholar] [CrossRef]
- Jemperli (Dostarlimab) RUBY Phase III Trial Met Its Primary Endpoint in a Planned Interim Analysis in Patients with Primary Advanced or Recurrent Endometrial Cancer|GSK. Available online: https://www.gsk.com/en-gb/media/press-releases/jemperli-dostarlimab-ruby-phase-iii-trial-met-its-primary-endpoint-in-a-planned-interim-analysis-in-patients-with-primary-advanced-or-recurrent-endometrial-cancer/ (accessed on 1 February 2023).
- Lheureux, S.; Matei, D.E.; Konstantinopoulos, P.A.; Wang, B.X.; Gadalla, R.; Block, M.S.; Jewell, A.; Gaillard, S.L.; McHale, M.; McCourt, C.; et al. Translational Randomized Phase II Trial of Cabozantinib in Combination with Nivolumab in Advanced, Recurrent, or Metastatic Endometrial Cancer. J. Immunother. Cancer 2022, 10, e004233. [Google Scholar] [CrossRef] [PubMed]
- Grandin, M.; Meier, M.; Delcros, J.G.; Nikodemus, D.; Reuten, R.; Patel, T.R.; Goldschneider, D.; Orriss, G.; Krahn, N.; Boussouar, A.; et al. Structural Decoding of the Netrin-1/UNC5 Interaction and Its Therapeutical Implications in Cancers. Cancer Cell 2016, 29, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Bruikman, C.S.; Zhang, H.; Kemper, A.M.; van Gils, J.M. Netrin Family: Role for Protein Isoforms in Cancer. J. Nucleic Acids 2019, 2019, 3947123. [Google Scholar] [CrossRef]
- Sun, Y.; Manceau, A.; Frydman, L.; Cappuccio, L.; Neves, D.; Basso, V.; Wang, H.; Fombonne, J.; Maisse, C.; Mehlen, P.; et al. Δ40p53 Isoform Up-Regulates Netrin-1/UNC5B Expression and Potentiates Netrin-1 pro-Oncogenic Activity. Proc. Natl. Acad. Sci. USA 2021, 118, e2103319118. [Google Scholar] [CrossRef]
- Adamczyk-Gruszka, O.; Horecka-Lewitowicz, A.; Gruszka, J.; Wawszczak-Kasza, M.; Strzelecka, A.; Lewitowicz, P. FGFR-2 and Epithelial–Mesenchymal Transition in Endometrial Cancer. J. Clin. Med. 2022, 11, 5416. [Google Scholar] [CrossRef]
- Winterhoff, B.; Konecny, G.E. Targeting Fibroblast Growth Factor Pathways in Endometrial Cancer. Curr. Probl. Cancer 2017, 41, 37–47. [Google Scholar] [CrossRef]
- Senol, S.; Ceyran, A.B.; Aydin, A.; Zemheri, E.; Ozkanli, S.; Kösemetin, D.; Sehitoglu, I.; Akalin, I. Folate Receptor α Expression and Significance in Endometrioid Endometrium Carcinoma and Endometrial Hyperplasia. Int. J. Clin. Exp. Pathol. 2015, 8, 5633–5641. [Google Scholar]
- Allouch, S.; Malki, A.; Allouch, A.; Gupta, I.; Vranic, S.; Al Moustafa, A.-E. High-Risk HPV Oncoproteins and PD-1/PD-L1 Interplay in Human Cervical Cancer: Recent Evidence and Future Directions. Front. Oncol. 2020, 10, 914. [Google Scholar] [CrossRef]
- Münger, K.; Howley, P.M. Human Papillomavirus Immortalization and Transformation Functions. Virus Res. 2002, 89, 213–228. [Google Scholar] [CrossRef]
- Tindle, R.W. Immune Evasion in Human Papillomavirus-Associated Cervical Cancer. Nat. Rev. Cancer 2002, 2, 59–64. [Google Scholar] [CrossRef]
- Piersma, S.J.; Jordanova, E.S.; van Poelgeest, M.I.E.; Kwappenberg, K.M.C.; van der Hulst, J.M.; Drijfhout, J.W.; Melief, C.J.M.; Kenter, G.G.; Fleuren, G.J.; Offringa, R.; et al. High Number of Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes Is Associated with the Absence of Lymph Node Metastases in Patients with Large Early-Stage Cervical Cancer. Cancer Res. 2007, 67, 354–361. [Google Scholar] [CrossRef] [Green Version]
- De Vos van Steenwijk, P.J.; Ramwadhdoebe, T.H.; Goedemans, R.; Doorduijn, E.M.; van Ham, J.J.; Gorter, A.; van Hall, T.; Kuijjer, M.L.; van Poelgeest, M.I.E.; van der Burg, S.H.; et al. Tumor-Infiltrating CD14-Positive Myeloid Cells and CD8-Positive T-Cells Prolong Survival in Patients with Cervical Carcinoma: Clinical Benefit of CD14+ Cells in Cervical Cancer. Int. J. Cancer 2013, 133, 2884–2894. [Google Scholar] [CrossRef]
- Jordanova, E.S.; Gorter, A.; Ayachi, O.; Prins, F.; Durrant, L.G.; Kenter, G.G.; van der Burg, S.H.; Fleuren, G.J. Human Leukocyte Antigen Class I, MHC Class I Chain-Related Molecule A, and CD8+/Regulatory T-Cell Ratio: Which Variable Determines Survival of Cervical Cancer Patients? Clin. Cancer Res. 2008, 14, 2028–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Lü, W.; Zhang, X.; Lü, B. Tumor-Infiltrating CD8+ and FOXP3+ Lymphocytes before and after Neoadjuvant Chemotherapy in Cervical Cancer. Diagn. Pathol. 2018, 13, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, W.; Yan, X.; Jing, L.; Zhou, Y.; Chen, H.; Wang, Y. A Reversed CD4/CD8 Ratio of Tumor-Infiltrating Lymphocytes and a High Percentage of CD4+FOXP3+ Regulatory T Cells Are Significantly Associated with Clinical Outcome in Squamous Cell Carcinoma of the Cervix. Cell. Mol. Immunol. 2011, 8, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.R.; Machado, C.M.T.; Coxir, S.A.; de Oliveira, A.J.; Moreira, T.B.; Campos, L.S.; Alcântara, R.; de Paula, S.O.C.; de Oliveira Salles, P.G.; Gollob, K.J.; et al. Cervical Cancer Patients That Respond to Chemoradiation Therapy Display an Intense Tumor Infiltrating Immune Profile before Treatment. Exp. Mol. Pathol. 2019, 111, 104314. [Google Scholar] [CrossRef]
- Dorta-Estremera, S.; Colbert, L.E.; Nookala, S.S.; Yanamandra, A.V.; Yang, G.; Delgado, A.; Mikkelson, M.; Eifel, P.; Jhingran, A.; Lilie, L.L.; et al. Kinetics of Intratumoral Immune Cell Activation During Chemoradiation for Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, M.; Jing, Y.; Cheng, J.; Zhang, C.; Cheng, L.; Lu, H.; Cai, M.-C.; Wu, J.; Wang, W.; et al. Baseline Immunity and Impact of Chemotherapy on Immune Microenvironment in Cervical Cancer. Br. J. Cancer 2021, 124, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Effectiveness and Safety of Camrelizumab Combined with Concurrent Chemoradiotherapy for FIGO IB2-IIIB Cervical Cancer: A Single-Center, Single-Arm, Open-Phase II Clinical Study; Clinical Trial Registration NCT05311566; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05311566 (accessed on 3 January 2023).
- Huang, X. An Open-Label, Phase 2 Randomized Trial of Camrelizumab (SHR1210) Plus Apatinib versus Paclitaxel and Cisplatin/Carboplatin Plus Bevacizumab as a First-Line Therapy in Patients with Stage IVB, Recurrent, or Persistent Cervical Cancer; Clinical Trial Registration NCT04974944; clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04974944 (accessed on 3 January 2023).
- First Affiliated Hospital of Guangxi Medical University. Efficacy and Safety of Tislelizumab Combined with Concurrent Chemoradiotherapy as First-Line Treatment for Stage IIIC2 Cervical Cancer; Clinical Trial Registration NCT05511623; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05511623 (accessed on 3 January 2023).
- Biocad. An International Randomized Double-Blind Clinical Trial of BCD-100 Plus Platinum-Based Chemotherapy with and without Bevacizumab versus Placebo Plus Platinum-Based Chemotherapy with and without Bevacizumab as First-Line Treatment of Subjects with Advanced Cervical Cancer; Clinical Trial Registration NCT03912415; clinicaltrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03912415 (accessed on 3 January 2023).
- Grupo Español de Investigación en Cáncer de Ovario. A Randomized Phase III Trial of Platinum Chemotherapy Plus Paclitaxel with Bevacizumab and Atezolizumab versus Platinum Chemotherapy Plus Paclitaxel and Bevacizumab in Metastatic (Stage IVB), Persistent, or Recurrent Carcinoma of the Cervix; Clinical Trial Registration NCT03556839; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03556839 (accessed on 31 January 2023).
- Akeso. A Randomized, Double-Blind, Placebo-Controlled Phase III Study to Evaluate AK104 Plus Platinum-Containing Chemotherapy with or without Bevacizumab as First-Line Treatment for Persistent, Recurrent, or Metastatic Cervical Cancer; Clinical Trial Registration NCT04982237; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04982237 (accessed on 31 January 2023).
- Peking Union Medical College Hospital. A Single-Arm, Multicenter, Phase II Study to Investigate Efficacy and Safety of Toripalimab Combined with Chemotherapy and Bevacizumab as First-Line Treatment in Patients with Recurrent, Refractory and Metastatic Cervical Cancer; Clinical Trial Registration NCT04973904; clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04973904 (accessed on 3 January 2023).
- Australia New Zealand Gynaecological Oncology Group. A Phase II Trial of Tislelizumab in Combination with Sitravatinib for Recurrent/Metastatic Cervical Cancer after Platinum-Based Chemotherapy; Clinical Trial Registration NCT05614453; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05614453 (accessed on 3 January 2023).
- Iovance Biotherapeutics, Inc. A Phase 2, Multicenter Study to Evaluate the Efficacy and Safety Using Autologous Tumor Infiltrating Lymphocytes (LN-145) in Patients with Recurrent, Metastatic or Persistent Cervical Carcinoma; Clinical Trial Registration NCT03108495; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03108495 (accessed on 3 January 2023).
- Regeneron Pharmaceuticals. A Phase 2 Study of Cemiplimab, an Anti-PD-1 Monoclonal Antibody, and ISA101b Vaccine in Patients with Recurrent/Metastatic HPV16 Cervical Cancer Who Have Experienced Disease Progression after First Line Chemotherapy; Clinical Trial Registration NCT04646005; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04646005 (accessed on 3 January 2023).
- Zhu, J. A Clinical Study to Explore the Efficacy and Safety of Tislelizumab in Combination with Bevacizumab and Chemotherapy in Patients with Persistent, Recurrent, or Metastatic Cervical Cancer; Clinical Trial Registration NCT05247619; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05247619 (accessed on 3 January 2023).
- Agenus Inc. An Open-Label, Multicenter Phase II Study Evaluating Balstilimab Alone or Balstilimab in Combination with Zalifrelimab in Patients with Advanced Cervical Cancer; Clinical Trial Registration NCT05033132; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05033132 (accessed on 3 January 2023).
- Tse, D.K.-Y. Avelumab with Axitinib in Persistent or Recurrent Cervical Cancer after Platinum-Based Chemotherapy–A Proof-of-Concept Study (ALARICE Study); Clinical Trial Registration NCT03826589; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03826589 (accessed on 3 January 2023).
- Genor Biopharma Co., Ltd. Phase II Clinical Study to Evaluate the Efficacy and Safety of GB226 in Treatment of Recurrent or Metastatic Cervical Cancer Patients with PD-L1 Positive Who Failed in Platinum-Based Chemotherapy; Clinical Trial Registration NCT03808857; clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03808857 (accessed on 3 January 2023).
- University of Oklahoma. Phase II Trial of Niraparib in Combination with Dostarlimab in Patients with Recurrent or Progressive Cervix Cancer; Clinical Trial Registration NCT04068753; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04068753 (accessed on 3 January 2023).
- Qilu Pharmaceutical Co., Ltd. A Randomized, Double-Blind, Placebo-Controlled Phase III Study to Evaluate QL1706 Plus Paclitaxel-Cisplatin/Carboplatin with or without Bevacizumab for the First-Line Treatment of Persistent, Recurrent or Metastatic Cervical Cancer; Clinical Trial Registration NCT05446883; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05446883 (accessed on 3 January 2023).
- Grupo Español de Investigación en Cáncer de Ovario. A Randomized, Open Label, Phase II Trial of Anti-PD1, TSR-042, as Maintenance Therapy for Patients with High-Risk Locally Advanced Cervical Cancer after Chemo-Radiation; Clinical Trial Registration NCT03833479; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03833479 (accessed on 3 January 2023).
- Roussy, G.; Cancer Campus, Grand Paris. Randomized Phase II Trial Assessing the Inhibitor of Programmed Cell Death Ligand 1 (PD-L1) Immune Checkpoint Atezolizumab in Locally Advanced Cervical Cancer; Clinical Trial Registration NCT03612791; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03612791 (accessed on 3 January 2023).
- Santin, A.D.; Deng, W.; Frumovitz, M.; Buza, N.; Bellone, S.; Huh, W.; Khleif, S.; Lankes, H.A.; Ratner, E.S.; O’Cearbhaill, R.E.; et al. Phase II Evaluation of Nivolumab in the Treatment of Persistent or Recurrent Cervical Cancer (NCT02257528/NRG-GY002). Gynecol. Oncol. 2020, 157, 161–166. [Google Scholar] [CrossRef]
- Naumann, R.W.; Hollebecque, A.; Meyer, T.; Devlin, M.-J.; Oaknin, A.; Kerger, J.; López-Picazo, J.M.; Machiels, J.-P.; Delord, J.-P.; Evans, T.R.J.; et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results from the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2825–2834. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Oaknin, A.; Monk, B.J.; Selle, F.; Rojas, C.; Gladieff, L.; Berton, D.; Leary, A.; Moore, K.N.; Estevez-Diz, M.D.P.; et al. Phase II Study of the Safety and Efficacy of the Anti-PD-1 Antibody Balstilimab in Patients with Recurrent and/or Metastatic Cervical Cancer. Gynecol. Oncol. 2021, 163, 274–280. [Google Scholar] [CrossRef]
- Mayadev, J.; Nunes, A.T.; Li, M.; Marcovitz, M.; Lanasa, M.C.; Monk, B.J. CALLA: Efficacy and Safety of Concurrent and Adjuvant Durvalumab with Chemoradiotherapy versus Chemoradiotherapy Alone in Women with Locally Advanced Cervical Cancer: A Phase III, Randomized, Double-Blind, Multicenter Study. Int. J. Gynecol. Cancer 2020, 30, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Update on CALLA Phase III trial of Concurrent Use of IMFINZI and Chemoradiotherapy in Locally Advanced Cervical Cancer. Available online: https://www.astrazeneca.com/media-centre/press-releases/2022/update-on-calla-phase-iii-trial-for-imfinzi.html (accessed on 1 February 2023).
- National Cancer Institute (NCI). Anti PD-L1 (Atezolizumab) as an Immune Primer and Concurrently with Extended Field Chemoradiotherapy for Node Positive Locally Advanced Cervical Cancer; Clinical Trial Registration NCT03738228; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03738228 (accessed on 1 February 2023).
- Lheureux, S.; Butler, M.O.; Clarke, B.; Cristea, M.C.; Martin, L.P.; Tonkin, K.S.; Fleming, G.F.; Tinker, A.; Hirte, H.W.; Tsoref, D.; et al. A Phase I/II Study of Ipilimumab in Women with Metastatic or Recurrent Cervical Carcinoma: A Study of the Princess Margaret and Chicago N01 Consortia. J. Clin. Oncol. 2015, 33 (Suppl. 15), 3061. [Google Scholar] [CrossRef]
- Mayadev, J.S.; Enserro, D.; Lin, Y.G.; Da Silva, D.M.; Lankes, H.A.; Aghajanian, C.; Ghamande, S.; Moore, K.N.; Kennedy, V.A.; Fracasso, P.M.; et al. Sequential Ipilimumab after Chemoradiotherapy in Curative-Intent Treatment of Patients with Node-Positive Cervical Cancer. JAMA Oncol. 2020, 6, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Shen, J.; Wang, Y.; Li, J.; Liu, Z.; He, M.; Cao, X.; Ling, J.; Huang, J.; Zheng, M.; et al. Camrelizumab Plus Apatinib in Patients with Advanced Cervical Cancer (CLAP): A Multicenter, Open-Label, Single-Arm, Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 4095–4106. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, Y.; Peng, H.; Yan, S.; Liu, Z.; Tong, C.; Huang, X. Multiparametric Immune Profiling of Advanced Cervical Cancer to Predict Response to Programmed Death-1 Inhibitor Combination Therapy: An Exploratory Study of the CLAP Trial. Clin. Transl. Oncol. 2022, 25, 256–268. [Google Scholar] [CrossRef]
- Bristol-Myers Squibb. Non-Comparative, Open-Label, Multiple Cohort, Phase 1/2 Study of Nivolumab Monotherapy and Nivolumab Combination Therapy in Subjects with Virus-Positive and Virus-Negative Solid Tumors; Clinical Trial Registration NCT02488759; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02488759 (accessed on 3 January 2023).
- O’Malley, D.M.; Neffa, M.; Monk, B.J.; Melkadze, T.; Huang, M.; Kryzhanivska, A.; Bulat, I.; Meniawy, T.M.; Bagameri, A.; Wang, E.W.; et al. Dual PD-1 and CTLA-4 Checkpoint Blockade Using Balstilimab and Zalifrelimab Combination as Second-Line Treatment for Advanced Cervical Cancer: An Open-Label Phase II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 762–771. [Google Scholar] [CrossRef]
- ARCAGY/GINECO GROUP. A Multicenter, Pilot Study Evaluating Immune Impact and Safety of Nivolumab in Combination with Ipilimumab (Immune Combination) before Initial RT-CT Treatment for Cervix Cancer.: The French GINECO–COLIBRI Study; Clinical Trial Registration NCT04256213; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04256213 (accessed on 19 January 2023).
- Hoffmann-La Roche. A Phase II, Safety, and Efficacy Study of Tiragolumab Plus Atezolizumab and Atezolizumab Monotherapy in Patients with Metastatic and/or Recurrent PD-L1-Positive Cervical Cancer; Clinical Trial Registration NCT04300647; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04300647 (accessed on 3 January 2023).
- Bristol-Myers Squibb. A Phase I/2a Dose Escalation and Cohort Expansion Study of the Safety, Tolerability, and Efficacy of Anti-LAG-3 Monoclonal Antibody (BMS-986016) Administered Alone and in Combination with Anti-PD-1 Monoclonal Antibody (Nivolumab, BMS-936558) in Advanced Solid Tumors; Clinical Trial Registration NCT01968109; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT01968109 (accessed on 5 January 2023).
- Friedman, C.F.; Snyder Charen, A.; Zhou, Q.; Carducci, M.A.; Buckley De Meritens, A.; Corr, B.R.; Fu, S.; Hollmann, T.J.; Iasonos, A.; Konner, J.A.; et al. Phase II Study of Atezolizumab in Combination with Bevacizumab in Patients with Advanced Cervical Cancer. J. Immunother. Cancer 2020, 8, e001126. [Google Scholar] [CrossRef] [PubMed]
- Seagen Inc. A Phase 1b/2 Open-Label Trial of Tisotumab Vedotin (HuMax®-TF-ADC) Monotherapy and in Combination with Other Agents in Subjects with Recurrent or Stage IVB Cervical Cancer; Clinical Trial Registration NCT03786081; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03786081 (accessed on 3 January 2023).
- Wang, R.; Pan, W.; Jin, L.; Huang, W.; Li, Y.; Wu, D.; Gao, C.; Ma, D.; Liao, S. Human Papillomavirus Vaccine against Cervical Cancer: Opportunity and Challenge. Cancer Lett. 2020, 471, 88–102. [Google Scholar] [CrossRef]
- Da Silva, D.M.; Skeate, J.G.; Chavez-Juan, E.; Lühen, K.P.; Wu, J.-M.; Wu, C.-M.; Kast, W.M.; Hwang, K. Therapeutic Efficacy of a Human Papillomavirus Type 16 E7 Bacterial Exotoxin Fusion Protein Adjuvanted with CpG or GPI-0100 in a Preclinical Mouse Model for HPV-Associated Disease. Vaccine 2019, 37, 2915–2924. [Google Scholar] [CrossRef]
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. A Pilot Clinical Trial Assessing the Safety and Feasibility of Intramuscular Administration of the TA-CIN Vaccine as Adjuvant Therapy for Patients with History of HPV16 Associated Cervical Cancer; Clinical Trial Registration NCT02405221; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02405221 (accessed on 5 January 2023).
- Centre Hospitalier Universitaire de Besancon. A Phase II Study Evaluating the Interest to Combine UCPVax a CD4 TH1-Inducer Cancer Vaccine and Atezolizumab for the Treatment of Human PapillomaVirus Positive Cancers; Clinical Trial Registration NCT03946358; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03946358 (accessed on 5 January 2023).
- Hancock, G.; Hellner, K.; Dorrell, L. Therapeutic HPV Vaccines. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 59–72. [Google Scholar] [CrossRef]
- ISA Pharmaceuticals. A Multicenter, Open Label Phase I/II Study to Determine the Safety and Immune Modulating Effects of the Therapeutic Human Papilloma Virus 16 (HPV16) E6/E7 Long Peptides Vaccine (ISA101/ISA101b) Immunotherapy in Combination with Standard of Care Therapy (Carboplatin and Paclitaxel with or without Bevacizumab) in Women with HPV16 Positive Advanced or Recurrent Cervical Cancer Who Have No Curative Treatment Options; Clinical Trial Registration NCT02128126; clinicaltrials.gov. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT02128126 (accessed on 5 January 2023).
- Marth, C.; Tarnawski, R.; Tyulyandina, A.; Pignata, S.; Gilbert, L.; Kaen, D.; Rubio, M.J.; Frentzas, S.; Beiner, M.; Magallanes-Maciel, M.; et al. Phase 3, Randomized, Open-Label Study of Pembrolizumab plus Lenvatinib versus Chemotherapy for First-Line Treatment of Advanced or Recurrent Endometrial Cancer: ENGOT-En9/LEAP-001. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2022, 32, 93–100. [Google Scholar] [CrossRef]
- Alaunos Therapeutics. Phase I/II Study of Autologous T Cells Engineered Using the Sleeping Beauty System to Express T-Cell Receptors (TCRs) Reactive against Cancer-Specific Mutations in Subjects with Solid Tumors; Clinical Trial Registration NCT05194735; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05194735 (accessed on 3 January 2023).
- Fang, W. A Phase I Clinical Study of CD70-Targeting CAR-T Therapy in the Treatment of CD70-Positive Advanced/Metastatic Solid Tumors; Clinical Trial Registration NCT05518253; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05518253 (accessed on 3 January 2023).
- Xencor, Inc. A Phase 2 Study of XmAb20717 in Patients with Selected Gynecological Malignancies and High-Risk Metastatic Castration-Resistant Prostate Cancer; Clinical Trial Registration NCT05032040; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05032040 (accessed on 3 January 2023).
- Xencor to Present Data from the Phase 1 Study of XmAb®20717 and Three Research Programs at the SITC Annual Meeting | Xencor, Inc. Available online: https://investors.xencor.com/news-releases/news-release-details/xencor-present-data-phase-1-study-xmabr20717-and-three-research (accessed on 5 January 2023).
- Knudson, K.M.; Hicks, K.C.; Luo, X.; Chen, J.-Q.; Schlom, J.; Gameiro, S.R. M7824, a Novel Bifunctional Anti-PD-L1/TGFβ Trap Fusion Protein, Promotes Anti-Tumor Efficacy as Monotherapy and in Combination with Vaccine. Oncoimmunology 2018, 7, e1426519. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute (NCI). Phase I/II Trial of HPV Vaccine PRGN-2009 Alone or in Combination with Anti-PD-L1/TGF-Beta Trap (M7824) in Subjects with HPV Positive Cancers; Clinical Trial Registration NCT04432597; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04432597 (accessed on 3 January 2023).
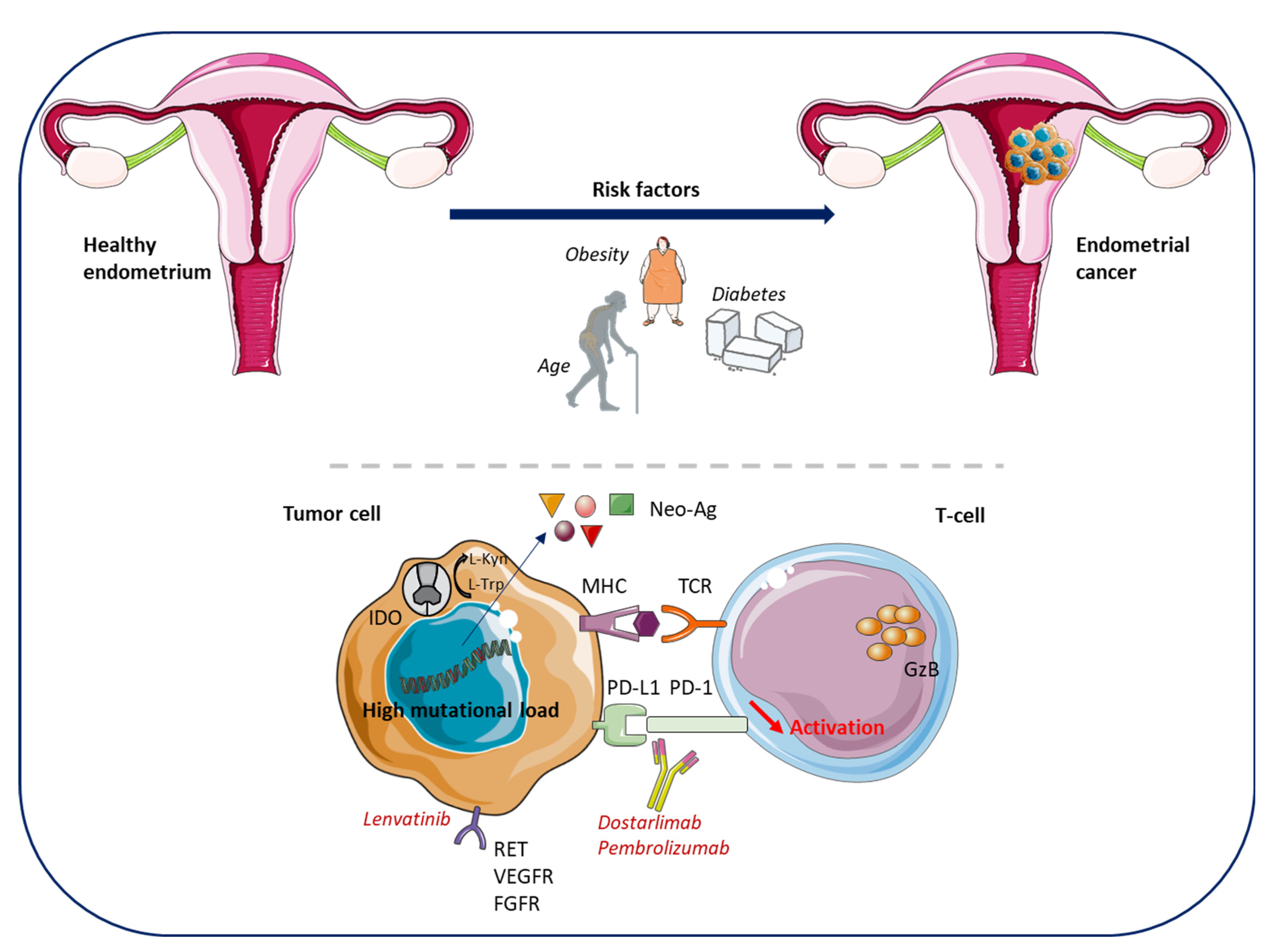

| Worldwide New Cases [1] | Worldwide Deaths [1] | Worldwide Female Cancer Rank [1] | 5-Year Survival Rates (United States) | |
|---|---|---|---|---|
| Cervical Cancers | 604,127 | 341,831 | 5th | ~66% in the whole population [3] Low stage: 92% and 58% if lymph nodes are invaded [4] |
| Endometrial Cancers | 417,367 | 97,370 | 7th | Early stages: >80% (95% for stage I) [3,5] Recurrent/advanced disease: 20-25% [5] |
| Ovarian Cancers | 313,959 | 207,252 | 9th | ~49.7% [3] |
| Vaginal Cancers | 17,908 | 7,995 | >10th | ~49% in the whole population with a variation between 35–78% [6,7] Early stage: ~85% [7] |
| Vulvar Cancers | 45,240 | 17,427 | >10th | ~70.3% [3] |
| Risk Group | Description |
|---|---|
| Low risk | Stage IA (G1-G2) with endometrioid type (dMMR or MSI-H and NSMP) and no or focal LVSI Stage I–III POLEmut EC |
| Intermediate risk | Stage IA G3 with endometrioid type (dMMR and NSMP) and no or focal LVSI Stage IA non-endometrioid type (serous, clear-cell, undifferentiated carcinoma, carcinosarcoma, mixed) and/or p53-abn cancers without myometrial invasion and no or focal LVSI Stage IB (G1-G2) with endometrioid type (dMMR and NSMP) and no or focal LVSI Stage II G1 endometrioid type (dMMR and NSMP) and no or focal LVSI |
| High-intermediate risk | Stage I endometrioid type (dMMR and NSMP) any grade and any depth of invasion with substantial LVSI Stage IB G3 with endometrioid type (dMMR and NSMP) regardless of LVSI Stage II G1 endometrioid type (dMMR and NSMP) with substantial LVSI Stage II G2-G3 endometrioid type (dMMR and NSMP) |
| High risk | All stages and all histologies with p53-abn and myometrial invasion All stages with serous or undifferentiated carcinoma including carcinosarcoma with myometrial invasion All stage III and IVA with no residual tumor, regardless of histology and regardless of molecular subtype |
| FIGO Stage | T Category | Description |
|---|---|---|
| Tx | Primary tumor cannot be assessed | |
| T0 | No evidence of primary tumor | |
| I | T1 | Cervical carcinoma confined to the uterus (extension to corpus should be disregarded) |
| IA | T1a | Invasive carcinoma diagnosed only by microscope. Stromal invasion with a maximum depth of 5.0 mm measured from the base of the epithelium and a horizontal spread of 7.0 mm or less; vascular space involvement, venous or lymphatic, does not affect classification |
| IA1 | T1a1 | Measured stromal invasion of 3.0 mm or less in depth and 7.0 mm or less in horizontal spread |
| IA2 | T1a2 | Measured stromal invasion of more than 3.0 mm and not more than 5.0 mm, with a horizontal spread of 7.0 mm or less |
| IB | T1b | Clinically visible lesion confined to the cervix or microscopic lesion greater than IA2 (T1a2). Includes all macroscopically visible lesions, even those with superficial invasion |
| IB1 | T1b1 | Clinically visible lesion 4.0 cm or less in greatest dimension |
| IB2 | T1b2 | Clinically visible lesion more than 4.0 cm or less in greatest dimension |
| II | T2 | Cervical carcinoma invading beyond the uterus but not to the pelvic wall or to lower third of the vagina |
| IIA | T2a | Tumor without parametrial invasion |
| IIA1 | T2a1 | Clinically visible lesion 4.0 cm or less in greatest dimension |
| IIA2 | T2a2 | Clinically visible lesion more than 4.0 cm in greatest dimension |
| IIB | T2b | Tumor with parametrial invasion |
| III | T3 | Tumor extending to the pelvic sidewall * and/or involving the lower third of the vagina and/or causing hydronephrosis or non-functioning kidney |
| IIIA | T3a | Tumor involving the lower third of the vagina but not extending to the pelvic wall |
| IIIB | T3b | Tumor extending to the pelvic wall and/or causing hydronephrosis or nonfunctioning kidney |
| IVA | T4 | Tumor invading the mucosa of the bladder or rectum and/or extending beyond the true pelvis (bullous edema is not sufficient to classify a tumor as T4) |
| IVB | Tumor invading distant organs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lainé, A.; Gonzalez-Lopez, A.M.; Hasan, U.; Ohkuma, R.; Ray-Coquard, I. Immune Environment and Immunotherapy in Endometrial Carcinoma and Cervical Tumors. Cancers 2023, 15, 2042. https://doi.org/10.3390/cancers15072042
Lainé A, Gonzalez-Lopez AM, Hasan U, Ohkuma R, Ray-Coquard I. Immune Environment and Immunotherapy in Endometrial Carcinoma and Cervical Tumors. Cancers. 2023; 15(7):2042. https://doi.org/10.3390/cancers15072042
Chicago/Turabian StyleLainé, Alexandra, Andrea M. Gonzalez-Lopez, Uzma Hasan, Ryotaro Ohkuma, and Isabelle Ray-Coquard. 2023. "Immune Environment and Immunotherapy in Endometrial Carcinoma and Cervical Tumors" Cancers 15, no. 7: 2042. https://doi.org/10.3390/cancers15072042
APA StyleLainé, A., Gonzalez-Lopez, A. M., Hasan, U., Ohkuma, R., & Ray-Coquard, I. (2023). Immune Environment and Immunotherapy in Endometrial Carcinoma and Cervical Tumors. Cancers, 15(7), 2042. https://doi.org/10.3390/cancers15072042






