Survival Differences by Comorbidity Burden among Patients with Stage I/II Non-Small-Cell Lung Cancer after Thoracoscopic Resection
Abstract
:Simple Summary
Abstract
1. Introduction
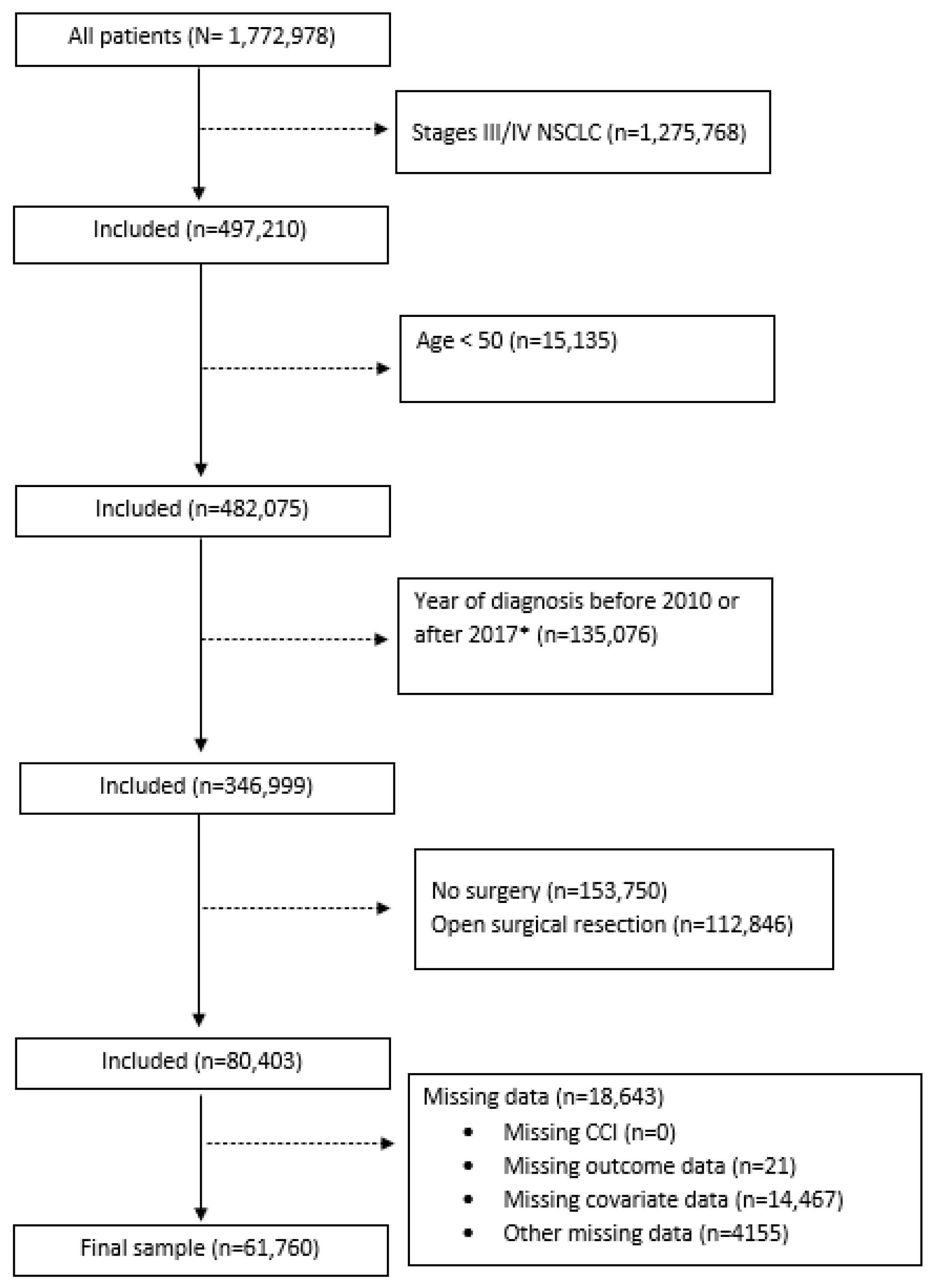
2. Methods
2.1. Data Source and Patient Selection
2.2. Exposure, Outcome, and Covariates
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Stage at Diagnosis. 2022. Available online: https://progressreport.cancer.gov/diagnosis/stage (accessed on 22 April 2022).
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Bendixen, M.; Jorgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.P.; Chang, E.; Senagore, A.J.; Broder, M. Clinical outcomes and resource utilization associated with laparoscopic and open colectomy using a large national database. Ann. Surg. 2008, 247, 819–824. [Google Scholar] [CrossRef]
- Boffa, D.J.; Dhamija, A.; Kosinski, A.S.; Kim, A.W.; Detterbeck, F.C.; Mitchell, J.D.; Onaitis, M.W.; Paul, S. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J. Thorac. Cardiovasc. Surg. 2014, 148, 637–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Feng, M.; Tan, L.; Wang, Q. Comparison of the short-term quality of life in patients with esophageal cancer after subtotal esophagectomy via video-assisted thoracoscopic or open surgery. Dis. Esophagus 2010, 23, 408–414. [Google Scholar] [CrossRef]
- Dziedzic, R.; Marjanski, T.; Binczyk, F.; Polanska, J.; Sawicka, W.; Rzyman, W. Favourable outcomes in patients with early-stage non-small-cell lung cancer operated on by video-assisted thoracoscopic surgery: A propensity score-matched analysis. Eur. J. Cardiothorac. Surg. 2018, 54, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y. Video-assisted thoracoscopic surgery for non-small-cell lung cancer is beneficial to elderly patients. Int. J. Clin. Exp. Med. 2015, 8, 13604–13609. [Google Scholar]
- Laursen, L.O.; Petersen, R.H.; Hansen, H.J.; Jensen, T.K.; Ravn, J.; Konge, L. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur. J. Cardiothorac. Surg. 2016, 49, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Akhtar-Danseh, G.G.; Akhtar-Danesh, N.; Finley, C. Uptake and survival effects of minimally invasive surgery for lung cancer: A population-based study. Eur. J. Surg. Oncol. 2021, 47, 1791–1796. [Google Scholar] [CrossRef]
- Yamashita, S.I.; Tokuishi, K.; Moroga, T.; Nagata, A.; Imamura, N.; Miyahara, S.; Yoshida, Y.; Waseda, R.; Sato, T.; Shiraishi, T.; et al. Long-term survival of thoracoscopic surgery compared with open surgery for clinical N0 adenocarcinoma. J. Thorac. Dis. 2020, 12, 6523–6532. [Google Scholar] [CrossRef]
- Taioli, E.; Lee, D.S.; Lesser, M.; Flores, R. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: A meta-analysis. Eur. J. Cardiothorac. Surg. 2013, 44, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Cancer Institute. Surveillance, Epidemiology and End Results Program. Cancer Stat Facts: Lung and Bronchus Cancer. 2022. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 6 May 2022).
- Battafarano, R.J.; Piccirillo, J.F.; Meyers, B.F.; Hsu, H.S.; Guthrie, T.J.; Cooper, J.D.; Patterson, G.A. Impact of comorbidity on survival after surgical resection in patients with stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2002, 123, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Mallin, K.; Browner, A.; Palis, B.; Gay, G.; McCabe, R.; Nogueira, L.; Yabroff, R.; Shulman, L.; Facktor, M.; Winchester, D.P.; et al. Incident Cases Captured in the National Cancer Database Compared with Those in U.S. Population Based Central Cancer Registries in 2012-2014. Ann. Surg. Oncol. 2019, 26, 1604–1612. [Google Scholar] [CrossRef]
- Boffa, D.J.; Rosen, J.E.; Mallin, K.; Loomis, A.; Gay, G.; Palis, B.; Thoburn, K.; Gress, D.; McKellar, D.P.; Shulman, L.N.; et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017, 3, 1722–1728. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann. Surg. Oncol. 2008, 15, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, F.G.; Force, S.D.; Pickens, A.; Kilgo, P.D.; Luu, T.; Miller, D.L. Impact of laterality on early and late survival after pneumonectomy. Ann. Thorac. Surg. 2011, 92, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Blom, E.F.; Ten Haaf, K.; Arenberg, D.A.; de Koning, H.J. Uptake of minimally invasive surgery and stereotactic body radiation therapy for early stage non-small cell lung cancer in the USA: An ecological study of secular trends using the National Cancer Database. BMJ Open Respir. Res. 2020, 7, e000603. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, K.; Cochuyt, J.; Hodge, D.; Qin, H.; Manochakian, R.; Zhao, Y.; Ailawadhi, S.; Adjei, A.A.; Lou, Y. Survival of Black and White Patients With Stage IV Small Cell Lung Cancer. Front. Oncol. 2021, 11, 773958. [Google Scholar] [CrossRef]
- Shi, R.; Diaz, R.; Shi, Z.; Duvall, E.; Mills, G. The Effect of Payer Status on Survival of Patients with Stage I/II Non-small Cell Lung Cancer: NCDB 1998-2011. Anticancer Res. 2016, 36, 319–326. [Google Scholar] [PubMed]
- SCHOENFELD, D. Partial residuals for the proportional hazards regression model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]
- Gordon, E.H.; Peel, N.M.; Samanta, M.; Theou, O.; Howlett, S.E.; Hubbard, R.E. Sex differences in frailty: A systematic review and meta-analysis. Exp. Gerontol. 2017, 89, 30–40. [Google Scholar] [CrossRef]
- Oertelt-Prigione, S.; de Rooij, B.H.; Mols, F.; Oerlemans, S.; Husson, O.; Schoormans, D.; Haanen, J.B.; van de Poll-Franse, L.V. Sex-differences in symptoms and functioning in >5000 cancer survivors: Results from the PROFILES registry. Eur. J. Cancer 2021, 156, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mobley, E.M.; Manini, T.M.; Leeuwenburgh, C.; Anton, S.D.; Washington, C.J.; Zhou, D.; Parker, A.S.; Okunieff, P.G.; Bian, J.; et al. Frailty and risk of mortality in older cancer survivors and adults without a cancer history: Evidence from the National Health and Nutrition Examination Survey, 1999–2014. Cancer 2022, 128, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Jawitz, O.K.; Wang, Z.; Boffa, D.J.; Detterbeck, F.C.; Blasberg, J.D.; Kim, A.W. The differential impact of preoperative comorbidity on perioperative outcomes following thoracoscopic and open lobectomies. Eur. J. Cardiothorac. Surg. 2017, 51, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Luchtenborg, M.; Jakobsen, E.; Krasnik, M.; Linklater, K.M.; Mellemgaard, A.; Moller, H. The effect of comorbidity on stage-specific survival in resected non-small cell lung cancer patients. Eur. J. Cancer 2012, 48, 3386–3395. [Google Scholar] [CrossRef] [PubMed]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Chkhotua, A.B.; Gabusi, E.; Altimari, A.; D’Errico, A.; Yakubovich, M.; Vienken, J.; Stefoni, S.; Chieco, P.; Yussim, A.; Grigioni, W.F. Increased expression of p16(INK4a) and p27(Kip1) cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am. J. Kidney Dis. 2003, 41, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Li, A.; Lei, Q.; Zhang, Y. Tumor-intrinsic signaling pathways: Key roles in the regulation of the immunosuppressive tumor microenvironment. J. Hematol. Oncol. 2019, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Webster, M.R.; Marchbank, K.; Behera, R.; Ndoye, A.; Kugel, C.H., 3rd; Dang, V.M.; Appleton, J.; O’Connell, M.P.; Cheng, P.; et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 2016, 532, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef]
- Kim, Y.; Bayona, P.W.; Kim, M.; Chang, J.; Hong, S.; Park, Y.; Budiman, A.; Kim, Y.J.; Choi, C.Y.; Kim, W.S.; et al. Macrophage Lamin A/C Regulates Inflammation and the Development of Obesity-Induced Insulin Resistance. Front. Immunol. 2018, 9, 696. [Google Scholar] [CrossRef] [Green Version]
- Agere, S.A.; Akhtar, N.; Watson, J.M.; Ahmed, S. RANTES/CCL5 Induces Collagen Degradation by Activating MMP-1 and MMP-13 Expression in Human Rheumatoid Arthritis Synovial Fibroblasts. Front. Immunol. 2017, 8, 1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espigol-Frigole, G.; Planas-Rigol, E.; Lozano, E.; Corbera-Bellalta, M.; Terrades-Garcia, N.; Prieto-Gonzalez, S.; Garcia-Martinez, A.; Hernandez-Rodriguez, J.; Grau, J.M.; Cid, M.C. Expression and Function of IL12/23 Related Cytokine Subunits (p35, p40, and p19) in Giant-Cell Arteritis Lesions: Contribution of p40 to Th1- and Th17-Mediated Inflammatory Pathways. Front. Immunol. 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negera, E.; Tilahun, M.; Bobosha, K.; Lambert, S.M.; Walker, S.L.; Spencer, J.S.; Aseffa, A.; Dockrell, H.M.; Lockwood, D.N. The effects of prednisolone treatment on serological responses and lipid profiles in Ethiopian leprosy patients with Erythema Nodosum Leprosum reactions. PLoS Negl. Trop. Dis. 2018, 12, e0007035. [Google Scholar] [CrossRef] [Green Version]
- Nold-Petry, C.A.; Nold, M.F.; Levy, O.; Kliger, Y.; Oren, A.; Borukhov, I.; Becker, C.; Wirtz, S.; Sandhu, M.K.; Neurath, M.; et al. Gp96 Peptide Antagonist gp96-II Confers Therapeutic Effects in Murine Intestinal Inflammation. Front. Immunol. 2017, 8, 1531. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, S.; Kobayashi, K.; Kasahara, C.; Onitsuka, T.; Matsuo, M.; Nakagawa, M.; Sugaya, M. Long-term impact of complications after lung resections in non-small cell lung cancer. J. Thorac. Dis. 2019, 11, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Sase, K.; Fujisaka, Y.; Shoji, M.; Mukai, M. Cardiovascular Complications Associated with Contemporary Lung Cancer Treatments. Curr. Treat. Options Oncol. 2021, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Heiden, B.T.; Eaton, D.B., Jr.; Chang, S.H.; Yan, Y.; Schoen, M.W.; Chen, L.S.; Smock, N.; Patel, M.R.; Kreisel, D.; Nava, R.G.; et al. The Impact of Persistent Smoking After Surgery on Long-term Outcomes After Stage I Non-small Cell Lung Cancer Resection. Chest 2022, 161, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Grosu, H.B.; Manzanera, A.; Shivakumar, S.; Sun, S.; Noguras Gonzalez, G.; Ost, D.E. Survival disparities following surgery among patients with different histological types of non-small cell lung cancer. Lung Cancer 2020, 140, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Rim, S.H.; Guy, G.P., Jr.; Yabroff, K.R.; McGraw, K.A.; Ekwueme, D.U. The impact of chronic conditions on the economic burden of cancer survivorship: A systematic review. Expert Rev. Pharm. Outcomes Res. 2016, 16, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nipp, R.D.; Shui, A.M.; Perez, G.K.; Kirchhoff, A.C.; Peppercorn, J.M.; Moy, B.; Kuhlthau, K.; Park, E.R. Patterns in Health Care Access and Affordability Among Cancer Survivors During Implementation of the Affordable Care Act. JAMA Oncol. 2018, 4, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Robinson, L.A.; Jensen, R.E.; Smith, T.G.; Yabroff, K.R. Factors Associated with Health-Related Quality of Life among Cancer Survivors in the United States. JNCI Cancer Spectr. 2021, 5, pkaa123. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | All (N = 61,760) | CCI 0 (N = 31,623) | CCI 1 (N = 19,640) | CCI 2+ (N = 10,497) |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Age, mean (SD), y | 69.1 (8.5) | 68.8 (8.8) | 69.0 (8.4) | 70.0 (8.1) |
| Age group | ||||
| 50–64 | 18,327 (29.7) | 9994 (31.6) | 5753 (29.3) | 2580 (24.6) |
| 65–74 | 26,088 (42.2) | 12,829 (40.6) | 8534 (43.5) | 4725 (45.0) |
| ≥75 | 17,345 (28.1) | 8800 (27.8) | 5353 (27.3) | 3192 (30.4) |
| Sex | ||||
| Male | 26,612 (43.1) | 12,630 (39.9) | 8687 (44.2) | 5295 (50.4) |
| Female | 35,148 (56.9) | 18,993 (60.1) | 10,953 (55.8) | 5202 (49.6) |
| Race/Ethnicity | ||||
| NH White | 54,043 (87.5) | 27,634 (87.4) | 17,299 (88.1) | 9110 (86.8) |
| NH Black | 4787 (7.8) | 2314 (7.3) | 1508 (7.7) | 965 (9.2) |
| American Indian | 141 (0.2) | 61 (0.2) | 43 (0.2) | 37 (0.4) |
| Asian/Pacific Islander | 573 (0.9) | 415 (1.3) | 119 (0.6) | 39 (0.4) |
| Hispanic | 2106 (3.4) | 1140 (3.6) | 636 (3.2) | 330 (3.1) |
| Other | 110 (0.2) | 59 (0.2) | 35 (0.2) | 16 (0.2) |
| Income # | ||||
| ≥USD 63,333 | 25,163 (40.7) | 13,946 (44.1) | 7492 (38.2) | 3725 (35.5) |
| USD 50,354–USD 63,332 | 14,473 (23.4) | 7304 (23.1) | 4601 (23.4) | 2569 (24.5) |
| USD 40,227–USD 50,353 | 12,444 (20.2) | 5935 (18.8) | 4179 (21.3) | 2330 (22.2) |
| <USD 40,227 | 9680 (15.7) | 4438 (14.0) | 3368 (17.2) | 1874 (17.9) |
| Insurance | ||||
| Medicare | 41,242 (66.8) | 20,141 (63.7) | 13,350 (68.0) | 7751 (73.8) |
| Medicaid | 2606 (4.2) | 1213 (3.8) | 917 (4.7) | 476 (4.5) |
| Private | 16,603 (26.9) | 9627 (30.4) | 4901 (25.0) | 2075 (19.8) |
| Not Insured | 628 (1.0) | 309 (1.0) | 238 (1.2) | 81 (0.8) |
| Other | 681 (1.1) | 333 (1.1) | 234 (1.2) | 114 (1.1) |
| Facility Type | ||||
| Academic facility | 27,339 (44.3) | 14,653 (46.3) | 8271 (42.1) | 4415 (42.1) |
| Non-academic facility | 34,421 (55.7) | 16,970 (53.7) | 11,369 (57.9) | 6082 (57.9) |
| Histology | ||||
| Adenocarcinoma | 33,260 (53.9) | 18,003 (53.9) | 10,241 (52.1) | 5016 (47.8) |
| Squamous cell | 12,435 (20.1) | 5127 (16.2) | 4437 (22.6) | 2871 (27.4) |
| Large cell | 307 (0.5) | 138 (0.4) | 110 (0.6) | 59 (0.6) |
| Other ¥ | 15,758 (25.5) | 8355 (26.4) | 4852 (24.7) | 2551 (24.3) |
| TNM Stage | ||||
| I | 54,674 (88.5) | 28,027 (88.6) | 17,362 (88.4) | 9285 (88.5) |
| II | 7086 (11.5) | 3596 (11.4) | 2278 (11.6) | 1212 (11.6) |
| Adjuvant Chemotherapy | ||||
| Yes | 9677 (15.7) | 5043 (16.0) | 3107 (15.8) | 1527 (14.6) |
| No | 52,083 (84.3) | 26,580 (84.1) | 16,533 (84.2) | 8970 (85.5) |
| Days from Diagnosis to Surgery | ||||
| 0–29 | 31,174 (50.5) | 16,551 (52.3) | 9678 (49.3) | 4945 (47.1) |
| 30–59 | 18,006 (29.2) | 9050 (28.6) | 5846 (29.8) | 3110 (29.6) |
| 60–89 | 7313 (11.8) | 3493 (11.1) | 2380 (12.1) | 1440 (13.7) |
| ≥90 | 5267 (8.5) | 2529 (8.0) | 1736 (8.8) | 1002 (9.6) |
| Surgical Margins | ||||
| No residual tumor | 59,033 (95.6) | 30,371 (96.0) | 18,695 (95.2) | 9967 (95.0) |
| Residual tumor, NOS | 774 (1.3) | 365 (1.2) | 269 (1.4) | 140 (1.3) |
| Microscopic residuals | 1082 (1.8) | 482 (1.5) | 392 (2.0) | 208 (2.0) |
| Macroscopic residuals | 116 (0.2) | 45 (0.1) | 41 (0.2) | 30 (0.3) |
| Indeterminate | 755 (1.2) | 360 (1.1) | 243 (1.2) | 152 (1.5) |
| Extent of Resection | ||||
| Sublobar resection | 18,007 (29.2) | 8580 (27.2) | 5996 (30.5) | 3431 (32.7) |
| Lobectomy/bilobectomy | 42,544 (68.9) | 22,421 (70.9) | 13,277 (67.6) | 6846 (65.2) |
| Pneumonectomy | 455 (0.7) | 264 (0.8) | 123 (0.6) | 68 (0.7) |
| Other | 754 (1.2) | 358 (1.1) | 244 (1.2) | 152 (1.5) |
| Laterality | ||||
| Right | 36,374 (58.9) | 18,642 (59.0) | 11,632 (59.2) | 6100 (58.1) |
| Left | 25,131 (40.7) | 12,853 (40.6) | 7931 (40.4) | 4347 (41.4) |
| Other | 255 (0.4) | 128 (0.4) | 77 (0.4) | 50 (0.5) |
| No. Death/Person-Years | Mortality Rate (Per 1000 Person-Years) (95% CI) | Age-Adjusted HR (95% CI) | aHR (95% CI) † | aHR (95% CI) § | |
|---|---|---|---|---|---|
| CCI | Overall: 18,833/225,222.3 | Overall: 83.6 (82.5–84.8) | |||
| 0 | 8073/117,522.5 | 68.7 (67.3–70.2) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 1 | 6650/72,935.6 | 91.2 (89.1–93.3) | 1.29 (1.25–1.34) | 1.25 (1.21–1.29) | 1.24 (1.20–1.28) |
| 2+ | 4110/34,764.2 | 118.2 (114.8–121.7) | 1.62 (1.56–1.68) | 1.55 (1.49–1.61) | 1.51 (1.45–1.57) |
| p-trend < 0.01 | p-trend < 0.01 | p-trend < 0.01 |
| CCI = 0 | CCI = 1 | CCI = 2+ | |||||
|---|---|---|---|---|---|---|---|
| Subgroup | No. Death/Person Years | aHR (95% CI) | No. Death/Person Years | aHR (95% CI) | No. Death/Person Years | aHR (95% CI) | p-interaction |
| Sex | |||||||
| Female (N = 35,148) | 3914/73,545.4 | 1 (Reference) | 3127/42,290.4 | 1.31 (1.25–1.37) | 1766/18,122.4 | 1.65 (1.56–1.75) | |
| Male (N = 26,612) | 4159/43,977.1 | 1 (Reference) | 3523/30,645.1 | 1.17 (1.12–1.22) | 2344/16,641.9 | 1.40 (1.33–1.47) | |
| <0.01 | |||||||
| Age | |||||||
| 50–64 (N = 18,327) | 1821/38,888.4 | 1 (Reference) | 1497/22,383.8 | 1.26 (1.17–1.35) | 754/9070.7 | 1.43 (1.31–1.56) | |
| 65–74 (N = 26,088) | 3082/47,666.8 | 1 (Reference) | 2763/31,758.1 | 1.27 (1.21–1.34) | 1770/15,994.1 | 1.57 (1.48–1.67) | |
| 75+ (N = 17,345) | 3170/30,967.3 | 1(Reference) | 2390/18,793.7 | 1.17 (1.11–1.24) | 1586/9699.4 | 1.46 (1.38–1.55) | |
| 0.11 | |||||||
| Days from Diagnosis to Surgery | |||||||
| <30 (N = 31,174) | 3984/64,292.1 | 1 (Reference) | 3144/37,340.7 | 1.25 (1.19–1.31) | 1890/16,899.0 | 1.55 (1.47–1.64) | |
| ≥30 (N = 30,586) | 4089/53,230.3 | 1 (Reference) | 3506/35,594.8 | 1.22 (1.16–1.27) | 2220/17,865.2 | 1.47 (1.40–1.55) | |
| <0.01 | |||||||
| Facility Type | |||||||
| Academic (N = 27,339) | 3416/55,558.6 | 1 (Reference) | 2684/30,998.4 | 1.29 (1.23–1.36) | 1673/14,887.5 | 1.58 (1.49–1.68) | |
| Non-academic (N = 30,586) | 4657/61,963.9 | 1 (Reference) | 3966/41,937.2 | 1.19 (1.14–1.25) | 2437/19,876.8 | 1.46 (1.39–1.53) | |
| <0.01 | |||||||
| Laterality | |||||||
| Left (N = 36,374) | 3389/47,939.3 | 1 (Reference) | 2728/29,463.2 | 1.22 (1.16–1.28) | 1718/14,295.8 | 1.50 (1.42–1.59) | |
| Right (N = 25,131) | 4637/69,201.6 | 1 (Reference) | 3885/43,243.3 | 1.25 (1.20–1.31) | 2355/20,350.7 | 1.52 (1.44–1.60) | |
| 0.07 | |||||||
| Type of Surgery | |||||||
| Sublobar resection (N = 18,007) | 2490/377,006.8 | 1 (Reference) | 2309/260,478.5 | 1.25 (1.18–1.32) | 1505/134,750.9 | 1.50 (1.40–1.60) | |
| Lobectomy/bilobectomy (N = 42,544) | 5249/1010,663.0 | 1 (Reference) | 4127/601,114.5 | 1.22 (1.17–1.27) | 2455/275,842.3 | 1.49 (1.42–1.57) | |
| Pneumonectomy (N = 455) | 107/10,272.3 | 1 (Reference) | 53/4826.6 | 1.08 (0.76–1.53) | 37/2324.9 | 1.26 (0.85–1.88) | |
| Other (N = 754) | 227/12,327.0 | 1 (Reference) | 161/8807.2 | 0.97 (0.79–1.20) | 113/4252.6 | 1.30 (1.03–1.66) | |
| <0.01 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wheeler, M.; Karanth, S.D.; Mehta, H.J.; Yang, D.; Aduse-Poku, L.; Washington, C.; Hong, Y.-R.; Zhang, D.; Gould, M.K.; Braithwaite, D. Survival Differences by Comorbidity Burden among Patients with Stage I/II Non-Small-Cell Lung Cancer after Thoracoscopic Resection. Cancers 2023, 15, 2075. https://doi.org/10.3390/cancers15072075
Wheeler M, Karanth SD, Mehta HJ, Yang D, Aduse-Poku L, Washington C, Hong Y-R, Zhang D, Gould MK, Braithwaite D. Survival Differences by Comorbidity Burden among Patients with Stage I/II Non-Small-Cell Lung Cancer after Thoracoscopic Resection. Cancers. 2023; 15(7):2075. https://doi.org/10.3390/cancers15072075
Chicago/Turabian StyleWheeler, Meghann, Shama D. Karanth, Hiren J. Mehta, Danting Yang, Livingstone Aduse-Poku, Caretia Washington, Young-Rock Hong, Dongyu Zhang, Michael K. Gould, and Dejana Braithwaite. 2023. "Survival Differences by Comorbidity Burden among Patients with Stage I/II Non-Small-Cell Lung Cancer after Thoracoscopic Resection" Cancers 15, no. 7: 2075. https://doi.org/10.3390/cancers15072075







