Nanosecond Pulsed Electric Field Induces an Antitumor Effect in Triple-Negative Breast Cancer via CXCL9 Axis Dependence in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Detection of Apoptosis by Flow Cytometry
2.3. Inoculation of Tumor and Tumor Ablation by nsPEF
2.4. Experiments of CXCL9 Deletion in Animals
2.5. Hematoxylin and Eosin (HE) Staining and Immunohistochemistry (IHC)
2.6. TUNEL
2.7. Transcriptome Analysis and Infiltrated Immune Cells Analysis
2.8. Inflammatory Cytokine Micro-Array
2.9. Flow Cytometry
2.10. Detection of CXCL9 by ELISA
2.11. Statistical Analysis
3. Results
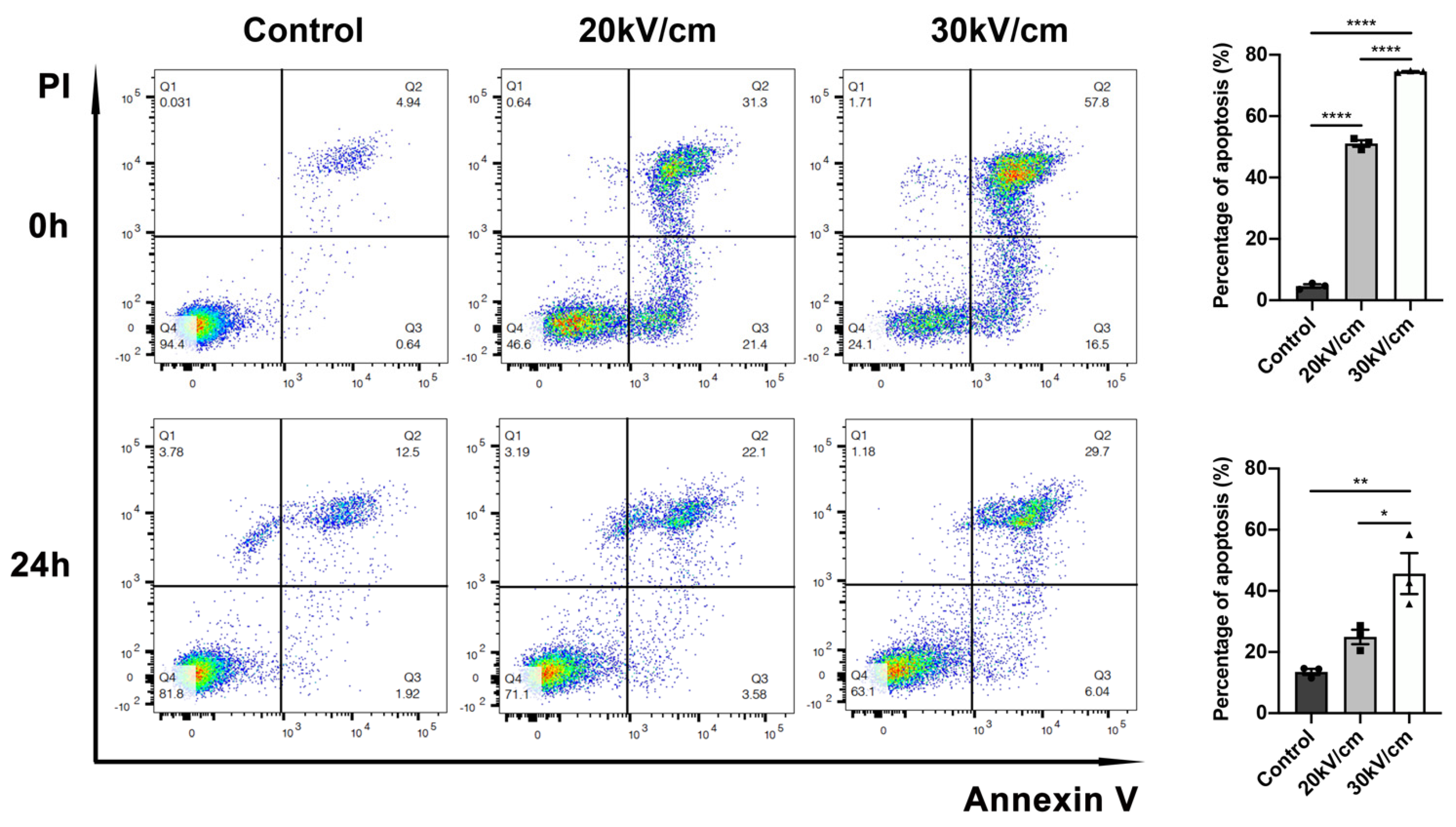
3.1. NsPEF Induces Apoptosis of Solid Tumor
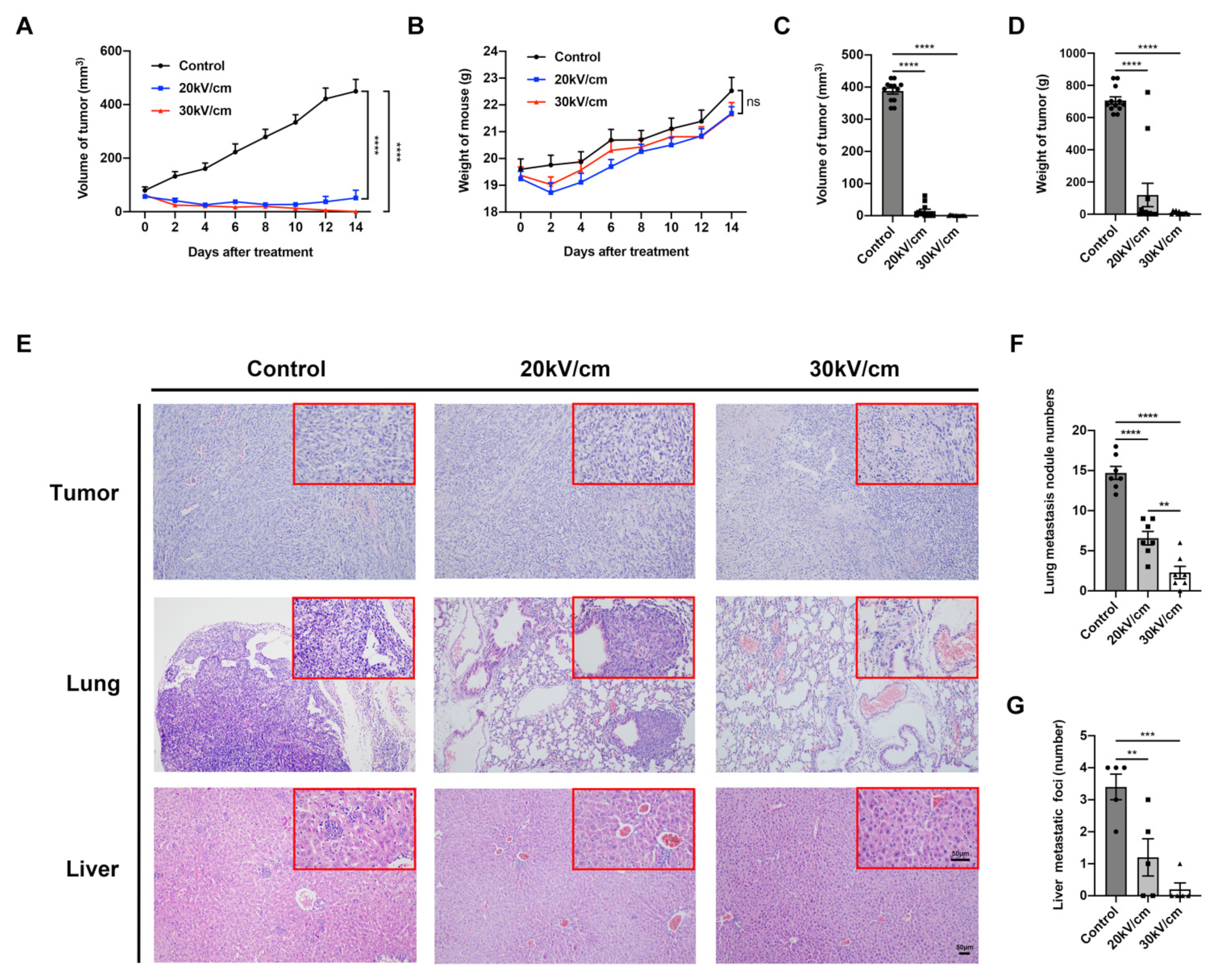
3.2. NsPEF Effectively Inhibits Tumor Growth and Distant Metastasis
3.3. Fifty Pulses of nsPEF Are Sufficient to Induce High Level of Apoptosis in 4T1 Cells
3.4. NsPEF Activates Apoptosis-Related Pathways
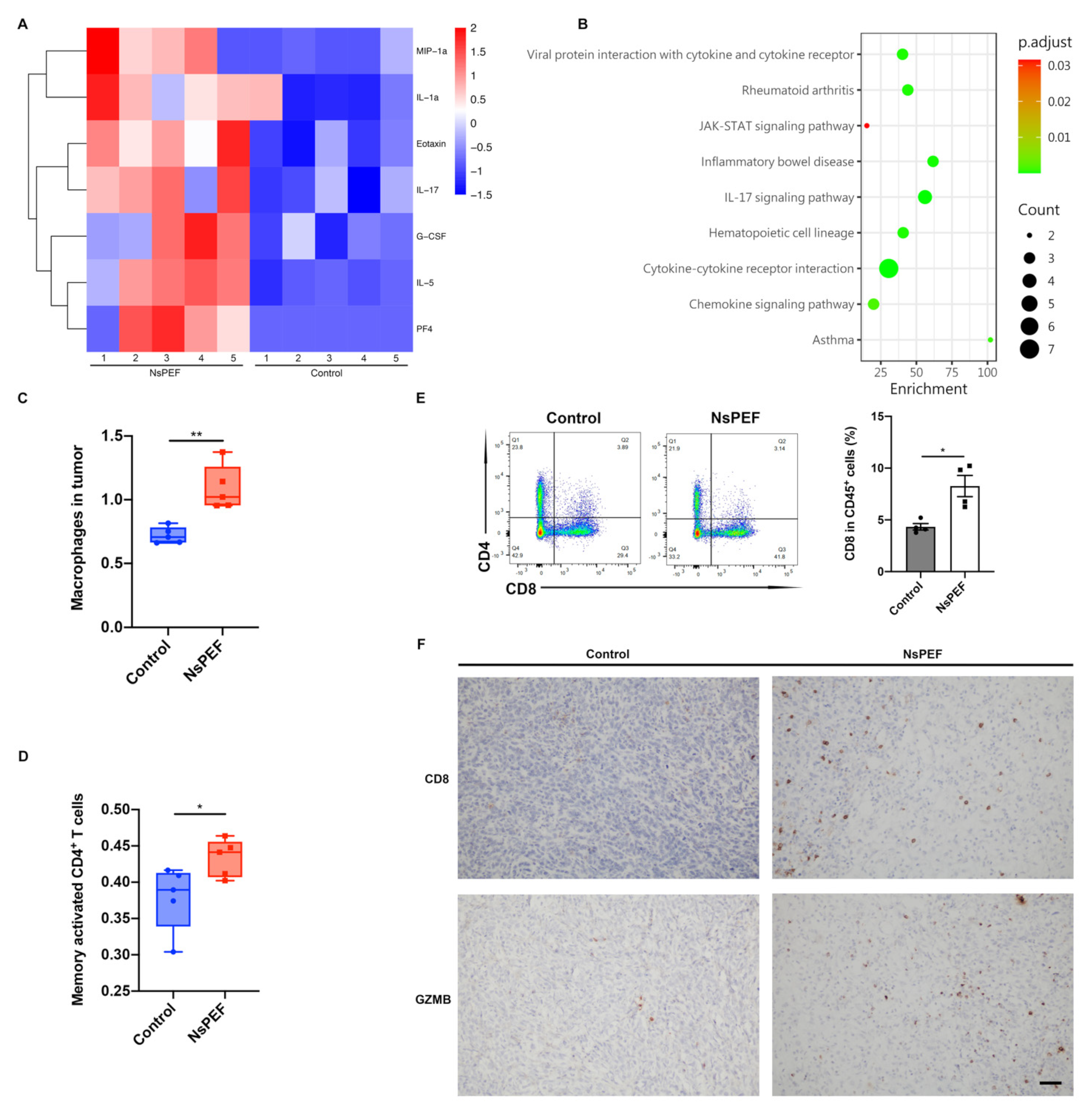
3.5. NsPEF Induced the Release of Inflammatory Cytokines and the Infiltration of Immune Cells into Tumor
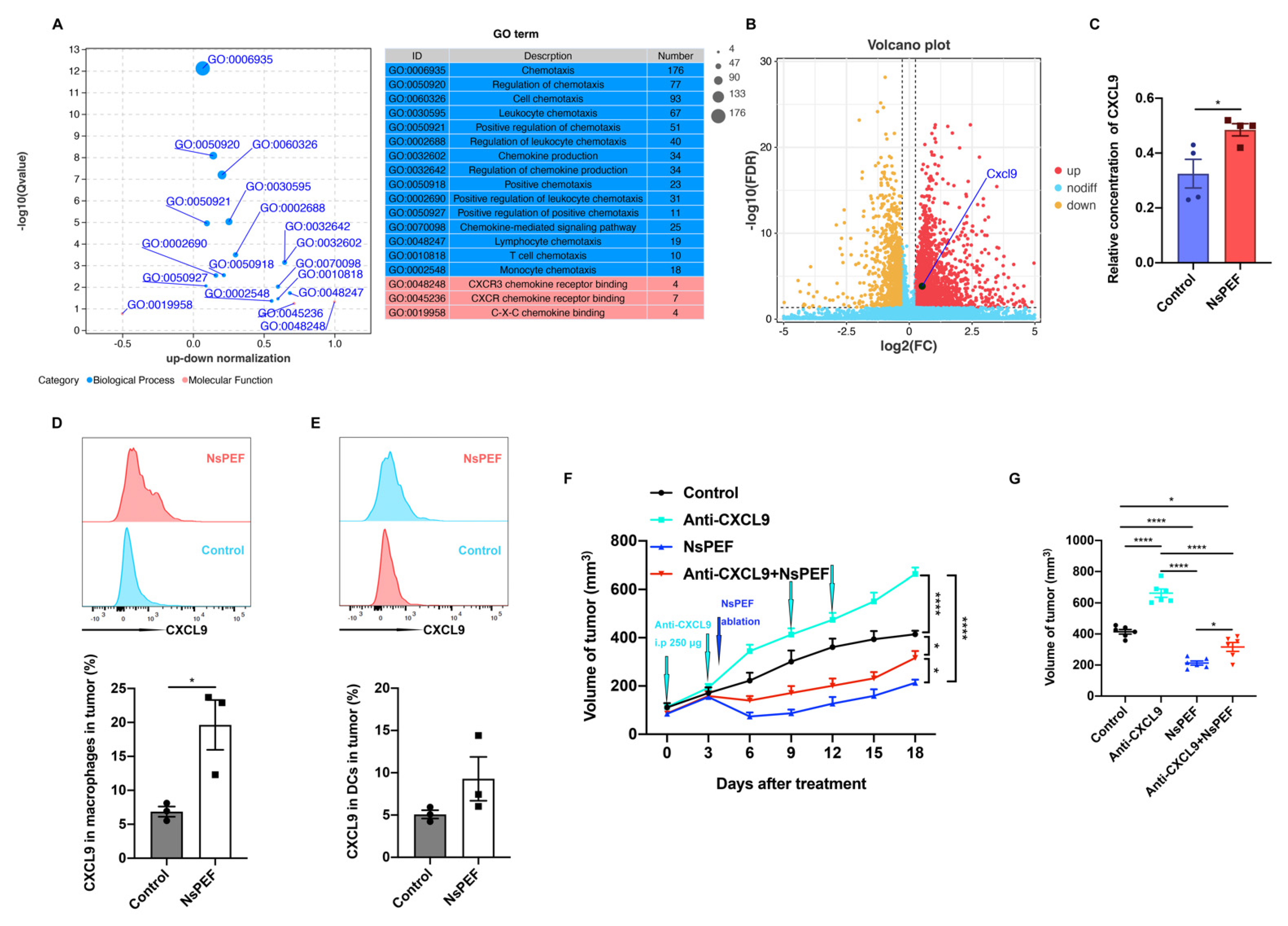
3.6. NsPEF Inhibits the Growth of Residual Breast Cancer via CXCL9 Axis Dependence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Maddiboyina, B.; Arora, S.; Riadi, Y.; Shadab; Alhakamy, N.A.; Kesharwani, P. The emerging role of immune checkpoint inhibitors in the treatment of triple-negative breast cancer. Drug Discov. Today 2021, 26, 1721–1727. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, M.; Mao, X.; Pan, H.; Tang, X.; Wang, J.; Che, N.; Xie, H.; Ling, L.; Zhao, Y.; et al. Landscape of the Peripheral Immune Response Induced by Local Microwave Ablation in Patients with Breast Cancer. Adv. Sci. 2022, 9, 2200033. [Google Scholar] [CrossRef]
- Ford, W.E.; Ren, W.; Blackmore, P.F.; Schoenbach, K.H.; Beebe, S.J. Nanosecond pulsed electric fields stimulate apoptosis without release of pro-apoptotic factors from mitochondria in B16f10 melanoma. Arch. Biochem. Biophys. 2010, 497, 82–89. [Google Scholar] [CrossRef]
- Beebe, S.J.; Fox, M.P.; Rec, L.J.; Willis, E.L.K.; Schoenbach, K.H. Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J. 2003, 17, 1493–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuccitelli, R.; Lui, K.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanosecond pulsed electric field stimulation of reactive oxygen species in human pancreatic cancer cells is Ca2+-dependent. Biochem. Biophys. Res. Commun. 2013, 435, 580–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morotomi-Yano, K.; Akiyama, H.; Yano, K.-I. Different involvement of extracellular calcium in two modes of cell death induced by nanosecond pulsed electric fields. Arch. Biochem. Biophys. 2014, 555–556, 47–54. [Google Scholar] [CrossRef]
- Wang, X.; He, Q.; Zhou, C.; Xu, Y.; Liu, D.; Fujiwara, N.; Kubota, N.; Click, A.; Henderson, P.; Vancil, J.; et al. Prolonged hypernutrition impairs TREM2-dependent efferocytosis to license chronic liver inflammation and NASH development. Immunity 2022, 56, 58–77. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and Chemokine Receptors: Positioning Cells for Host Defense and Immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [Green Version]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Jing, Y.; Burcus, N.I.; Lassiter, B.P.; Tanaz, R.; Heller, R.; Beebe, S.J. Nano-pulse stimulation induces potent immune responses, eradicating local breast cancer while reducing distant metastases. Int. J. Cancer 2017, 142, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Nuccitelli, R.; Tran, K.; Athos, B.; Kreis, M.; Nuccitelli, P.; Chang, K.S.; Epstein, E.H.; Tang, J.Y. Nanoelectroablation therapy for murine basal cell carcinoma. Biochem. Biophys. Res. Commun. 2012, 424, 446–450. [Google Scholar] [CrossRef] [Green Version]
- Nuccitelli, R.; Berridge, J.C.; Mallon, Z.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanoelectroablation of Murine Tumors Triggers a CD8-Dependent Inhibition of Secondary Tumor Growth. PLoS ONE 2015, 10, e0134364. [Google Scholar] [CrossRef] [Green Version]
- Haberkorn, I.; Siegenthaler, L.; Buchmann, L.; Neutsch, L.; Mathys, A. Enhancing single-cell bioconversion efficiency by harnessing nanosecond pulsed electric field processing. Biotechnol. Adv. 2021, 53, 107780. [Google Scholar] [CrossRef] [PubMed]
- Pliquett, U.; Nuccitelli, R. Measurement and simulation of Joule heating during treatment of B-16 melanoma tumors in mice with nanosecond pulsed electric fields. Bioelectrochemistry 2014, 100, 62–68. [Google Scholar] [CrossRef]
- Chen, R.; Sain, N.M.; Harlow, K.T.; Chen, Y.-J.; Shires, P.K.; Heller, R.; Beebe, S.J. A protective effect after clearance of orthotopic rat hepatocellular carcinoma by nanosecond pulsed electric fields. Eur. J. Cancer 2014, 50, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Yin, S.; Shao, Z.; Zhang, Y.; Chen, X. Nanosecond pulsed electric field inhibits proliferation and induces apoptosis in human osteosarcoma. J. Orthop. Surg. Res. 2015, 10, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Xu, D.; Dong, G.; Ren, Z.; Zhang, W.; Aji, T.; Zhao, Q.; Chen, X.; Jiang, T. The Safety and Efficacy of Nanosecond Pulsed Electric Field in Patients with Hepatocellular Carcinoma: A Prospective Phase 1 Clinical Study Protocol. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Fesik, S.W. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer 2005, 5, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Bergkamen, H.; Krammer, H. Apoptosis in cancer--implications for therapy. Semin. Oncol. 2004, 31, 90–119. [Google Scholar] [CrossRef]
- Baell, J.B.; Huang, D.C. Prospects for targeting the Bcl-2 family of proteins to develop novel cytotoxic drugs. Biochem. Pharmacol. 2002, 64, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Vaux, D.L.; Cory, S.; Adams, J.M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988, 335, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a Mitochondrial Protein that Promotes Cytochrome c–Dependent Caspase Activation by Eliminating IAP Inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Nuccitelli, R.; McDaniel, A.; Anand, S.; Cha, J.; Mallon, Z.; Berridge, J.C.; Uecker, D. Nano-Pulse Stimulation is a physical modality that can trigger immunogenic tumor cell death. J. Immunother. Cancer 2017, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.; Pakhomova, O.N.; Mollica, P.A.; Casciola, M.; Mangalanathan, U.; Pakhomov, A.G.; Muratori, C. Nanosecond Pulsed Electric Fields Induce Endoplasmic Reticulum Stress Accompanied by Immunogenic Cell Death in Murine Models of Lymphoma and Colorectal Cancer. Cancers 2019, 11, 2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbachev, A.V.; Kobayashi, H.; Kudo, D.; Tannenbaum, C.; Finke, J.; Shu, S.; Farber, J.; Fairchild, R. CXC chemokine ligand 9/monokine induced by IFN-gamma production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J. Immunol. 2007, 178, 2278–2286. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.-J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Peter Carmeliet, R.K.J. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Zeng, X.; Liao, G.; Li, S.; Liu, H.; Zhao, X.; Li, S.; Lei, K.; Zhu, S.; Chen, Z.; Zhao, Y.; et al. Eliminating METTL1-mediated accumulation of PMN-MDSCs prevents hepatocellular carcinoma recurrence after radiofrequency ablation. Hepatology 2022, 77, 1122–1138. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Pan, C.; Chen, L.; Qian, J.; Chen, X.; Zhou, L.; Zheng, S. Nanosecond Pulsed Electric Field Induces an Antitumor Effect in Triple-Negative Breast Cancer via CXCL9 Axis Dependence in Mice. Cancers 2023, 15, 2076. https://doi.org/10.3390/cancers15072076
Xu Z, Pan C, Chen L, Qian J, Chen X, Zhou L, Zheng S. Nanosecond Pulsed Electric Field Induces an Antitumor Effect in Triple-Negative Breast Cancer via CXCL9 Axis Dependence in Mice. Cancers. 2023; 15(7):2076. https://doi.org/10.3390/cancers15072076
Chicago/Turabian StyleXu, Zhentian, Caixu Pan, Luyan Chen, Junjie Qian, Xinhua Chen, Lin Zhou, and Shusen Zheng. 2023. "Nanosecond Pulsed Electric Field Induces an Antitumor Effect in Triple-Negative Breast Cancer via CXCL9 Axis Dependence in Mice" Cancers 15, no. 7: 2076. https://doi.org/10.3390/cancers15072076




