Tumor-Infiltrating B Lymphocytes: Promising Immunotherapeutic Targets for Primary Liver Cancer Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Background of B Lymphocytes
2.1. B-Cell Biology and Maturation Process
2.2. B-Cell Function in Adaptive Immune Response
3. Effect of B Cells on Tumor
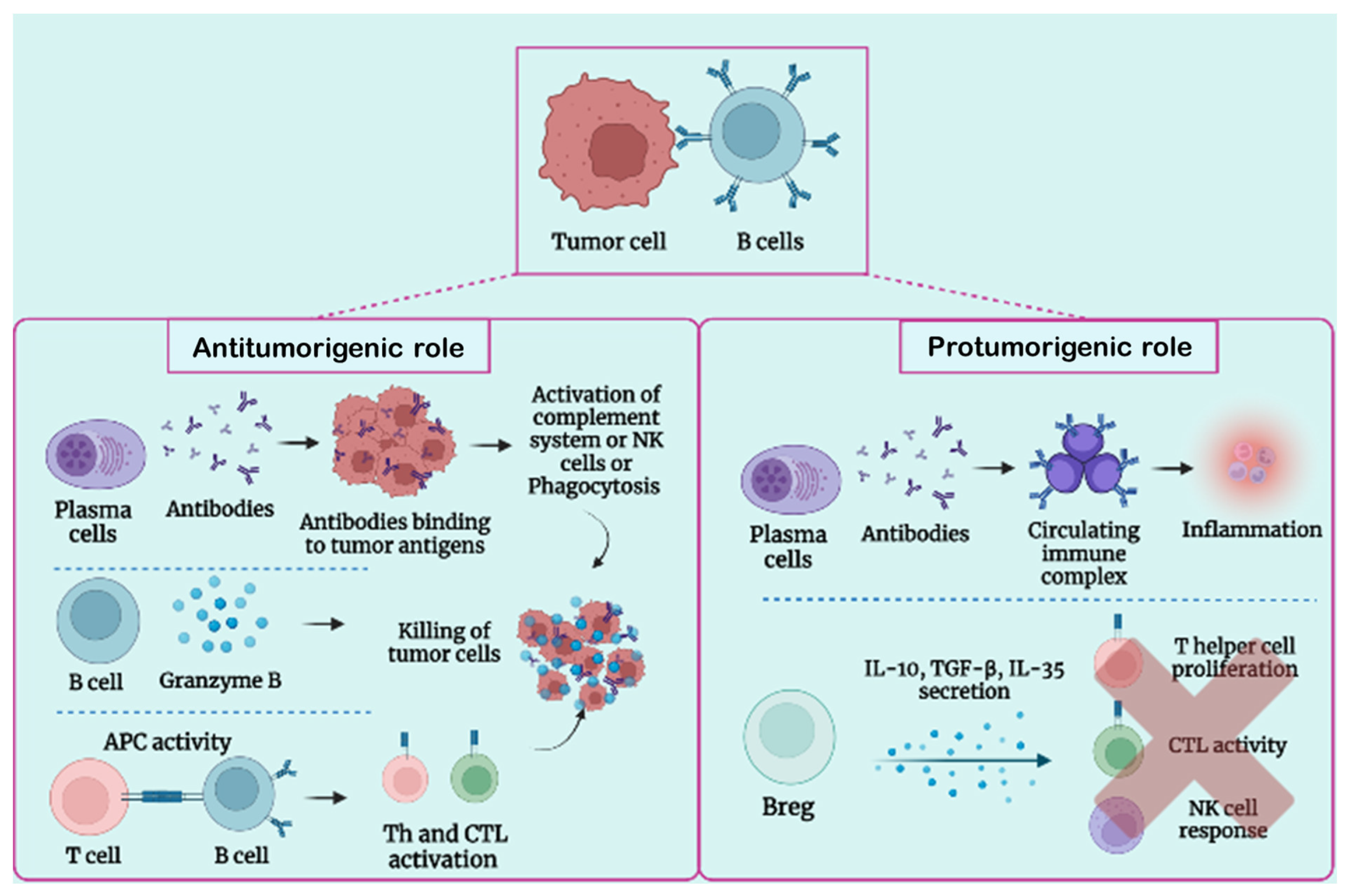
3.1. B-Cell Function in Tumor Condition
3.2. Protumorigenic Function of B Lymphocytes
3.3. Antitumorigenic Role of B Lymphocytes
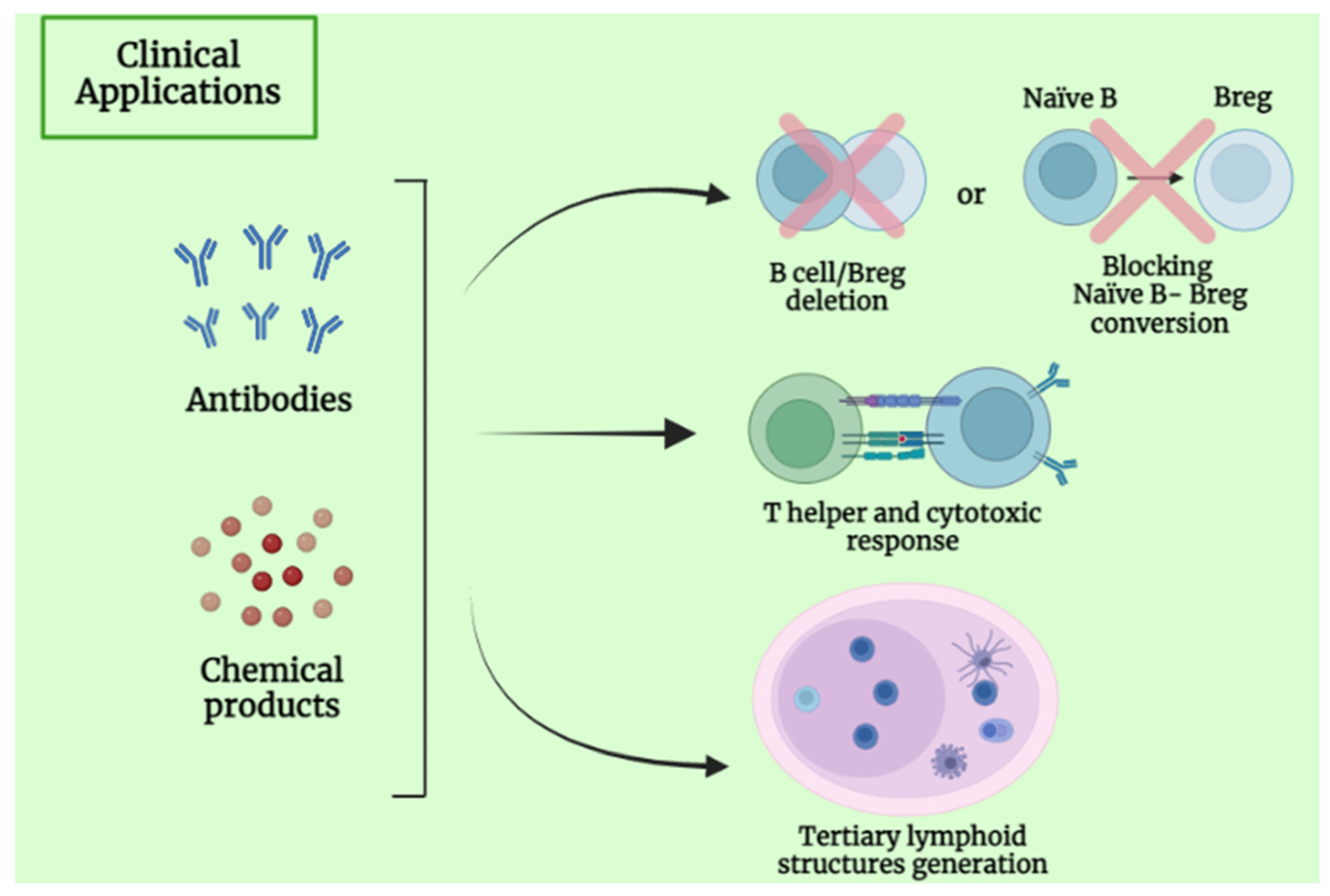
4. Clinical Application and Future Perspective of B Lymphocytes
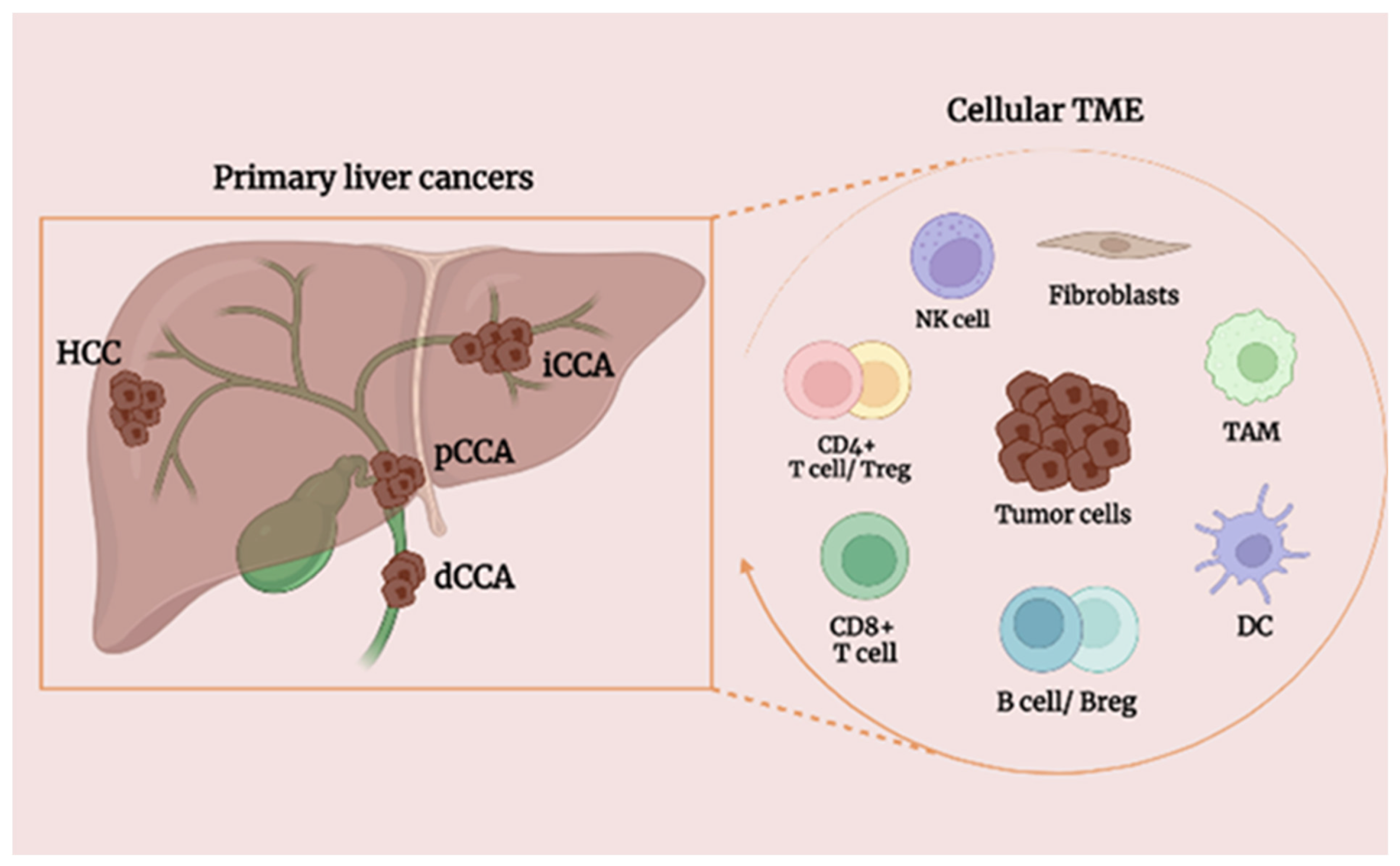
5. Effect of B Cells on Primary Liver Tumors
5.1. Background of HCC
5.2. B Lymphocytes in HCC
5.3. Background of CCA
5.4. B Lymphocytes in CCA
5.5. B-Cell-Based Immunotherapeutic Strategies in Primary Liver Cancer
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | hepatocellular carcinoma |
| CCA | cholangiocarcinoma |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| ASH | alcoholic steatohepatitis |
| NASH | nonalcoholic steatohepatitis |
| TILs | tumor-infiltrating lymphocytes |
| OS | overall survival |
| TME | tumor microenvironment |
| ICB | immune checkpoint blockade |
| iCCA | intrahepatic cholangiocarcinoma |
| pCCA | perihilar cholangiocarcinoma |
| dCCA | distal cholangiocarcinoma |
| DC | dendritic cell |
| NK | natural killer cell |
| TAM | tumor-associated macrophage |
| Breg | regularity B cell |
| Treg | regularity T cell |
| BM | bone marrow |
| HSCs | hematopoietic stem cells |
| Pro-B | progenitor B |
| Pre-B | precursor B |
| BCR | B cell receptor |
| SLO | secondary lymphoid organs |
| PAMPS | pathogen-associated molecular patterns |
| GC | germinal centers |
| Tfh | T follicular helper |
| FDCs | follicular dendritic cells |
| CSR | class-switch recombination |
| SHM | somatic hypermutation |
| PCs | plasma cells |
| TGF | transforming growth factor |
| Th | T helper |
| APC | antigen-presenting cell |
| M-CSF | macrophage colony stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-activating factor |
| GCSF | granulocyte colony-stimulating factor |
| MDSC | myeloid-derived suppressor cells |
| FcγR | Fcγ receptors |
| CICs | circulating immune complexes |
| TLS | tertiary lymphoid structures |
| CLL | chronic lymphocytic leukemia |
| ADCC | antibody-dependent cellular cytotoxicity |
| CDC | complement-dependent cytotoxicity |
| CAFs | cancer-associated fibroblasts |
| TAMs | tumor-associated macrophages |
| TANs | tumor-associated neutrophils |
| ECM | extracellular matrix |
| ACT | adoptive cell therapy |
| RFS | recurrence-free survival |
| BN | B naïve |
| SM B | switched memory B |
| PSC | primary sclerosing cholangitis |
| CHC | chronic hepatitis C |
References
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The Immunological and Metabolic Landscape in Primary and Metastatic Liver Cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Sarcognato, S.; Sacchi, D.; Fassan, M.; Fabris, L.; Cadamuro, M.; Zanus, G.; Cataldo, I.; Capelli, P.; Baciorri, F.; Cacciatore, M.; et al. Cholangiocarcinoma. Pathologica 2021, 113, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Liang, L. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Physiol. Behav. 2018, 155, 1828–1837.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protzer, U.; Maini, M.K.; Knolle, P.A. Living in the Liver: Hepatic Infections. Nat. Rev. Immunol. 2012, 12, 201–213. [Google Scholar] [CrossRef]
- Feng, M.Y.; Chan, L.L.; Chan, S.L. Drug Treatment for Advanced Hepatocellular Carcinoma: First-Line and Beyond. Curr. Oncol. 2022, 29, 434. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.H. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. Physiol. Behav. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Villanueva, A. Randomized Trials and Endpoints in Advanced HCC: Role of PFS as a Surrogate of Survival. J. Hepatol. 2019, 70, 1262–1277. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [Green Version]
- Nault, J.C.; Cheng, A.L.; Sangro, B.; Llovet, J.M. Milestones in the Pathogenesis and Management of Primary Liver Cancer. J. Hepatol. 2020, 72, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma-Evolving Concepts and Therapeutic Strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [Green Version]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272e11. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.-J.; Cheng, J.-W.; Gao, P.-T.; Huang, A.; Sun, Y.-F.; Zhou, K.-Q.; Hu, B.; Qiu, S.-J.; Zhou, J.; Fan, J.; et al. Clinical Characteristics and Prognostic Factors of Patients with Intrahepatic Cholangiocarcinoma with Fever: A Propensity Score Matching Analysis. Oncologist 2019, 24, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor Immunotherapies by Immune Checkpoint Inhibitors (ICIs); the Pros and Cons. Cell Commun. Signal. 2022, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Paijens, S.T.; Vledder, A.; Bruyn, M.; Nijman, H.W. Tumor-Infiltrating Lymphocytes in the Immunotherapy Era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef] [PubMed]
- Gnjatic, S.; Bronte, V.; Brunet, L.R.; Butler, M.O.; Disis, M.L.; Galon, J.; Hakansson, L.G.; Hanks, B.A.; Karanikas, V.; Khleif, S.N.; et al. Identifying Baseline Immune-Related Biomarkers to Predict Clinical Outcome of Immunotherapy. J. Immunother. Cancer 2017, 5, 44. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, L.; Goswami, S.; Ma, J.; Zheng, B.; Duan, M.; Liu, L.; Zhang, L.; Shi, J.; Dong, L.; et al. Landscape of Infiltrating B Cells and Their Clinical Significance in Human Hepatocellular Carcinoma. Oncoimmunology 2019, 8, e1571388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Hernandez, M.O.; Zhao, Y.; Mehta, M.; Tran, B.; Kelly, M.; Rae, Z.; Hernandez, J.M.; Davis, J.L.; Martin, S.P.; et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell 2019, 36, 418–430. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019, 179, 829–845.e20. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Kasper, H.U.; Drebber, U.; Stippel, D.L.; Dienes, H.P.; Gillessen, A. Liver Tumor Infiltrating Lymphocytes: Comparison of Hepatocellular and Cholangiolar Carcinoma. World J. Gastroenterol. 2009, 15, 5053–5057. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Meylan, M.; Petitprez, F.; Sun, C.M.; Italiano, A.; Sautès-Fridman, C. B Cells and Tertiary Lymphoid Structures as Determinants of Tumour Immune Contexture and Clinical Outcome. Nat. Rev. Clin. Oncol. 2022, 19, 441–457. [Google Scholar] [CrossRef]
- Vigano, L.; Soldani, C.; Franceschini, B.; Cimino, M.; Lleo, A.; Donadon, M.; Roncalli, M.; Aghemo, A.; Di Tommaso, L.; Torzilli, G. Tumor-Infiltrating Lymphocytes and Macrophages in Intrahepatic Cholangiocellular Carcinoma. Impact on Prognosis after Complete Surgery. J. Gastrointest. Surg. 2019, 23, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, G.; Termanini, A.; Soldani, C.; Portale, F.; Carriero, R.; Pilipow, K.; Costa, G.; Polidoro, M.; Franceschini, B.; Malenica, I.; et al. Multimodal Single-Cell Profiling of Intrahepatic Cholangiocarcinoma Defines Hyperactivated Tregs as a Potential Therapeutic Target. J. Hepatol. 2022, 77, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Montecino-Rodriguez, E.; Dorshkind, K. B-1 B Cell Development in the Fetus and Adult Encarnacion. Immunity 2012, 36, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Holl, T.M.; Haynes, B.F.; Kelsoe, G. Stromal Cell Independent B Cell Development in Vitro: Generation and Recovery of Autoreactive Clones. J. Immunol. Methods 2010, 354, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Pieper, K.; Grimbacher, B.; Eibel, H. B-Cell Biology and Development. J. Allergy Clin. Immunol. 2013, 131, 959–971. [Google Scholar] [CrossRef]
- Patel, A.M.; Liu, Y.S.; Davies, S.P.; Brown, R.M.; Kelly, D.A.; Scheel-Toellner, D.; Reynolds, G.M.; Stamataki, Z. The Role of B Cells in Adult and Paediatric Liver Injury. Front. Immunol. 2021, 12, 729143. [Google Scholar] [CrossRef]
- Laumont, C.M.; Banville, A.C.; Gilardi, M.; Hollern, D.P.; Nelson, B.H. Tumour-Infiltrating B Cells: Immunological Mechanisms, Clinical Impact and Therapeutic Opportunities. Nat. Rev. Cancer 2023, 22, 414–430. [Google Scholar] [CrossRef]
- Carsetti, R.; Terreri, S.; Conti, M.G.; Fernandez Salinas, A.; Corrente, F.; Capponi, C.; Albano, C.; Piano Mortari, E. Comprehensive Phenotyping of Human Peripheral Blood B Lymphocytes in Healthy Conditions. Cytom. Part A 2022, 101, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Poirot, J.; Medvedovic, J.; Trichot, C.; Soumelis, V. Compartmentalized Multicellular Crosstalk in Lymph Nodes Coordinates the Generation of Potent Cellular and Humoral Immune Responses. Eur. J. Immunol. 2021, 51, 3146–3160. [Google Scholar] [CrossRef] [PubMed]
- Perez-Andres, M.; Paiva, B.; Nieto, W.G.; Caraux, A.; Schmitz, A.; Almeida, J.; Vogt, R.F.; Marti, G.E.; Rawstron, A.C.; Van Zelm, M.C.; et al. Human Peripheral Blood B-Cell Compartments: A Crossroad in B-Cell Traffic. Cytom. Part B Clin. Cytom. 2010, 78, S47–S60. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Cols, M.; Puga, I. Activation of B Cells by Non-Canonical Helper Signals. EMBO Rep. 2012, 13, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Wang, D.; Fang, Y.; Zheng, Z.; Liu, X.; Wu, F.; Wang, L.; Li, X.; Hui, B.; Ma, S.; et al. Current Perspectives on B Lymphocytes in the Immunobiology of Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 647854. [Google Scholar] [CrossRef]
- Andreu, P.; Johansson, M.; Affara, N.I.; Pucci, F.; Junankar, S.; Korets, L.; Lam, J.; Tawfik, D.; Denardo, D.G.; Naldini, L.; et al. FcRγ Activation Regulates Inflammation-Associated Squamous Carcinogenesis. Cancer Cell 2011, 17, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Palm, A.K.E.; Henry, C. Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front. Immunol. 2019, 10, 1787. [Google Scholar] [CrossRef] [Green Version]
- Elsner, R.A.; Shlomchik, M.J. Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity 2020, 53, 1136–1150. [Google Scholar] [CrossRef]
- Radbruch, A.; Muehlinghaus, G.; Luger, E.O.; Inamine, A.; Smith, K.G.C.; Dörner, T.; Hiepe, F. Competence and Competition: The Challenge of Becoming a Long-Lived Plasma Cell. Nat. Rev. Immunol. 2006, 6, 741–750. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An Introduction to Immunology and Immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Pinto, D.; Moreno, J. B Cells Can Prime Naive CD4+ T Cells in Vivo in the Absence of Other Professional Antigen-Presenting Cells in a CD154-CD40-Dependent Manner. Eur. J. Immunol. 2005, 35, 1097–1105. [Google Scholar] [CrossRef]
- Janeway, C.A.; Ron, J.; Katz, M.E. The B Cell Is the Initiating Antigen-Presenting Cell in Peripheral Lymph Nodes. J. Immunol. 1987, 138, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Ron, Y.; Sprent, J. T Cell Priming in Vivo: A Major Role for B Cells in Presenting Antigen to T Cells in Lymph Nodes. J. Immunol. 1987, 138, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Braza, F.; Chesne, J.; Castagnet, S.; Magnan, A.; Brouard, S. Regulatory Functions of B Cells in Allergic Diseases. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Komai, T.; Inoue, M.; Okamura, T.; Morita, K.; Iwasaki, Y.; Sumitomo, S.; Shoda, H.; Yamamoto, K.; Fujio, K. Transforming Growth Factor-β and Interleukin-10 Synergistically Regulate Humoral Immunity via Modulating Metabolic Signals. Front. Immunol. 2018, 9, 1364. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Flavell, R.A. TGF-β and Regulatory T Cell in Immunity and Autoimmunity. J. Clin. Immunol. 2008, 28, 647–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazqueza, M.; Catalan-Dibenea, J.; Zlotnik, A. B Cells Responses and Cytokine Production Are Regulated by Their Immune Microenvironmen. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.C.; Kroemer, G.; Zitvogel, L. Targeting the Tumor Microenvironment: Removing Obstruction to Anticancer Immune Responses and Immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
- Lebien, T.W.; Tedder, T.F. B Lymphocytes: How They Develop and Function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- Catalán, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef]
- Abebe, E.C.; Dejenie, T.A.; Ayele, T.M.; Baye, N.D.; Teshome, A.A.; Muche, Z.T. The Role of Regulatory B Cells in Health and Diseases: A Systemic Review. J. Inflamm. Res. 2021, 14, 75–84. [Google Scholar] [CrossRef]
- Yuen, G.J.; Demissie, E.; Pillai, S.; Burnet, M. B Lymphocytes and Cancer: A Love-Hate Relationship. Trends Cancer 2017, 2, 747–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridman, W.H.; Petitprez, F.; Meylan, M.; Chen, T.W.W.; Sun, C.M.; Roumenina, L.T.; Sautès-Fridman, C. B Cells and Cancer: To B or Not to B? J. Exp. Med. 2021, 218, e20200851. [Google Scholar] [CrossRef] [PubMed]
- Reuschenbach, M.; von Knebel Doeberitz, M.; Wentzensen, N. A Systematic Review of Humoral Immune Responses against Tumor Antigens. Cancer Immunol. Immunother. 2010, 58, 1535–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinker, G.S.; Vitiello, G.A.F.; Ferreira, W.A.S.; Chaves, A.S.; Cordeiro de Lima, V.C.; Medina, T.D.S. B Cell Orchestration of Anti-Tumor Immune Responses: A Matter of Cell Localization and Communication. Front. Cell Dev. Biol. 2021, 9, 678127. [Google Scholar] [CrossRef]
- Ramos, R.N.; Amano, M.T.; Paes Leme, A.F.; Fox, J.W.; de Oliveira, A.K. Editorial: Tumor Microenvironment (TME) and Tumor Immune Microenvironment (TIME): New Perspectives for Prognosis and Therapy. Front. Cell Dev. Biol. 2022, 10, 1516. [Google Scholar] [CrossRef]
- Chen, X.; Jensen, P.E. The Role of B Lymphocytes as Antigen-Presenting Cells. Arch. Immunol. Ther. Exp. 2008, 56, 77–83. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.K.; Cao, Y.; Hamel, K.M.; Doodes, P.D.; Hutas, G.; Finnegan, A. Expression of CD80/86 on B Cells Is Essential for Autoreactive T Cell Activation and the Development of Arthritis. J. Immunol. 2007, 179, 5109–5116. [Google Scholar] [CrossRef] [Green Version]
- Shen, P.; Fillatreau, S. Antibody-Independent Functions of B Cells: A Focus on Cytokines. Nat. Rev. Immunol. 2015, 15, 441–451. [Google Scholar] [CrossRef]
- Garaud, S.; Buisseret, L.; Solinas, C.; Gu-Trantien, C.; De Wind, A.; Van Den Eynden, G.; Naveaux, C.; Lodewyckx, J.N.; Boisson, A.; Duvillier, H.; et al. Tumor-Infiltrating B Cells Signal Functional Humoral Immune Responses in Breast Cancer. JCI Insight 2019, 4, e129641. [Google Scholar] [CrossRef] [Green Version]
- Tan, R.; Nie, M.; Long, W. The Role of B Cells in Cancer Development. Front. Oncol. 2022, 12, 958756. [Google Scholar] [CrossRef]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B Cell Regulation in Cancer and Anti-Tumor Immunity. Cell. Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, P.; Roch, T.; Lampropoulou, V.; O’Connor, R.A.; Stervbo, U.; Hilgenberg, E.; Ries, S.; Dang, V.D.; Jaimes, Y.; Daridon, C.; et al. IL-35-Producing B Cells Are Critical Regulators of Immunity during Autoimmune and Infectious Diseases. Nature 2014, 507, 366–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Borja, F.; Blair, P. Mechanisms of Induction of Regulatory B Cells in the Tumour Microenvironment and Their Contribution to Immunosuppression and Pro-Tumour Responses. Clin. Exp. Immunol. 2022, 209, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shi, S.; Ljunggren, H.G.; Cava, A.L.; Van Kaer, L.; Shi, F.D.; Liu, R. NK Cells Inhibit T-Bet-Deficient, Autoreactive Th17 Cells. Scand. J. Immunol. 2012, 76, 559–566. [Google Scholar] [CrossRef]
- Xue, V.W.; Chung, J.Y.F.; Córdoba, C.A.G.; Cheung, A.H.K.; Kang, W.; Lam, E.W.F.; Leung, K.T.; To, K.F.; Lan, H.Y.; Tang, P.M.K. Transforming Growth Factor-β: A Multifunctional Regulator of Cancer Immunity. Cancers 2020, 12, 3099. [Google Scholar] [CrossRef]
- Largeot, A.; Pagano, G.; Gonder, S.; Moussay, E.; Paggetti, J. The B-Side of Cancer Immunity: The Underrated Tune. Cells 2019, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- De Visser, K.E.; Korets, L.V.; Coussens, L.M. De Novo Carcinogenesis Promoted by Chronic Inflammation Is B Lymphocyte Dependent. Cancer Cell 2005, 7, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [Green Version]
- Bindea, G.; Mlecnik, B.; Angell, H.K.; Galon, J. The Immune Landscape of Human Tumors: Implications for Cancer Immunotherapy. Oncoimmunology 2014, 3, 12–14. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Galon, J.; Fridman, W.H.; Smyth, M.J. From Mice to Humans: Developments in Cancer Immunoediting. J. Clin. Investig. 2015, 125, 3338–3346. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Mao, R.; Yang, J. NF- κ B and STAT3 Signaling Pathways Collaboratively Link in Fl Ammation to Cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, Z.; Wang, Y.; Liu, L.; Li, L.; Yeh, S.; Qi, L.; Chang, C. Tumor Microenvironment B Cells Increase Bladder Cancer Metastasis via Modulation of the IL-8/Androgen Receptor (AR)/MMPs Signals. Oncotarget 2015, 6, 26065–26078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sautès-Fridman, C.; Verneau, J.; Sun, C.M.; Moreira, M.; Chen, T.W.W.; Meylan, M.; Petitprez, F.; Fridman, W.H. Tertiary Lymphoid Structures and B Cells: Clinical Impact and Therapeutic Modulation in Cancer. In Seminars in Immunology; Academic Press: Cambridge, UK, 2020; p. 48. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Thommen, D.S. Tertiary Lymphoid Structures in Cancer. Science 2022, 375, eabf9419. [Google Scholar] [CrossRef] [PubMed]
- Gago da Graça, C.; van Baarsen, L.G.M.; Mebius, R.E. Tertiary Lymphoid Structures: Diversity in Their Development, Composition, and Role. J. Immunol. 2021, 206, 273–281. [Google Scholar] [CrossRef]
- Qin, M.; Jin, Y.; Pan, L.Y. Tertiary Lymphoid Structure and B-Cell-Related Pathways: A Potential Target in Tumor Immunotherapy. Oncol. Lett. 2021, 22, 836. [Google Scholar] [CrossRef]
- Teillaud, J.L.; Dieu-Nosjean, M.C. Tertiary Lymphoid Structures: An Anti-Tumor School for Adaptive Immune Cells and an Antibody Factory to Fight Cancer? Front. Immunol. 2017, 8, 830. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Ding, H.; Lin, Z.B.; Qiu, S.H.; Zhang, Y.R.; Guo, Y.G.; Chu, X.D.; Sam, L.I.; Pan, J.H.; Pan, Y.L. Relationship between Tertiary Lymphoid Structure and the Prognosis and Clinicopathologic Characteristics in Solid Tumors. Int. J. Med. Sci. 2021, 18, 2327–2338. [Google Scholar] [CrossRef]
- Deola, S.; Panelli, M.C.; Maric, D.; Selleri, S.; Dmitrieva, N.I.; Voss, C.Y.; Klein, H.; Stroncek, D.; Wang, E.; Marincola, F.M. Helper B Cells Promote Cytotoxic T Cell Survival and Proliferation Independently of Antigen Presentation through CD27/CD70 Interactions. J. Immunol. 2008, 180, 1362–1372. [Google Scholar] [CrossRef] [Green Version]
- Germain, C.; Gnjatic, S.; Dieu-Nosjean, M.C. Tertiary Lymphoid Structure-Associated B Cells Are Key Players in Anti-Tumor Immunity. Front. Immunol. 2015, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.F.; Cui, J.W. The Role of Tumor-Infiltrating B Cells in Tumor Immunity. J. Oncol. 2019, 2019, 2592419 . [Google Scholar] [CrossRef]
- Garnelo, M.; Tan, A.; Her, Z.; Yeong, J.; Lim, C.J.; Chen, J.; Lim, K.H.; Weber, A.; Chow, P.; Chung, A.; et al. Interaction between Tumour-Infiltrating B Cells and T Cells Controls the Progression of Hepatocellular Carcinoma. Gut 2017, 66, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, J.S.; Nelson, B.H. Tumor-Infiltrating B Cells and T Cells: Working Together to Promote Patient Survival. Oncoimmunology 2012, 1, 1623–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milo, R. Therapies for Multiple Sclerosis Targeting B Cells. Croat. Med. J. 2019, 60, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouaziz, J.D.; Yanaba, K.; Venturi, G.M.; Wang, Y.; Tisch, R.M.; Poe, J.C.; Tedder, T.F. Therapeutic B Cell Depletion Impairs Adaptive and Autoreactive CD4+ T Cell Activation in Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 20878–20883. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, A.E.; Karagiannis, P.; Dodev, T.; Koers, A.; Lacy, K.; Josephs, D.H.; Takhar, P.; Geh, J.L.C.; Healy, C.; Harries, M.; et al. Monitoring the Systemic Human Memory B Cell Compartment of Melanoma Patients for Anti-Tumor IgG Antibodies. PLoS ONE 2011, 6, e19330. [Google Scholar] [CrossRef]
- Vourekas, A.; Alexiou, P.; Vrettos, N.; Maragkakis, M.; Mourelatos, Z.; Medicine, T. Allogeneic IgG Combined with Dendritic Cell Stimuli Induces Anti- Tumor T Cell Immunity. Nature 2016, 531, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.Y.; Gao, Q.; Wang, Z.C.; Zhou, J.; Wang, X.Y.; Min, Z.H.; Shi, Y.H.; Shi, G.M.; Ding, Z.B.; Ke, A.W.; et al. Margin-Infiltrating CD20+ B Cells Display an Atypical Memory Phenotype and Correlate with Favorable Prognosis in Hepatocellular Carcinoma. Clin. Cancer Res. 2013, 19, 5994–6005. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Lao, X.; Pan, Q.; Ning, N.; Yet, J.; Xu, Y.; Li, S.; Chang, A.E. Adoptive Transfer of Tumor Reactive B Cells Confers Host T Cell Immunity and Tumor Regression. Clin. Cancer Res. 2011, 17, 4987–4995. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.L.; Khan, N.; Basu, S.; Soni, V. B-Cell-Based Immunotherapy: A Promising New Alternative. Vaccines 2022, 10, 879. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, G.; Lv, H.; Xu, L.; Liu, H.; Chen, T.; Qu, L.; Wang, J.; Cheng, L.; Hu, S.; et al. CD4-Targeted T Cells Rapidly Induce Remissions in Mice with T Cell Lymphoma. Biomed Res. Int. 2021, 2021, 6614784. [Google Scholar] [CrossRef] [PubMed]
- Heo, C.K.; Bahk, Y.Y.; Cho, E.W. Tumor-Associated Autoantibodies as Diagnostic and Prognostic Biomarkers. BMB Rep. 2012, 45, 677–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Peng, H.; Gao, J.; Nong, A.; Hua, H.; Yang, S.; Chen, L.; Wu, X.; Zhang, H.; Wang, J. Current Insights into the Expression and Functions of Tumor-Derived Immunoglobulins. Cell Death Discov. 2021, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B Cells and Tertiary Lymphoid Structures Promote Immunotherapy Response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary Lymphoid Structures Improve Immunotherapy and Survival in Melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B Cells Are Associated with Survival and Immunotherapy Response in Sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Munoz-Erazo, L.; Rhodes, J.L.; Marion, V.C.; Kemp, R.A. Tertiary Lymphoid Structures in Cancer-Considerations for Patient Prognosis. Cell. Mol. Immunol. 2020, 17, 570–575. [Google Scholar] [CrossRef]
- Mak, D.; Kramvis, A. Epidemiology and Aetiology of Hepatocellular Carcinoma in Sub-Saharan Africa. Hepatoma Res. 2021, 7, 10-20517. [Google Scholar] [CrossRef]
- Brown, Z.J.; Tsung, A. Adjuvant Treatment of Hepatocellular Carcinoma after Resection. Hepatoma Res. 2021, 7, 68. [Google Scholar] [CrossRef]
- Zhang, Q.; Lou, Y.; Bai, X.L.; Liang, T.B. Intratumoral Heterogeneity of Hepatocellular Carcinoma: From Singlecell to Population-Based Studies. World J. Gastroenterol. 2020, 26, 3720–3736. [Google Scholar] [CrossRef]
- Lu, C.; Rong, D.; Zhang, B.; Zheng, W.; Wang, X.; Chen, Z.; Tang, W. Current Perspectives on the Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma: Challenges and Opportunities. Mol. Cancer 2019, 18, 130. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Liu, S.; Zeng, S.; Shen, H. From Bench to Bed: The Tumor Immune Microenvironment and Current Immunotherapeutic Strategies for Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oura, K.; Morishita, A.; Tani, J.; Masaki, T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int. J. Mol. Sci. 2021, 22, 5801. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Chen, D.; Cheng, C.; Li, Z.; Chi, H.; Zhang, Y.; Zhang, X.; Tang, S.; Zhao, Q.; Ang, B.; et al. Immunosuppressive Landscape in Hepatocellular Carcinoma Revealed by Single-Cell Sequencing. Front. Immunol. 2022, 13, 950536. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Mukherjee, S. Targets of Immunotherapy for Hepatocellular Carcinoma: An Update. World J. Hepatol. 2022, 14, 140–157. [Google Scholar] [CrossRef]
- Guizhen, Z.; Guanchang, J.; Liwen, L.; Huifen, W.; Zhigang, R.; Ranran, S.; Zujiang, Y. The Tumor Microenvironment of Hepatocellular Carcinoma and Its Targeting Strategy by CAR-T Cell Immunotherapy. Front. Endocrinol. 2022, 13, 141. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 41–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Lou, Y.; Yang, J.; Wang, J.; Feng, J.; Zhao, Y.; Wang, L.; Huang, X.; Fu, Q.; Ye, M.; et al. Integrated Multiomic Analysis Reveals Comprehensive Tumour Heterogeneity and Novel Immunophenotypic Classification in Hepatocellular Carcinomas. Gut 2019, 68, 2019–2031. [Google Scholar] [CrossRef]
- Giraud, J.; Chalopin, D.; Blanc, J.F.; Saleh, M. Hepatocellular Carcinoma Immune Landscape and the Potential of Immunotherapies. Front. Immunol. 2021, 12, 655697. [Google Scholar] [CrossRef]
- Donne, R.; Lujambio, A. The Liver Cancer Immune Microenvironment: Therapeutic Implications for Hepatocellular Carcinoma. Hepatology 2022, in press. [Google Scholar] [CrossRef]
- Shimada, S.; Mogushi, K.; Akiyama, Y.; Furuyama, T.; Watanabe, S.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; et al. Comprehensive Molecular and Immunological Characterization of Hepatocellular Carcinoma. EBioMedicine 2019, 40, 457–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorosito Serrán, M.; Fiocca Vernengo, F.; Beccaria, C.G.; Acosta Rodriguez, E.V.; Montes, C.L.; Gruppi, A. The Regulatory Role of B Cells in Autoimmunity, Infections and Cancer: Perspectives beyond IL10 Production. FEBS Lett. 2015, 589, 3362–3369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Lo, C.M.; Ling, C.C.; Liu, X.B.; Ng, K.T.P.; Chu, A.C.Y.; Ma, Y.Y.; Li, C.X.; Fan, S.T.; Man, K. Regulatory B Cells Accelerate Hepatocellular Carcinoma Progression via CD40/CD154 Signaling Pathway. Cancer Lett. 2014, 355, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.J.; Liu, J.N.; Wu, R.; Long, Z.X.; Zhang, J.Z.; Tao, D.; Liu, Y.P. Change of the Peripheral Blood Immune Pattern and Its Correlation with Prognosis in Patients with Liver Cancer Treated by Sorafenib. Asian Pac. J. Trop. Med. 2016, 9, 592–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetta, H.F.; Mekky, M.A.; Zahran, A.M.; Abdel-Malek, M.O.; Ramadan, H.K.; Shafik, E.A.; Abbas, W.A.; El-Masry, M.A.; Mohamed, N.A.; Kamel, A.A.; et al. Regulatory b Cells and Their Cytokine Profile in HCV-Related Hepatocellular Carcinoma: Association with Regulatory t Cells and Disease Progression. Vaccines 2020, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.Z.; Wu, R.Q.; Wei, Y.; Liu, R.X.; Yang, D.; Xiao, X.; Zheng, L.; Li, B.; Lao, X.M.; Kuang, D.M. Dendritic Cell-Elicited B-Cell Activation Fosters Immune Privilege via IL-10 Signals in Hepatocellular Carcinoma. Nat. Commun. 2016, 7, 13453. [Google Scholar] [CrossRef]
- Jeannet, G.; Coudert, J.D.; Held, W. T and B Lymphocytes Exert Distinct Effects on the Homeostasis on NK Cells. Eur. J. Immunol. 2006, 36, 2725–2734. [Google Scholar] [CrossRef]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical Diagnosis and Staging of Cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert Consensus Document: Cholangiocarcinoma: Current Knowledge and Future Perspectives Consensus Statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Gupta, A.; Dixon, E. Epidemiology and Risk Factors: Intrahepatic Cholangiocarcinoma. HepatoBiliary Surg. Nutr. 2017, 6, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.; Gores, G.J. Pathogenesis, Diagnosis, and Management of Cholangiocarcinoma. Gastroenterol. Clin. N. Am. 2013, 145, 1215–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Song, Y.; Liu, S. The New Insight of Treatment in Cholangiocarcinoma. J. Cancer 2022, 13, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Macias, R.I.R.; Andersen, J.B.; Braconi, C.; Carpino, G.; Alvaro, D.; Calvisi, D.F. Cholangiocarcinoma: State-of-the-Art Knowledge and Challenges. Liver Int. 2019, 39, 5–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch-Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Chun, Y.S.; Javle, M. Systemic and Adjuvant Therapies for Intrahepatic Cholangiocarcinoma. Cancer Control. 2017, 24, 729241. [Google Scholar] [CrossRef]
- Sarantis, P.; Tzanetatou, E.D.; Ioakeimidou, E.; Vallilas, C.; Androutsakos, T.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Papavassiliou, A.G.; Karamouzis, M.V. Cholangiocarcinoma: The Role of Genetic and Epigenetic Factors; Current and Prospective Treatment with Checkpoint Inhibitors and Immunotherapy. Am. J. Transl. Res. 2021, 13, 13246–13260. [Google Scholar]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Ethics Self-Cultiv. 2018, 24, 30–46. [Google Scholar] [CrossRef]
- Gros, A.; Robbins, P.F.; Yao, X.; Li, Y.F.; Turcotte, S.; Tran, E.; Wunderlich, J.R.; Mixon, A.; Farid, S.; Dudley, M.E.; et al. PD-1 Identifies the Patient-Specific CD8+ Tumor-Reactive Repertoire Infiltrating Human Tumors. J. Clin. Investig. 2014, 124, 2246–2259. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-Institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and Safety of Pembrolizumab for the Treatment of Advanced Biliary Cancer: Results from the KEYNOTE-158 and KEYNOTE-028 Studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Lang, S.A.; Ulmer, T.F.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The Role of Tumor-Infiltrating Lymphocytes in Cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 127. [Google Scholar] [CrossRef] [PubMed]
- Goeppert, B.; Roessler, S.; Renner, M.; Singer, S.; Mehrabi, A.; Vogel, M.N.; Pathil, A.; Czink, E.; Köhler, B.; Springfeld, C.; et al. Mismatch Repair Deficiency Is a Rare but Putative Therapeutically Relevant Finding in Non-Liver Fluke Associated Cholangiocarcinoma. Br. J. Cancer 2019, 120, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Ding, G.Y.; Ma, J.Q.; Yun, J.P.; Chen, X.; Ling, Y.; Zhang, S.; Shi, J.Y.; Chang, Y.Q.; Ji, Y.; Wang, X.Y.; et al. Distribution and Density of Tertiary Lymphoid Structures Predict Clinical Outcome in Intrahepatic Cholangiocarcinoma. J. Hepatol. 2022, 76, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.W.; Rojas, O.L.; Gommerman, J.L. B Cell Depletion Therapies in Autoimmune Disease: Advances and Mechanistic Insights. Nat. Rev. Drug Discov. 2021, 20, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, F.A.; Hassanein, K.M.; Hetta, H.F.; Abdelmalek, M.O.; Zahran, A.M.; El-Badawy, O. Impact of Deranged B Cell Subsets Distribution in the Development of HCV-Related Cirrhosis and HCC in Type Two Diabetes Mellitus. Sci. Rep. 2020, 10, 20383. [Google Scholar] [CrossRef]
- Faggioli, F.; Palagano, E.; Di Tommaso, L.; Donadon, M.; Marrella, V.; Recordati, C.; Mantero, S.; Villa, A.; Vezzoni, P.; Cassani, B. B Lymphocytes Limit Senescence-Driven Fibrosis Resolution and Favor Hepatocarcinogenesis in Mouse Liver Injury. Hepatology 2018, 67, 1970–1985. [Google Scholar] [CrossRef] [Green Version]
- Weinmann, A.S. Molecular Mechanism and Function of CD40/CD40L Engagement in the Immune System. Group 2004, 4, 152–172. [Google Scholar] [CrossRef] [Green Version]
- Vonderheide, R.H.; Glennie, M.J. Agonistic CD40 Antibodies and Cancer Therapy. Clin. Cancer Res. 2013, 19, 1035–1043. [Google Scholar] [CrossRef] [Green Version]
- Diggs, L.P.; Ruf, B.; Ma, C.; Heinrich, B.; Cui, L.; Zhang, Q.; McVey, J.C.; Wabitsch, S.; Heinrich, S.; Rosato, U.; et al. CD40-Mediated Immune Cell Activation Enhances Response to Anti-PD-1 in Murine Intrahepatic Cholangiocarcinoma. J. Hepatol. 2021, 74, 1145–1154. [Google Scholar] [CrossRef]
- Vitale, L.A.; Thomas, L.J.; He, L.Z.; O’Neill, T.; Widger, J.; Crocker, A.; Sundarapandiyan, K.; Storey, J.R.; Forsberg, E.M.; Weidlick, J.; et al. Development of CDX-1140, an Agonist CD40 Antibody for Cancer Immunotherapy. Cancer Immunol. Immunother. 2019, 68, 233–245. [Google Scholar] [CrossRef]
- Zahran, A.M.; Hetta, H.F.; Rayan, A.; Eldin, A.S.; Hassan, E.A.; Fakhry, H.; Soliman, A.; El-Badawy, O. Differential Expression of Tim-3, PD-1, and CCR5 on Peripheral T and B Lymphocytes in Hepatitis C Virus-Related Hepatocellular Carcinoma and Their Impact on Treatment Outcomes. Cancer Immunol. Immunother. 2020, 69, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Alemohammad, H.; Najafzadeh, B.; Asadzadeh, Z.; Baghbanzadeh, A.; Ghorbaninezhad, F.; Najafzadeh, A.; Safarpour, H.; Bernardini, R.; Brunetti, O.; Sonnessa, M.; et al. The Importance of Immune Checkpoints in Immune Monitoring: A Future Paradigm Shift in the Treatment of Cancer. Biomed. Pharmacother. 2022, 146, 112516. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Peng, H.; Fu, Y.X. PD-1 Shapes B Cells as Evildoers in the Tumor Microenvironment. Physiol. Behav. 2019, 176, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.M.; Nair, S.S.; O’Leary, J.G.; Thompson-Snipes, L.A.; Nyarige, V.; Wang, J.; Park, W.; Stegall, M.; Heilman, R.; Klintmalm, G.B.; et al. Implication of TIGIT+ Human Memory B Cells in Immune Regulation. Nat. Commun. 2021, 12, 1534. [Google Scholar] [CrossRef]
- Pikarsky, E.; Heikenwalder, M. Focal and Local: Ectopic Lymphoid Structures and Aggregates of Myeloid and Other Immune Cells in Liver. Gastroenterology 2016, 151, 780–783. [Google Scholar] [CrossRef] [Green Version]
- Meylan, M.; Petitprez, F.; Lacroix, L.; Di Tommaso, L.; Roncalli, M.; Bougouin, A.; Laurent, A.; Amaddeo, G.; Sommacale, D.; Regnault, H.; et al. Early Hepatic Lesions Display Immature Tertiary Lymphoid Structures and Show Elevated Expression of Immune Inhibitory and Immunosuppressive Molecules. Clin. Cancer Res. 2020, 26, 4381–4389. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Zhang, T.; Yao, Q.; Li, J.; Nie, Y.; Lei, X.; Mao, Z.; Wang, Y.; Shi, W.; Song, W. Tertiary Lymphatic Structures in Primary Hepatic Carcinoma: Controversy Cannot Overshadow Hope. Front. Immunol. 2022, 13, 3212. [Google Scholar] [CrossRef]
- Bader, J.E.; Voss, K.; Rathmell, J.C. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, F.; Lyu, K.; Chang, A.E.; Li, Q. Emerging Concepts Regarding Pro- and Anti Tumor Properties of B Cells in Tumor Immunity. Front. Immunol. 2022, 13, 4117. [Google Scholar] [CrossRef]
- Xiao, X.; Lao, X.M.; Chen, M.M.; Liu, R.X.; Wei, Y.; Ouyang, F.Z.; Chen, D.P.; Zhao, X.Y.; Zhao, Q.; Li, X.F.; et al. PD-1hi Identifi Es a Novel Regulatory b-Cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov. 2016, 6, 546–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.; Chen, Y.; Lei, P.; Sheldon, M.; Sun, Y.; Yao, F.; Ma, L. Immunotherapeutic Approaches for Treating Hepatocellular Carcinoma. Cancers 2022, 14, 5013. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milardi, G.; Lleo, A. Tumor-Infiltrating B Lymphocytes: Promising Immunotherapeutic Targets for Primary Liver Cancer Treatment. Cancers 2023, 15, 2182. https://doi.org/10.3390/cancers15072182
Milardi G, Lleo A. Tumor-Infiltrating B Lymphocytes: Promising Immunotherapeutic Targets for Primary Liver Cancer Treatment. Cancers. 2023; 15(7):2182. https://doi.org/10.3390/cancers15072182
Chicago/Turabian StyleMilardi, Giulia, and Ana Lleo. 2023. "Tumor-Infiltrating B Lymphocytes: Promising Immunotherapeutic Targets for Primary Liver Cancer Treatment" Cancers 15, no. 7: 2182. https://doi.org/10.3390/cancers15072182
APA StyleMilardi, G., & Lleo, A. (2023). Tumor-Infiltrating B Lymphocytes: Promising Immunotherapeutic Targets for Primary Liver Cancer Treatment. Cancers, 15(7), 2182. https://doi.org/10.3390/cancers15072182





