Detection and Molecular Characterization of Circulating Tumour Cells: Challenges for the Clinical Setting
Abstract
Simple Summary
Abstract
1. Introduction
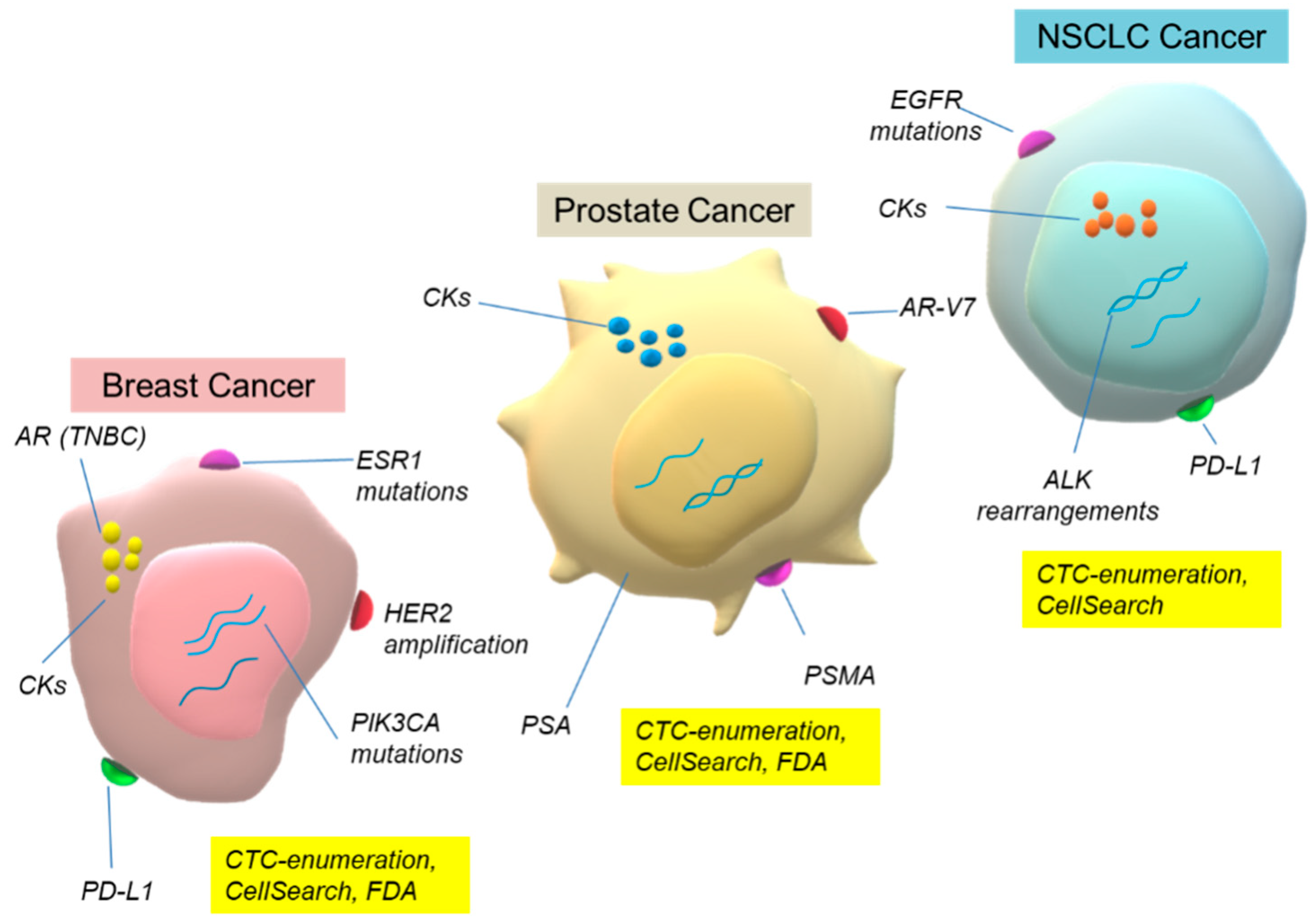
2. CTC: Challenges for the Management of Cancer Patients
2.1. Breast Cancer (BrCa)
2.1.1. Early Breast Cancer
CTC Enumeration
CTC Biomarkers
Clinical Trials
2.1.2. Metastatic Breast Cancer (MBC)
CTC Enumeration
HER2
ESR1 Mutations and Resistance to Estrogen Deprivation Therapy (EDT)
PIK3CA Mutations
Biomarkers for Immunotherapy
CTCs Biomarkers
Clinical Trials
2.2. Prostate Cancer (PCa)
2.2.1. Non-Metastatic
2.2.2. Metastatic Prostate Cancer (mPCa)
CTC Enumeration
AR-V7
PD-L1
Detection of Biomarkers in CTCs
Clinical Trials
2.3. NSCLC
2.3.1. Early Stage
2.3.2. Advanced NSCLC
CTC Enumeration
PD-L1
Anaplastic Lymphoma Kinase (ALK) Rearrangements
EGFR–TKIs-Osimertinib
3. CTC: Ongoing Clinical Trials, Novel Dimensions and Future Challenges
4. Conclusions—Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lianidou, E.; Hoon, D. Circulating Tumor Cells and circulating Tumor DNA. In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th ed.; Rifai, N., Horvath, A.-R., Wittwer, C., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 1111–1144. [Google Scholar]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Rushton, A.J.; Nteliopoulos, G.; Shaw, J.A.; Coombes, R.C. A Review of Circulating Tumour Cell Enrichment Technologies. Cancers 2021, 13, 970. [Google Scholar] [CrossRef] [PubMed]
- Mendelaar, P.A.J.; Kraan, J.; Van, M.; Zeune, L.L.; Terstappen, L.W.M.M.; Oomen-de Hoop, E.; Martens, J.W.M.; Sleijfer, S. Defining the dimensions of circulating tumor cells in a large series of breast, prostate, colon, and bladder cancer patients. Mol. Oncol. 2021, 15, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Vidlarova, M.; Rehulkova, A.; Stejskal, P.; Prokopova, A.; Slavik, H.; Hajduch, M.; Srovnal, J. Recent Advances in Methods for Circulating Tumor Cell Detection. Int. J. Mol. Sci. 2023, 24, 3902. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, C.; Qi, M.; Cheng, L.; Wang, L.; Li, C.; Dong, B. Recent progress of nanostructure-based enrichment of circulating tumor cells and downstream analysis. Lab Chip 2023, 23, 1493–1523. [Google Scholar] [CrossRef]
- Chowdhury, T.; Cressiot, B.; Parisi, C.; Smolyakov, G.; Thiébot, B.; Trichet, L.; Fernandes, F.M.; Pelta, J.; Manivet, P. Circulating Tumor Cells in Cancer Diagnostics and Prognostics by Single-Molecule and Single-Cell Characterization. ACS Sens. 2023, 8, 406–426. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, H.; Cheng, W.; Ni, Z.; Xiang, N. Droplet microfluidics for CTC-based liquid biopsy: A review. Analyst 2023, 148, 203–221. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Edd, J.F.; Mishra, A.; Smith, K.C.; Kapur, R.; Maheswaran, S.; Haber, D.A.; Toner, M. Isolation of circulating tumor cells. iScience 2022, 25, 104696. [Google Scholar] [CrossRef]
- Dirix, L.; Buys, A.; Oeyen, S.; Peeters, D.; Liègeois, V.; Prové, A.; Rondas, D.; Vervoort, L.; Mariën, V.; Van Laere, S.; et al. Circulating tumor cell detection: A prospective comparison between CellSearch® and RareCyte® platforms in patients with progressive metastatic breast cancer. Breast Cancer Res. Treat. 2022, 193, 437–444. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Crucial roles of circulating tumor cells in the metastatic cascade and tumor immune escape: Biology and clinical translation. J. Immunother. Cancer 2022, 10, e005615. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.-C.; Michiels, S.; Riethdorf, S.; Mueller, V.; Esserman, L.J.; Lucci, A.; Naume, B.; Horiguchi, J.; Gisbert-Criado, R.; Sleijfer, S.; et al. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Trapp, E.K.; Fasching, P.A.; Fehm, T.; Schneeweiss, A.; Mueller, V.; Harbeck, N.; Lorenz, R.; Schumacher, C.; Heinrich, G.; Schochter, F.; et al. Does the Presence of Circulating Tumor Cells in High-Risk Early Breast Cancer Patients Predict the Site of First Metastasis-Results from the Adjuvant SUCCESS A Trial. Cancers 2022, 14, 3949. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulou, D.; Markou, A.; Strati, A.; Zavridou, M.; Tzanikou, E.; Mastoraki, S.; Kallergi, G.; Georgoulias, V.; Lianidou, E. Comprehensive liquid biopsy analysis as a tool for the early detection of minimal residual disease in breast cancer. Sci. Rep. 2023, 13, 1258. [Google Scholar] [CrossRef]
- Matikas, A.; Kotsakis, A.; Apostolaki, S.; Politaki, H.; Perraki, M.; Kalbakis, K.; Nikolaou, M.; Economopoulou, P.; Hatzidaki, D.; Georgoulias, V. Detection of circulating tumour cells before and following adjuvant chemotherapy and long-term prognosis of early breast cancer. Br. J. Cancer 2022, 126, 1563–1569. [Google Scholar] [CrossRef]
- Strati, A.; Nikolaou, M.; Georgoulias, V.; Lianidou, E.S.; Strati, A.; Nikolaou, M.; Georgoulias, V.; Lianidou, E.S. Prognostic Significance of TWIST1, CD24, CD44, and ALDH1 Transcript Quantification in EpCAM-Positive Circulating Tumor Cells from Early Stage Breast Cancer Patients. Cells 2019, 8, 652. [Google Scholar] [CrossRef]
- Kasimir-Bauer, S.; Keup, C.; Hoffmann, O.; Hauch, S.; Kimmig, R.; Bittner, A.K. Circulating Tumor Cells Expressing the Prostate Specific Membrane Antigen (PSMA) Indicate Worse Outcome in Primary, Non-Metastatic Triple-Negative Breast Cancer. Front. Oncol. 2020, 10, 1658. [Google Scholar] [CrossRef]
- Tzanikou, E.; Markou, A.; Politaki, E.; Koutsopoulos, A.; Psyrri, A.; Mavroudis, D.; Georgoulias, V.; Lianidou, E. PIK3CA hotspot mutations in circulating tumor cells and paired circulating tumor DNA in breast cancer: A direct comparison study. Mol. Oncol. 2019, 13, 2515–2530. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Koutsopoulos, A.V.; Tsoulfas, P.G.; Lagoudaki, E.; Aggouraki, D.; Monastirioti, A.; Koutoulaki, C.; Apostolopoulou, C.A.; Merodoulaki, A.C.; Papadaki, C.; et al. Clinical Relevance of Immune Checkpoints on Circulating Tumor Cells in Breast Cancer. Cancers 2020, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.R.; Seagle, B.L.L.; Friedl, T.W.P.; Rack, B.; Lato, K.; Fink, V.; Cristofanilli, M.; Donnelly, E.D.; Janni, W.; Shahabi, S.; et al. Association of Circulating Tumor Cell Status with Benefit of Radiotherapy and Survival in Early-Stage Breast Cancer. JAMA Oncol. 2018, 4, e180163. [Google Scholar] [CrossRef]
- Trapp, E.; Janni, W.; Schindlbeck, C.; Jückstock, J.; Andergassen, U.; de Gregorio, A.; Alunni-Fabbroni, M.; Tzschaschel, M.; Polasik, A.; Koch, J.G.; et al. Presence of Circulating Tumor Cells in High-Risk Early Breast Cancer During Follow-Up and Prognosis. JNCI J. Natl. Cancer Inst. 2019, 111, 380–387. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Pierga, J.Y.; Reuben, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit. Rev. Oncol. Hematol. 2019, 134, 39–45. [Google Scholar] [PubMed]
- Magbanua, M.J.M.; Hendrix, L.H.; Hyslop, T.; Barry, W.T.; Winer, E.P.; Hudis, C.; Toppmeyer, D.; Carey, L.A.; Partridge, A.H.; Pierga, J.Y.; et al. Serial Analysis of Circulating Tumor Cells in Metastatic Breast Cancer Receiving First-Line Chemotherapy. J. Natl. Cancer Inst. 2021, 113, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Bortolini Silveira, A.; Bidard, F.C.; Tanguy, M.L.; Girard, E.; Trédan, O.; Dubot, C.; Jacot, W.; Goncalves, A.; Debled, M.; Levy, C.; et al. Multimodal liquid biopsy for early monitoring and outcome prediction of chemotherapy in metastatic breast cancer. NPJ Breast Cancer 2021, 7, 115. [Google Scholar]
- Franken, A.; Kraemer, A.; Sicking, A.; Watolla, M.; Rivandi, M.; Yang, L.; Warfsmann, J.; Polzer, B.M.; Friedl, T.W.P.; Meier-Stiegen, F.; et al. Comparative analysis of EpCAM high-expressing and low-expressing circulating tumour cells with regard to their clonal relationship and clinical value. Br. J. Cancer 2023. [Google Scholar] [CrossRef]
- Nanou, A.; Miao, J.; Coumans, F.A.W.; Dolce, E.M.; Darga, E.; Barlow, W.; Smerage, J.B.; Paoletti, C.; Godwin, A.K.; Pusztai, L.; et al. Tumor-Derived Extracellular Vesicles as Complementary Prognostic Factors to Circulating Tumor Cells in Metastatic Breast Cancer. JCO Precis. Oncol. 2023, 7, e2200372. [Google Scholar] [CrossRef]
- Kaur, P.; Campo, D.; Porras, T.B.; Ring, A.; Lu, J.; Chairez, Y.; Su, Y.; Kang, I.; Lang, J.E. A Pilot Study for the Feasibility of Exome-Sequencing in Circulating Tumor Cells Versus Single Metastatic Biopsies in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 4826. [Google Scholar] [CrossRef]
- Deutsch, T.M.; Riethdorf, S.; Fremd, C.; Feisst, M.; Nees, J.; Fischer, C.; Hartkopf, A.D.; Pantel, K.; Trumpp, A.; Schütz, F.; et al. HER2-targeted therapy influences CTC status in metastatic breast cancer. Breast Cancer Res. Treat. 2020, 182, 127–136. [Google Scholar] [CrossRef]
- Müller, V.; Banys-Paluchowski, M.; Friedl, T.W.P.; Fasching, P.A.; Schneeweiss, A.; Hartkopf, A.; Wallwiener, D.; Rack, B.; Meier-Stiegen, F.; Huober, J.; et al. Prognostic relevance of the HER2 status of circulating tumor cells in metastatic breast cancer patients screened for participation in the DETECT study program. ESMO Open 2021, 6, 100299. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mu, Z.; Ye, Z.; Zhang, Z.; Abu-Khalaf, M.M.; Silver, D.P.; Palazzo, J.P.; Jagannathan, G.; Fellin, F.M.; Bhattacharya, S.; et al. Prognostic value of HER2 status on circulating tumor cells in advanced-stage breast cancer patients with HER2-negative tumors. Breast Cancer Res. Treat. 2020, 181, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Y.; Yates, M.E.; Tasdemir, N.; Bahreini, A.; Chen, J.; Levine, K.M.; Priedigkeit, N.M.; Nasrazadani, A.; Ali, S.; et al. Hotspot ESR1 mutations are multimodal and contextual modulators of breast cancer metastasis. Cancer Res. 2022, 82, 1321–1339. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, T.K.; Dubash, T.D.; Zheng, Z.; Bardia, A.; Wittner, B.S.; Aceto, N.; Silva, E.J.; Fox, D.B.; Liebers, M.; Kapur, R.; et al. Evaluation of endocrine resistance using ESR1 genotyping of circulating tumor cells and plasma DNA. Breast Cancer Res. Treat. 2021, 188, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulou, D.; Markou, A.; Tzanikou, E.; Ladas, I.; Mike Makrigiorgos, G.; Georgoulias, V.; Lianidou, E. ESR1 NAPA Assay: Development and Analytical Validation of a Highly Sensitive and Specific Blood-Based Assay for the Detection of ESR1 Mutations in Liquid Biopsies. Cancers 2021, 13, 556. [Google Scholar] [CrossRef]
- Rothé, F.; Venet, D.; Peeters, D.; Rouas, G.; Rediti, M.; Smeets, D.; Dupont, F.; Campbell, P.; Lambrechts, D.; Dirix, L.; et al. Interrogating breast cancer heterogeneity using single and pooled circulating tumor cell analysis. NPJ Breast Cancer 2022, 8, 79. [Google Scholar]
- Cani, A.K.; Dolce, E.M.; Darga, E.P.; Hu, K.; Liu, C.J.; Pierce, J.; Bradbury, K.; Kilgour, E.; Aung, K.; Schiavon, G.; et al. Serial monitoring of genomic alterations in circulating tumor cells of ER-positive/HER2-negative advanced breast cancer: Feasibility of precision oncology biomarker detection. Mol. Oncol. 2022, 16, 1969–1985. [Google Scholar] [CrossRef]
- Mastoraki, S.; Strati, A.; Tzanikou, E.; Chimonidou, M.; Politaki, E.; Voutsina, A.; Psyrri, A.; Georgoulias, V.; Lianidou, E. ESR1 Methylation: A Liquid Biopsy–Based Epigenetic Assay for the Follow-up of Patients with Metastatic Breast Cancer Receiving Endocrine Treatment. Clin. Cancer Res. 2018, 24, 1500–1510. [Google Scholar] [CrossRef]
- Ningsi, R.; Elazezy, M.; Stegat, L.; Laakmann, E.; Peine, S.; Riethdorf, S.; Müller, V.; Pantel, K.; Joosse, S.A. Detection and Characterization of Estrogen Receptor α Expression of Circulating Tumor Cells as a Prognostic Marker. Cancers 2022, 14, 2621. [Google Scholar] [CrossRef]
- Jacot, W.; Mazel, M.; Mollevi, C.; Pouderoux, S.; D’Hondt, V.; Cayrefourcq, L.; Bourgier, C.; Boissiere-Michot, F.; Berrabah, F.; Lopez-Crapez, E.; et al. Clinical Correlations of Programmed Cell Death Ligand 1 Status in Liquid and Standard Biopsies in Breast Cancer. Clin. Chem. 2020, 66, 1093–1101. [Google Scholar] [CrossRef]
- Darga, E.P.; Dolce, E.M.; Fang, F.; Kidwell, K.M.; Gersch, C.L.; Kregel, S.; Thomas, D.G.; Gill, A.; Brown, M.E.; Gross, S.; et al. PD-L1 expression on circulating tumor cells and platelets in patients with metastatic breast cancer. PLoS ONE 2021, 16, e0260124. [Google Scholar] [CrossRef]
- Kallergi, G.; Tsintari, V.; Sfakianakis, S.; Bei, E.; Lagoudaki, E.; Koutsopoulos, A.; Zacharopoulou, N.; Alkahtani, S.; Alarifi, S.; Stournaras, C.; et al. The prognostic value of JUNB-positive CTCs in metastatic breast cancer: From bioinformatics to phenotypic characterization. Breast Cancer Res. 2019, 21, 86. [Google Scholar]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating Tumor Cells with Stemness and Epithelial-to-Mesenchymal Transition Features Are Chemoresistant and Predictive of Poor Outcome in Metastatic Breast Cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, M.A.; Mala, A.; Merodoulaki, A.C.; Vassilakopoulou, M.; Mavroudis, D.; Agelaki, S. Investigating the Role of CTCs with Stem/EMT-like Features in Metastatic Breast Cancer Patients Treated with Eribulin Mesylate. Cancers 2022, 14, 3903. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, M.A.; Monastirioti, A.; Apostolopoulou, C.A.; Aggouraki, D.; Papadaki, C.; Michaelidou, K.; Vassilakopoulou, M.; Alexakou, K.; Mavroudis, D.; Agelaki, S. TLR4 and pSTAT3 Expression on Circulating Tumor Cells (CTCs) and Immune Cells in the Peripheral Blood of Breast Cancer Patients: Prognostic Implications. Cancers 2022, 14, 1053. [Google Scholar] [CrossRef]
- Strati, A.; Nikolaou, M.; Georgoulias, V.; Lianidou, E.S. RNA-Based CTC Analysis Provides Prognostic Information in Metastatic Breast Cancer. Diagnostics 2021, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.T.; Bardia, A.; Spring, L.M.; Giobbie-Hurder, A.; Kalinich, M.; Dubash, T.; Sundaresan, T.; Hong, X.; Licausi, J.A.; Ho, U.; et al. A digital rna signature of circulating tumor cells predicting early therapeutic response in localized and metastatic breast cancer. Cancer Discov. 2018, 8, 1286–1299. [Google Scholar] [CrossRef]
- Strati, A.; Zavridou, M.; Kallergi, G.; Politaki, E.; Kuske, A.; Gorges, T.M.; Riethdorf, S.; Joosse, S.A.; Koch, C.; Bohnen, A.L.; et al. A Comprehensive Molecular Analysis of in Vivo Isolated EpCAM-Positive Circulating Tumor Cells in Breast Cancer. Clin. Chem. 2021, 67, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Welsch, E.; Schuster, E.; Krainer, M.; Marhold, M.; Bartsch, R.; Fischer, M.B.; Hermann, M.; Hastermann, G.; Uher, H.; Sliutz, G.; et al. Comparison of RNA Marker Panels for Circulating Tumor Cells and Evaluation of Their Prognostic Relevance in Breast Cancer. Cancers 2023, 15, 1271. [Google Scholar] [CrossRef]
- Keup, C.; Suryaprakash, V.; Hauch, S.; Storbeck, M.; Hahn, P.; Sprenger-Haussels, M.; Kolberg, H.C.; Tewes, M.; Hoffmann, O.; Kimmig, R.; et al. Integrative statistical analyses of multiple liquid biopsy analytes in metastatic breast cancer. Genome Med. 2021, 13, 85. [Google Scholar]
- Nair, M.G.; Somashekaraiah, V.M.; Ramamurthy, V.; Prabhu, J.S.; Sridhar, T.S. miRNAs: Critical mediators of breast cancer metastatic programming. Exp. Cell Res. 2021, 401, 112518. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Turchinovich, A.; Feisst, M.; Riedel, F.; Haßdenteufel, K.; Scharli, P.; Hartkopf, A.D.; Brucker, S.Y.; Michel, L.; Burwinkel, B.; et al. Circulating miR-200 Family and CTCs in Metastatic Breast Cancer before, during, and after a New Line of Systemic Treatment. Int. J. Mol. Sci. 2022, 23, 9535. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, A.; Kiavue, N.; Bidard, F.C.; Pierga, J.Y.; Cabel, L. Clinical utility of circulating tumor cells: An update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- Jacot, W.; Cottu, P.; Berger, F.; Dubot, C.; Venat-Bouvet, L.; Lortholary, A.; Bourgeois, H.; Bollet, M.; Servent, V.; Luporsi, E.; et al. Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: The CirCe T-DM1 trial. Breast Cancer Res. 2019, 21, 121. [Google Scholar]
- Gerratana, L.; Pierga, J.Y.; Reuben, J.M.; Davis, A.A.; Wehbe, F.H.; Dirix, L.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. Modeling the Prognostic Impact of Circulating Tumor Cells Enumeration in Metastatic Breast Cancer for Clinical Trial Design Simulation. Oncologist 2022, 27, e561–e570. [Google Scholar] [CrossRef]
- Bidard, F.C.; Jacot, W.; Kiavue, N.; Dureau, S.; Kadi, A.; Brain, E.; Bachelot, T.; Bourgeois, H.; Gonçalves, A.; Ladoire, S.; et al. Efficacy of Circulating Tumor Cell Count-Driven vs Clinician-Driven First-line Therapy Choice in Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: The STIC CTC Randomized Clinical Trial. JAMA Oncol. 2021, 7, 34–41. [Google Scholar] [CrossRef]
- Cohen, E.N.; Jayachandran, G.; Moore, R.G.; Cristofanilli, M.; Lang, J.E.; Khoury, J.D.; Press, M.F.; Kim, K.K.; Khazan, N.; Zhang, Q.; et al. A Multi-Center Clinical Study to Harvest and Characterize Circulating Tumor Cells from Patients with Metastatic Breast Cancer Using the Parsortix® PC1 System. Cancers 2022, 14, 5238. [Google Scholar] [CrossRef]
- Rzhevskiy, A.S.; Kapitannikova, A.Y.; Butnaru, D.V.; Shpot, E.V.; Joosse, S.A.; Zvyagin, A.V.; Ebrahimi Warkiani, M. Liquid Biopsy in Diagnosis and Prognosis of Non-Metastatic Prostate Cancer. Biomedicines 2022, 10, 3115. [Google Scholar] [CrossRef]
- Budna-Tukan, J.; Świerczewska, M.; Mazel, M.; Cieślikowski, W.A.; Ida, A.; Jankowiak, A.; Antczak, A.; Nowicki, M.; Pantel, K.; Azria, D.; et al. Analysis of Circulating Tumor Cells in Patients with Non-Metastatic High-Risk Prostate Cancer before and after Radiotherapy Using Three Different Enumeration Assays. Cancers 2019, 11, 802. [Google Scholar] [CrossRef]
- Chen, S.; Tauber, G.; Langsenlehner, T.; Schmölzer, L.M.; Pötscher, M.; Riethdorf, S.; Kuske, A.; Leitinger, G.; Kashofer, K.; Czyż, Z.T.; et al. In Vivo Detection of Circulating Tumor Cells in High-Risk Non-Metastatic Prostate Cancer Patients Undergoing Radiotherapy. Cancers 2019, 11, 933. [Google Scholar] [CrossRef]
- Joosse, S.A.; Beyer, B.; Gasch, C.; Nastały, P.; Kuske, A.; Isbarn, H.; Horst, L.J.; Hille, C.; Gorges, T.M.; Cayrefourcq, L.; et al. Tumor-Associated Release of Prostatic Cells into the Blood after Transrectal Ultrasound-Guided Biopsy in Patients with Histologically Confirmed Prostate Cancer. Clin. Chem. 2020, 66, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mandel, P.C.; Huland, H.; Tiebel, A.; Haese, A.; Salomon, G.; Budäus, L.; Tilki, D.; Chun, F.; Heinzer, H.; Graefen, M.; et al. Enumeration and Changes in Circulating Tumor Cells and Their Prognostic Value in Patients Undergoing Cytoreductive Radical Prostatectomy for Oligometastatic Prostate Cancer-Translational Research Results from the Prospective ProMPT trial. Eur. Urol. Focus 2021, 7, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sumanasuriya, S.; Omlin, A.; Armstrong, A.; Attard, G.; Chi, K.N.; Bevan, C.L.; Shibakawa, A.; IJzerman, M.J.; De Laere, B.; Lolkema, M.; et al. Consensus Statement on Circulating Biomarkers for Advanced Prostate Cancer. Eur. Urol. Oncol. 2018, 1, 151–159. [Google Scholar] [CrossRef]
- Lorente, D.; Olmos, D.; Mateo, J.; Dolling, D.; Bianchini, D.; Seed, G.; Flohr, P.; Crespo, M.; Figueiredo, I.; Miranda, S.; et al. Circulating tumour cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1554–1560. [Google Scholar] [CrossRef]
- Klusa, D.; Lohaus, F.; Franken, A.; Baumbach, M.; Cojoc, M.; Dowling, P.; Linge, A.; Offermann, A.; Löck, S.; Hušman, D.; et al. Dynamics of CXCR4 positive circulating tumor cells in prostate cancer patients during radiotherapy. Int. J. Cancer 2023, 1–16. [Google Scholar] [CrossRef]
- Brown, L.C.; Lu, C.; Antonarakis, E.S.; Luo, J.; Armstrong, A.J. Androgen receptor variant-driven prostate cancer II: Advances in clinical investigation. Prostate Cancer Prostatic Dis. 2020, 23, 367–380. [Google Scholar] [CrossRef]
- Strati, A.; Zavridou, M.; Bournakis, E.; Mastoraki, S.; Lianidou, E. Expression pattern of androgen receptors, AR-V7 and AR-567es, in circulating tumor cells and paired plasma-derived extracellular vesicles in metastatic castration resistant prostate cancer. Analyst 2019, 144, 6671–6680. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Welti, J.C.; Lambros, M.B.K.; Dolling, D.; Rodrigues, D.N.; Pope, L.; Aversa, C.; Figueiredo, I.; Fraser, J.; Ahmad, Z.; et al. Clinical Utility of Circulating Tumour Cell Androgen Receptor Splice Variant-7 Status in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 76, 676–685. [Google Scholar] [CrossRef]
- Graf, R.P.; Hullings, M.; Barnett, E.S.; Carbone, E.; Dittamore, R.; Scher, H.I. Clinical Utility of the Nuclear-localized AR-V7 Biomarker in Circulating Tumor Cells in Improving Physician Treatment Choice in Castration-resistant Prostate Cancer. Eur. Urol. 2020, 77, 170–177. [Google Scholar] [CrossRef]
- Sperger, J.M.; Emamekhoo, H.; McKay, R.R.; Stahlfeld, C.N.; Singh, A.; Chen, X.E.; Kwak, L.; Gilsdorf, C.S.; Wolfe, S.K.; Wei, X.X.; et al. Prospective Evaluation of Clinical Outcomes Using a Multiplex Liquid Biopsy Targeting Diverse Resistance Mechanisms in Metastatic Prostate Cancer. J. Clin. Oncol. 2021, 39, 2926–2937. [Google Scholar] [CrossRef]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A.; et al. What Do We Have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review (Part 6): Correlation of PD-L1 Expression with the Status of Mismatch Repair System, BRCA, PTEN, and Other Genes. Biomedicines 2022, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Du, X.; Lu, C.; Zhang, Z.; Huang, C.Y.; Yang, L.; Warren, S.; Kuczler, M.D.; Reyes, D.K.; Luo, J.; et al. RNA profiling of circulating tumor cells systemically captured from diagnostic leukapheresis products in prostate cancer patients. Mater. Today Bio 2022, 17, 100474. [Google Scholar] [CrossRef] [PubMed]
- Zavridou, M.; Strati, A.; Bournakis, E.; Smilkou, S.; Tserpeli, V.; Lianidou, E. Prognostic significance of gene expression and DNA methylation markers in circulating tumor cells and paired plasma derived exosomes in metastatic castration resistant prostate cancer. Cancers 2021, 13, 780. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Lazaridou, M.; Paraskevopoulos, P.; Chen, S.; Świerczewska, M.; Budna, J.; Kuske, A.; Gorges, T.M.; Joosse, S.A.; Kroneis, T.; et al. Multiplex Gene Expression Profiling of In Vivo Isolated Circulating Tumor Cells in High-Risk Prostate Cancer Patients. Clin. Chem. 2018, 64, 297–306. [Google Scholar] [CrossRef]
- Heller, G.; McCormack, R.; Kheoh, T.; Molina, A.; Smith, M.R.; Dreicer, R.; Saad, F.; De Wit, R.; Aftab, D.T.; Hirmand, M.; et al. Circulating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: A Comparison with Prostate-Specific Antigen Across Five Randomized Phase III Clinical Trials. J. Clin. Oncol. 2018, 36, 572–580. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Luo, J.; Nanus, D.M.; Giannakakou, P.; Szmulewitz, R.Z.; Danila, D.C.; Healy, P.; Anand, M.; Berry, W.R.; Zhang, T.; et al. Prospective Multicenter Study of Circulating Tumor Cell AR-V7 and Taxane Versus Hormonal Treatment Outcomes in Metastatic Castration-Resistant Prostate Cancer. JCO Precis. Oncol. 2020, 4, 1285–1301. [Google Scholar] [CrossRef]
- Chemi, F.; Rothwell, D.G.; McGranahan, N.; Gulati, S.; Abbosh, C.; Pearce, S.P.; Zhou, C.; Wilson, G.A.; Jamal-Hanjani, M.; Birkbak, N.; et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat. Med. 2019, 25, 1534–1539. [Google Scholar] [CrossRef]
- Lindsay, C.R.; Blackhall, F.H.; Carmel, A.; Fernandez-Gutierrez, F.; Gazzaniga, P.; Groen, H.J.M.; Hiltermann, T.J.N.; Krebs, M.G.; Loges, S.; López-López, R.; et al. EPAC-lung: Pooled analysis of circulating tumour cells in advanced non-small cell lung cancer. Eur. J. Cancer 2019, 117, 60–68. [Google Scholar] [CrossRef]
- de Wit, S.; Rossi, E.; Weber, S.; Tamminga, M.; Manicone, M.; Swennenhuis, J.F.; Groothuis-Oudshoorn, C.G.M.; Vidotto, R.; Facchinetti, A.; Zeune, L.L.; et al. Single tube liquid biopsy for advanced non-small cell lung cancer. Int. J. Cancer 2019, 144, 3127–3137. [Google Scholar] [CrossRef]
- Janning, M.; Kobus, F.; Babayan, A.; Wikman, H.; Velthaus, J.L.; Bergmann, S.; Schatz, S.; Falk, M.; Berger, L.A.; Böttcher, L.M.; et al. Determination of PD-L1 Expression in Circulating Tumor Cells of NSCLC Patients and Correlation with Response to PD-1/PD-L1 Inhibitors. Cancers 2019, 11, 835. [Google Scholar] [CrossRef]
- Sinoquet, L.; Jacot, W.; Gauthier, L.; Pouderoux, S.; Viala, M.; Cayrefourcq, L.; Quantin, X.; Alix-Panabières, C. Programmed Cell Death Ligand 1-Expressing Circulating Tumor Cells: A New Prognostic Biomarker in Non-Small Cell Lung Cancer. Clin. Chem. 2021, 67, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, T.H.; Fouladdel, S.; Zhang, Z.; Soni, P.; Qin, A.; Zhao, L.; Azizi, E.; Lawrence, T.S.; Ramnath, N.; et al. PD-L1 Expression in Circulating Tumor Cells Increases during Radio(chemo)therapy and Indicates Poor Prognosis in Non-small Cell Lung Cancer. Sci. Rep. 2019, 9, 566. [Google Scholar] [CrossRef]
- Manjunath, Y.; Upparahalli, S.V.; Avella, D.M.; Deroche, C.B.; Kimchi, E.T.; Staveley-O’carroll, K.F.; Smith, C.J.; Li, G.; Kaifi, J.T. PD-L1 Expression with Epithelial Mesenchymal Transition of Circulating Tumor Cells Is Associated with Poor Survival in Curatively Resected Non-Small Cell Lung Cancer. Cancers 2019, 11, 806. [Google Scholar] [CrossRef]
- Spiliotaki, M.; Neophytou, C.M.; Vogazianos, P.; Stylianou, I.; Gregoriou, G.; Constantinou, A.I.; Deltas, C.; Charalambous, H. Dynamic monitoring of PD-L1 and Ki67 in circulating tumor cells of metastatic non-small cell lung cancer patients treated with pembrolizumab. Mol. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ntzifa, A.; Strati, A.; Kallergi, G.; Kotsakis, A.; Georgoulias, V.; Lianidou, E. Gene expression in circulating tumor cells reveals a dynamic role of EMT and PD-L1 during osimertinib treatment in NSCLC patients. Sci. Rep. 2021, 11, 2313. [Google Scholar] [CrossRef]
- Pailler, E.; Faugeroux, V.; Oulhen, M.; Mezquita, L.; Laporte, M.; Honore, A.; Lecluse, Y.; Queffelec, P.; NgoCamus, M.; Nicotra, C.; et al. Acquired Resistance Mutations to ALK Inhibitors Identified by Single Circulating Tumor Cell Sequencing in ALK-Rearranged Non-Small-Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 6671–6682. [Google Scholar] [CrossRef] [PubMed]
- Oulhen, M.; Pawlikowska, P.; Tayoun, T.; Garonzi, M.; Buson, G.; Forcato, C.; Manaresi, N.; Aberlenc, A.; Mezquita, L.; Lecluse, Y.; et al. Circulating tumor cell copy-number heterogeneity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. NPJ Precis. Oncol. 2021, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Buszka, K.; Ntzifa, A.; Owecka, B.; Kamińska, P.; Kolecka-Bednarczyk, A.; Zabel, M.; Nowicki, M.; Lianidou, E.; Budna-Tukan, J. Liquid Biopsy Analysis as a Tool for TKI-Based Treatment in Non-Small Cell Lung Cancer. Cells 2022, 11, 2871. [Google Scholar] [CrossRef] [PubMed]
- Ntzifa, A.; Kotsakis, A.; Georgoulias, V.; Lianidou, E. Detection of EGFR Mutations in Plasma cfDNA and Paired CTCs of NSCLC Patients before and after Osimertinib Therapy Using Crystal Digital PCR. Cancers 2021, 13, 2736. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Duan, J.; Bai, H.; Wang, Z.; Wei, L.; Zhao, J.; Zhuo, M.; Wang, S.; Yang, L.; et al. DNA Methylation status of Wnt antagonist SFRP5 can predict the response to the EGFR-tyrosine kinase inhibitor therapy in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2012, 31, 80. [Google Scholar] [CrossRef]
- Ntzifa, A.; Londra, D.; Rampias, T.; Kotsakis, A.; Georgoulias, V.; Lianidou, E. DNA Methylation Analysis in Plasma Cell-Free DNA and Paired CTCs of NSCLC Patients before and after Osimertinib Treatment. Cancers 2021, 13, 5974. [Google Scholar] [CrossRef] [PubMed]
- Cayrefourcq, L.; Thomas, F.; Mazard, T.; Assenat, E.; Assou, S.; Alix-Panabières, C. Selective treatment pressure in colon cancer drives the molecular profile of resistant circulating tumor cell clones. Mol. Cancer 2021, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Tayoun, T.; Faugeroux, V.; Oulhen, M.; Aberlenc, A.; Pawlikowska, P.; Farace, F. CTC-Derived Models: A Window into the Seeding Capacity of Circulating Tumor Cells (CTCs). Cells 2019, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Mout, L.; van Dessel, L.F.; Kraan, J.; de Jong, A.C.; Neves, R.P.L.; Erkens-Schulze, S.; Beaufort, C.M.; Sieuwerts, A.M.; van Riet, J.; Woo, T.L.C.; et al. Generating human prostate cancer organoids from leukapheresis enriched circulating tumour cells. Eur. J. Cancer 2021, 150, 179–189. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112. [Google Scholar] [CrossRef]
- Krol, I.; Schwab, F.D.; Carbone, R.; Ritter, M.; Picocci, S.; De Marni, M.L.; Stepien, G.; Franchi, G.M.; Zanardi, A.; Rissoglio, M.D.; et al. Detection of clustered circulating tumour cells in early breast cancer. Br. J. Cancer 2021, 125, 23–27. [Google Scholar] [CrossRef]
- Reduzzi, C.; Di Cosimo, S.; Gerratana, L.; Motta, R.; Martinetti, A.; Vingiani, A.; D’amico, P.; Zhang, Y.; Vismara, M.; Depretto, C.; et al. Circulating Tumor Cell Clusters Are Frequently Detected in Women with Early-Stage Breast Cancer. Cancers 2021, 13, 2356. [Google Scholar] [CrossRef]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef]
- Radfar, P.; Aboulkheyr Es, H.; Salomon, R.; Kulasinghe, A.; Ramalingam, N.; Sarafraz-Yazdi, E.; Thiery, J.P.; Warkiani, M.E. Single-cell analysis of circulating tumour cells: Enabling technologies and clinical applications. Trends Biotechnol. 2022, 40, 1041–1060. [Google Scholar] [CrossRef]
- Neves, R.P.L.; Ammerlaan, W.; Andree, K.C.; Bender, S.; Cayrefourcq, L.; Driemel, C.; Koch, C.; Luetke-Eversloh, M.V.; Oulhen, M.; Rossi, E.; et al. Proficiency Testing to Assess Technical Performance for CTC-Processing and Detection Methods in CANCER-ID. Clin. Chem. 2021, 67, 631–641. [Google Scholar] [CrossRef]
- Connors, D.; Allen, J.; Alvarez, J.D.; Boyle, J.; Cristofanilli, M.; Hiller, C.; Keating, S.; Kelloff, G.; Leiman, L.; McCormack, R.; et al. International liquid biopsy standardization alliance white paper. Crit. Rev. Oncol. Hematol. 2020, 156, 103112. [Google Scholar] [CrossRef] [PubMed]
- IJzerman, M.J.; Berghuis, A.M.S.; de Bono, J.S.; Terstappen, L.W.M.M. Health economic impact of liquid biopsies in cancer management. Expert Rev. Pharmacoecon. Outcomes Res. 2018, 18, 593–599. [Google Scholar] [CrossRef] [PubMed]

| CTC Enrichment | CTC Detection | Biomarkers Tested | Patients (Positivity) | Clinical Significance | Ref. |
|---|---|---|---|---|---|
| Early breast cancer | |||||
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 1574: ≥ 5 CTCs (25.2%) | Yes: DFS, p < 0.001 Yes: OS, p < 0.001 | [15] |
| EpCAM-based immunomagnetic enrichment | RT-qPCR | CK-19 | 1220 (39.6%) | Yes: OS, p < 0.001 | [18] |
| EpCAM-based immunomagnetic enrichment | Multiplex RT-qPCR | CD24, CD44, ALDH1, TWIST1 | 100: TWIST1 (19%), CD24−/low/CD44high (15%), CD24−/low/ALDH1high (9%) | Yes: DFS, p < 0.001 Yes: OS, p < 0.001 | [19] |
| CellSearch/DNA isolation from cartridges | Droplet digital PCR (ddPCR) | PIK3CA hotspot mutations | 56 (66.1%) | no information | [21] |
| Ficoll-hypaque density gradient | Triple IF | CD47/PD-L1/Cytokeratins | 100 CK+ (15%) CD47 (8%) PDL1 (2%) | PFS: n.s O.S: n.s | [22] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 3213: ≥5 CTCs (21.6%) | Yes: OS, p < 0.001 | [23] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 1087: ≥5 CTCs (18.2%) | Yes: DFS, p < 0.001 Yes: OS, p < 0.001 | [24] |
| Metastatic breast cancer (MBC) | |||||
| CellSearch/DNA isolation from cartridges | ddPCR | PIK3CA hotspot Mutations | 22 (81.5%) | no information | [21] |
| Ficoll-hypaque density gradient | Triple IF | CD47/PD-L1/ Cytokeratins | 98: CK+ (22.4%) CD47 (14.8%) PDL1 (7.4%) | Yes: PFS, p = 0.010 No: OS, 0.184 | [22] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 1944: ≥ 5 CTCs (46.9%) | Yes: OS, p < 0.0001 | [25] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 469: ≥ 5 CTCs (53.3%) | Yes: DFS, p < 0.001 Yes: OS, p < 0.001 | [26] |
| CellSearch® | IF | CD45 CK8,18,19 | 510: ≥1 CTC (72%), ≥5 CTC (49%) | Yes:PFS, p < 0.0001 YES: OS, p < 0.0001 | [27] |
| Τargeted NGS | 54 genes for mutation analysis | 510 (28.8%) | |||
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 HER2 | 264: ≥5 CTCs; HER2 therapy (17.9%), New HER2 therapy (46.7%), and No HER2 therapy (46.2%) | no information | [31] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 1933: ≥1 CTC (63.0%) | Yes:OS, p = 0.013 | [32] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 105: ≥5 CTCs (59.0%) | Yes: PFS, p = 0.008 | [33] |
| CTC-iChip | ddPCR | ESR1 mutations | 55 (22%) | Yes: PFS, p = 0.0006 | [35] |
| EpCAM-based immunomagnetic enrichment | MSP | ESR1 methylation | 112 (23.3%) | Yes: PFS, p = 0.009 Yes: OS, p = 0.028 | [39] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 PD-L1 | 72: ≥5 CTCs (79.2%), PD-L1(+)-CTCs (36.1%) | Yes: PFS, p = 0.03 Yes: OS, p = 0.05 | [41] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 PD-L1 | 52: ≥1% PD-L1(+)-CTC (40%) | no information | [42] |
| Ficoll-hypaque density gradient | Confocal laser scanning microscopy | JUNB, YWHAB, TYROBP, NFYA, and PRDX1 | 100: JUNB (65%), TYROBP (75%), NFYA (14.3%), and PRDX (12.5%) | Yes: PFS, p = 0.015 Yes: OS, p = 0.002 | [43] |
| Ficoll-hypaque density gradient | Triple IF | Cytokeratins, ALDH1, and TWIST1 | 130 (27.7%) | Yes: PFS, p = 0.024 Yes: OS, p = 0.020 | [44] |
| EpCAM-based immunomagnetic enrichment | RT-qPCR | CD24, CD44, ALDH1, TWIST1, ESR1, PGR, HER2, EGFR, CK-19 | 46: TWIST1 (2.2%), CD24 (45.7%), CD44 (26.1%), ALDH1 (8.7%), ESR1 (13.0%), HER2 (17.4%), CK19 (21.7%), EGFR (0%) | Yes: OS, p = 0.001 | [47] |
| In vivo CellCollector | Multiplex Real-time PCR and ddPCR | CK8, CK18, CK19, ERBB2, TWIST1, VEGF, ESR1, PR, EGFR, CD44, CD24, ALDH1, VIM, and CDH2 PIK3CA and ESR1 mutations | 42: At least one gene before therapy (54.8%) 13: At least one gene after therapy(61.5%) | no information | [49] |
| CellSearch® | IF: CTC enumeration | HER2-positive CTC | 154: ≥1 CTC (78.7%), ≥5 CTC (57.3%), ≥1 HER2amp CTC (9.1%) | No: PFS No: OS | [55] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 391 CTC-driven choice arm (36.6%) | Yes: PFS, p < 0.05 No: OS, p > 0.05 | [57] |
| Parsortix device | RT-qPCR, RNA-seq, FISH | GYPA, PTPRC, EpCAM, KRT19, ERBB2, TWIST1, SNAI2 | 194 ≥ 5 CTC (22.7%) Gene expression (47.9%) | no information | [58] |
| CTC Enrichment | CTC Detection | Biomarkers Tested | Positivity (%) | Clinical Significance | Ref. |
|---|---|---|---|---|---|
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 177 ≥ 1 CTCs (14%) | no information | [60] |
| EPISPOT | Detection of secreted proteins | PSA/FGF2 | 192 ≥ 1 CTCs (42%) | ||
| In-vivo (CellCollector) | IF | panCK-, DAPI, CD45 | 190 ≥ 1 CTCs (48%) | ||
| In-vivo (CellCollector) | IF | panCK-, DAPI, CD45 | 51 (39.2%) | no information | [61] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 51 (18.6%) | ||
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 105 ≥ 1 CTCs(20%) | Yes: PFS, p = 0.0021 | [62] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 511 (49.7%) | Yes: OS, p < 0.001 | [64] |
| EpCAM-based immunomagnetic enrichment | Multiplex RT-qPCR | AR-FL, AR-V7, AR-567es and AR-total | 69: AR-FL (92.3%), AR-V7 (49.3%), AR-567es (23.2%), AR-total (89.9%) | no information | [68] |
| Positive immunomagnetic enrichment (AdnaTest) | Multiplex RT-qPCR | PSMA, PSA, EGFR | 227: CTC+/AR-7+ (35%) | Yes: OS, p = 0.02 | [69] |
| All cells analyzed (Epic technology) | IF | cytokeratins, CD45, and AR-V7 | 255 pts blinded to AR-V7 status | Yes: OS, p = 0.041 | [70] |
| EpCAM-based immunomagnetic enrichment | Multiplex RT-qPCR | CK-8, CK-18, TWIST1, PSMA, AR-FL, AR-V7, AR-567 and PD-L1 | 57 (85.9%) | Yes: OS, p = 0.001 | [74] |
| IF: CTC enumeration | panCK-, DAPI, CD45 | 57: ≥ 1 CTC (85.9%) | |||
| In-vivo (CellCollector) | Multiplex RT-qPCR | KRT19, EpCAM, CDH1, HMBS, PSCA, ALDH1A1, PROM1, HPRT1, TWIST1, VIM, CDH2, B2M, PLS3, and PSA | 74 (70.5%) | no information | [75] |
| CTC Enrichment | CTC Detection | Biomarkers Tested | Patients Positivity % | Clinical Significance | Ref. |
|---|---|---|---|---|---|
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 100 (48%) | Yes: PFS, p = 0.019 | [78] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 | 97: ≥2 EpCAMhigh CTC (21%), ≥2 EpCAMlow CTC (15%) | Yes: OS, p = 0.014 | [80] |
| CellSearch® | IF: CTC enumeration | panCK-, DAPI, CD45 PD-L1 | 54: CTCs(43.4%),PD-L1(+) CTCs (9.4%) | Yes: PFS, p = 0.006 Yes: OS, p = 0.002 | [82] |
| Microfluidic chip | IF | PD-L1 CK+/CD45−/DAPI+ | 38: PD-L1(+) CTCs (69.4%) | Yes: PFS, p = 0.017 | [83] |
| qPCR | PD-L1 | 38 (36.4%) | |||
| Size-based enrichment (Parsortix) | Multiplex RT-qPCR | CK-8, CK-18, CK-19), VIM, TWIST-1, AXL, ALDH-1, PD-L1 PIM-1 | 30: epithelial markers (37%), mesenchymal/EMT markers (65.4%), stem cell marker (29.6%) | no information | [86] |
| RosetteSep/DEPArray | Target NGS | ALK mutations | 14 (64.3%) | no information | [87] |
| FACS/DEPArray | IF | ALK/ cytokeratins/ CD45/ | 82 CTCs: ALK+/cytokeratins-(37%),ALK-/cytokeratins+ (56%), ALK+/cytokeratins+(5%) | no information | [88] |
| Size-based enrichment (Parsortix) | ddPCR | EGFR mutations | 64 (17.2%) | no information | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strati, A.; Markou, A.; Kyriakopoulou, E.; Lianidou, E. Detection and Molecular Characterization of Circulating Tumour Cells: Challenges for the Clinical Setting. Cancers 2023, 15, 2185. https://doi.org/10.3390/cancers15072185
Strati A, Markou A, Kyriakopoulou E, Lianidou E. Detection and Molecular Characterization of Circulating Tumour Cells: Challenges for the Clinical Setting. Cancers. 2023; 15(7):2185. https://doi.org/10.3390/cancers15072185
Chicago/Turabian StyleStrati, Areti, Athina Markou, Evgenia Kyriakopoulou, and Evi Lianidou. 2023. "Detection and Molecular Characterization of Circulating Tumour Cells: Challenges for the Clinical Setting" Cancers 15, no. 7: 2185. https://doi.org/10.3390/cancers15072185
APA StyleStrati, A., Markou, A., Kyriakopoulou, E., & Lianidou, E. (2023). Detection and Molecular Characterization of Circulating Tumour Cells: Challenges for the Clinical Setting. Cancers, 15(7), 2185. https://doi.org/10.3390/cancers15072185








