THY1 (CD90) Maintains the Adherens Junctions in Nasopharyngeal Carcinoma via Inhibition of SRC Activation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Chemicals
2.2. Establishment of THY1-Expressing NPC Cell Lines
2.3. Microscopy for Immunofluorescence (IF) Stained Samples and for Living Cells
2.4. Co-Immunoprecipitation (co-IP)
2.5. Western Blot (WB) Analysis
2.6. Cell Transfection
2.7. In Vivo Lung Metastasis Assay
2.8. Immunohistochemical (IHC) Staining
2.9. TOP/FOP-FLASH Assay
2.10. Protein Identification by Mass Spectrometry
2.11. Invasion Assay
2.12. MTT Assay
2.13. Statistics
3. Results
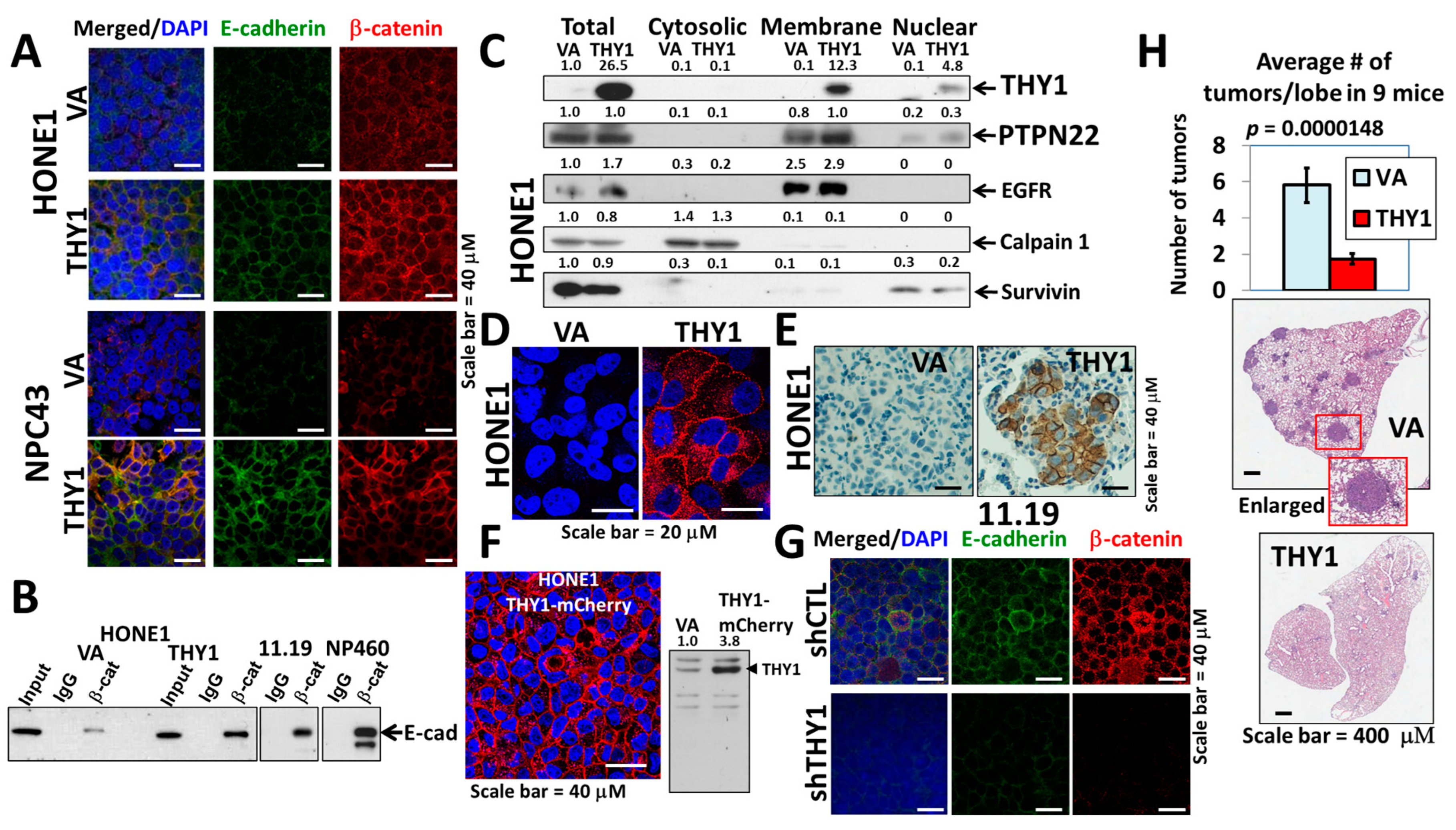
3.1. THY1 Is Involved in Maintaining Adherens Junctions of NPC Cells
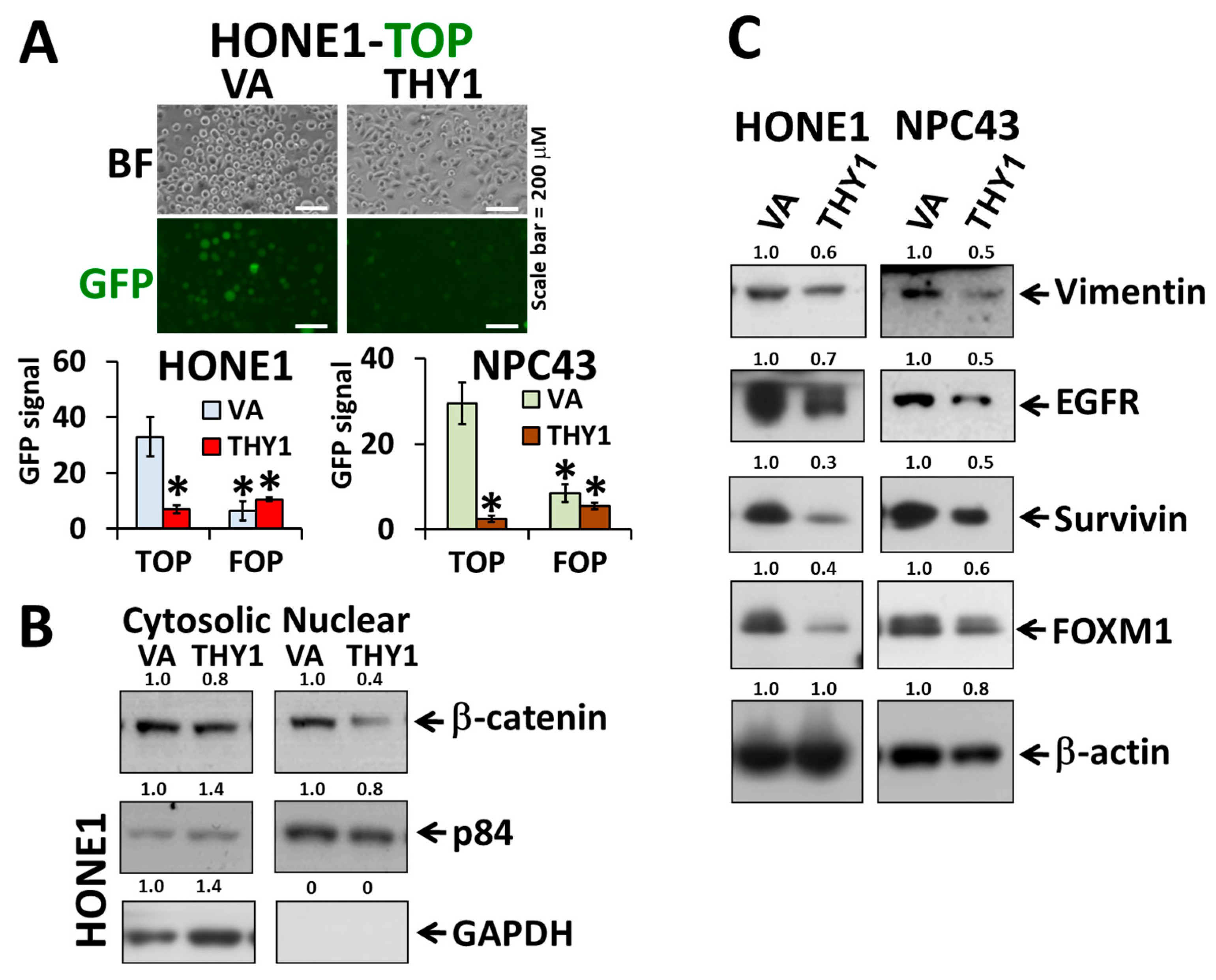
3.2. THY1 Inhibits Nuclear Translocation of β-Catenin and Inhibits EMT in NPC
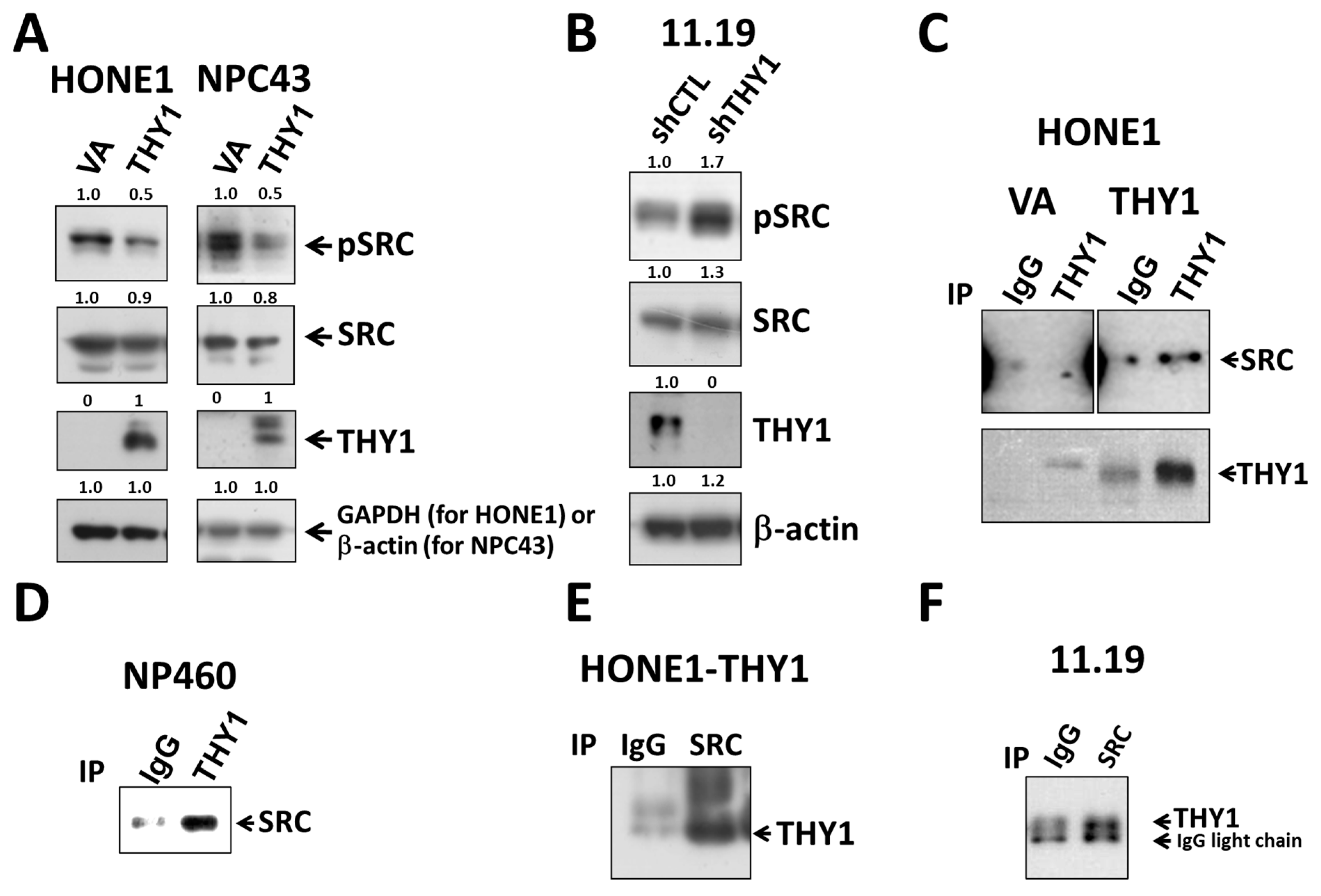
3.3. THY1 Inhibits SRC Activation in NPC
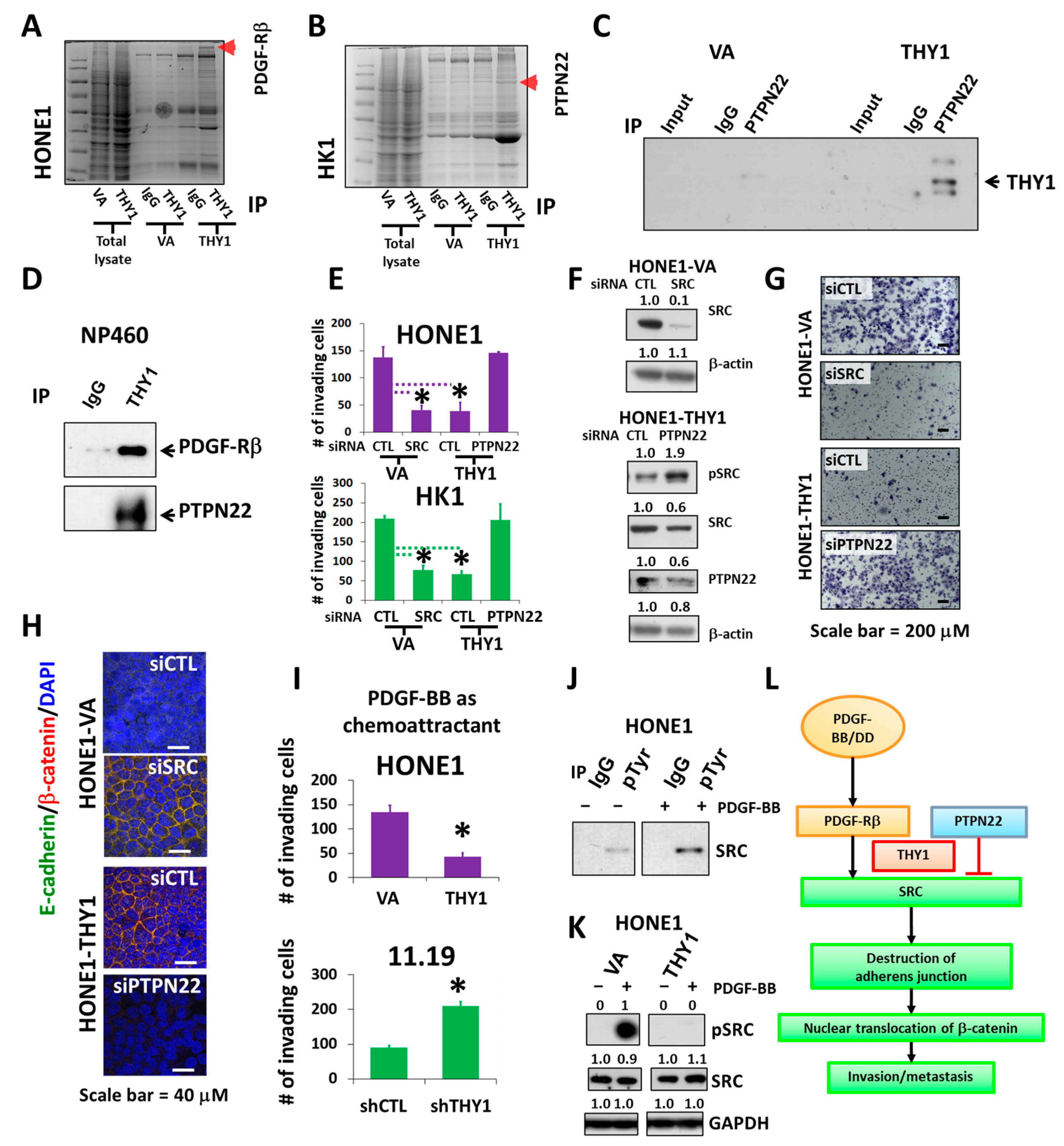
3.4. PTPN22 Is Essential for THY1 to Inhibit SRC Activation
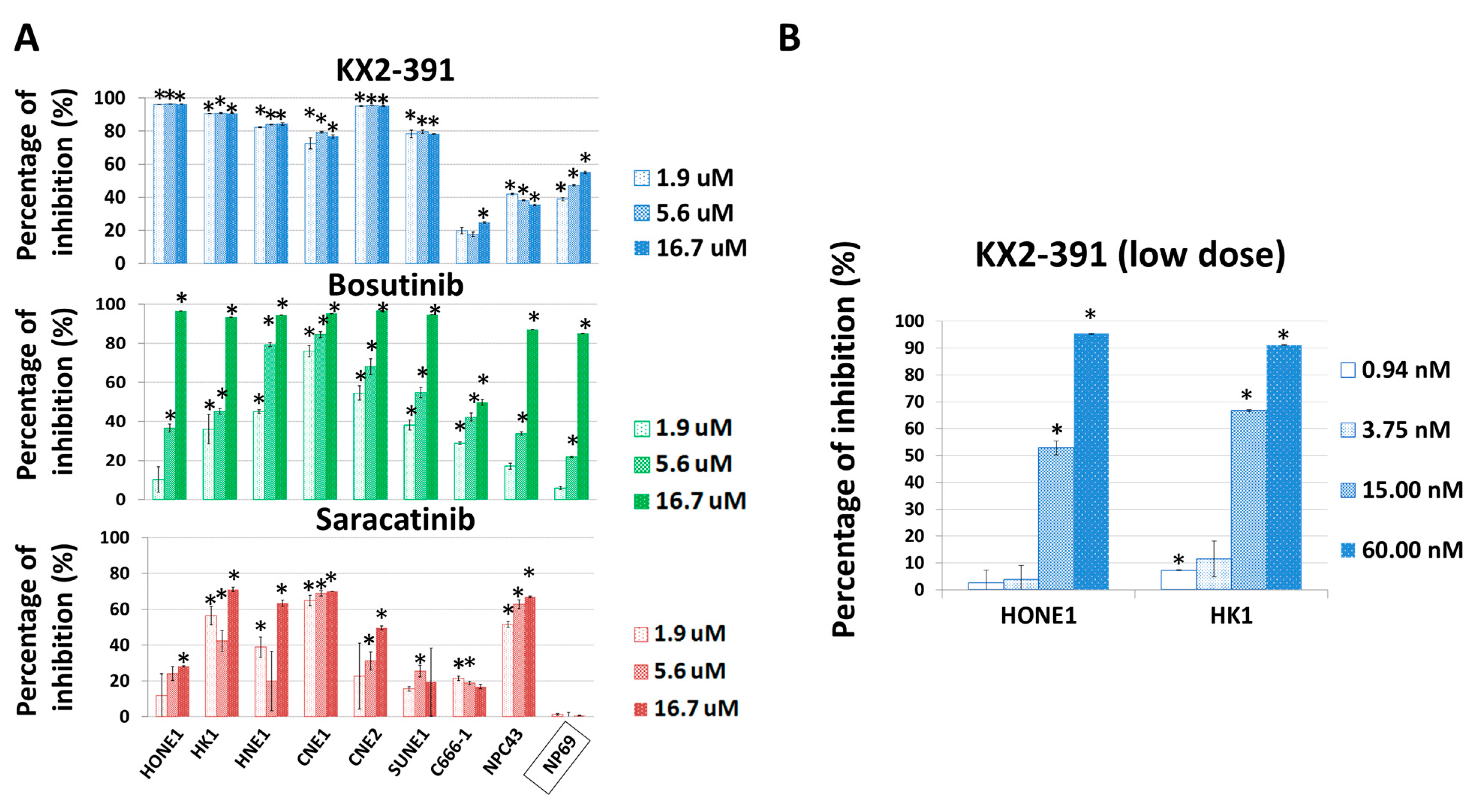
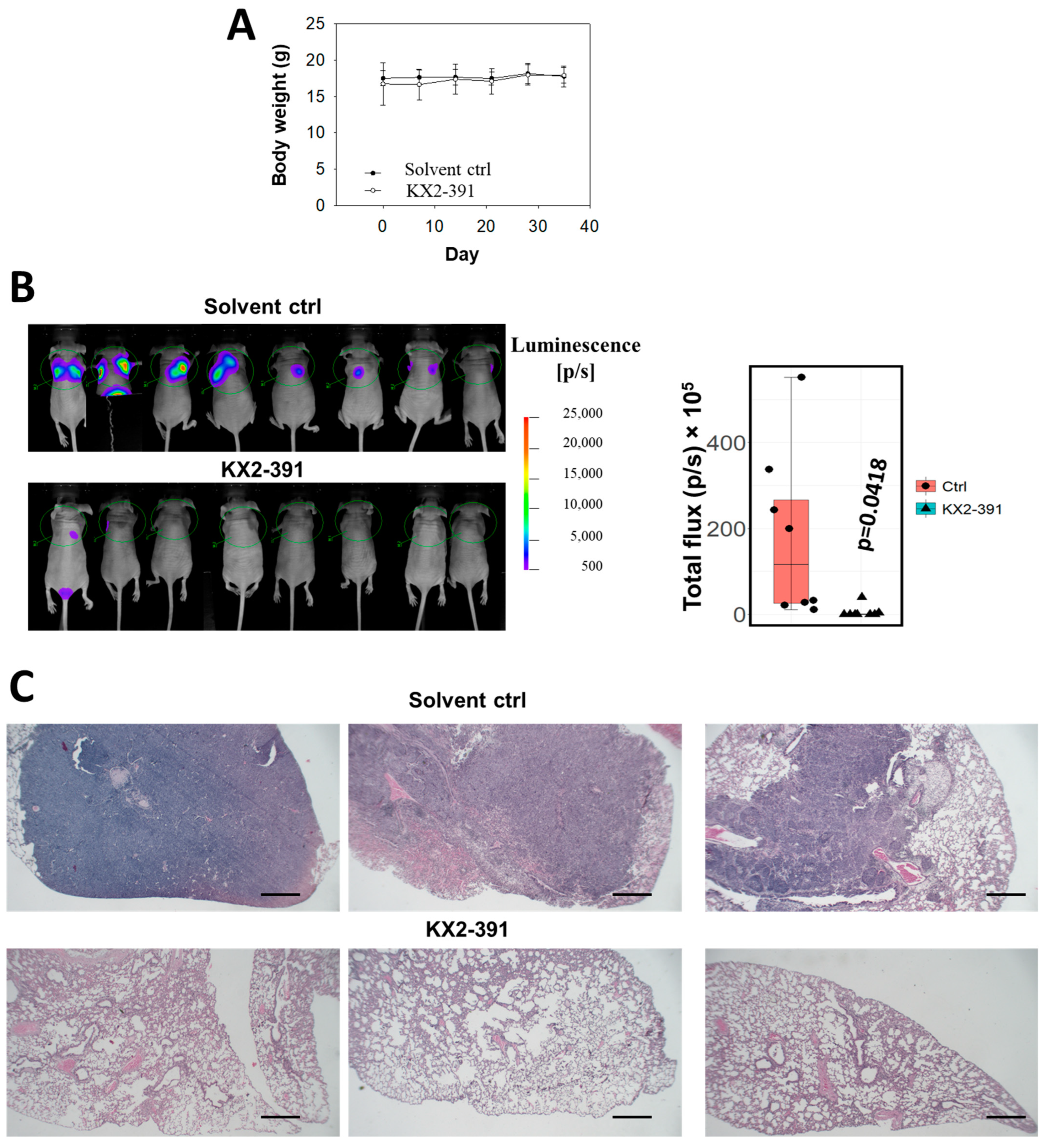
3.5. Inhibition of SRC Suppresses Metastasis of NPC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal Carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal Carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Khor, T.H.; Tan, B.C.; Chua, E.J.; Chia, K.B. Distant Metastases in Nasopharyngeal Carcinoma. Clin. Radiol. 1978, 29, 27–30. [Google Scholar] [CrossRef]
- Lung, H.L.; Bangarusamy, D.K.; Xie, D.; Cheung, A.K.; Cheng, Y.; Kumaran, M.K.; Miller, L.; Liu, E.T.; Guan, X.Y.; Sham, J.S.; et al. Thy1 Is a Candidate Tumour Suppressor Gene with Decreased Expression in Metastatic Nasopharyngeal Carcinoma. Oncogene 2005, 24, 6525–6532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lung, H.L.; Cheung, A.K.; Cheng, Y.; Kwong, F.M.; Lo, P.H.; Law, E.W.; Chua, D.; Zabarovsky, E.R.; Wang, N.; Tsao, S.W.; et al. Functional Characterization of Thy1 as a Tumor Suppressor Gene with Antiinvasive Activity in Nasopharyngeal Carcinoma. Int. J. Cancer 2010, 127, 304–312. [Google Scholar]
- Rege, T.A.; Hagood, J.S. Thy-1, a Versatile Modulator of Signaling Affecting Cellular Adhesion, Proliferation, Survival, and Cytokine/Growth Factor Responses. Biochim. Biophys. Acta 2006, 1763, 991–999. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Molina, R.; Valdivia, A.; Kong, M.; Alvarez, A.; Cardenas, A.; Quest, A.F.; Leyton, L. Thy-1-Interacting Molecules and Cellular Signaling in Cis and Trans. Int. Rev. Cell Mol. Biol. 2013, 305, 163–216. [Google Scholar]
- Shan, B.; Hagood, J.S.; Zhuo, Y.; Nguyen, H.T.; MacEwen, M.; Morris, G.F.; Lasky, J.A. Thy-1 Attenuates Tnf-Alpha-Activated Gene Expression in Mouse Embryonic Fibroblasts Via Src Family Kinase. PLoS ONE 2010, 5, e11662. [Google Scholar] [CrossRef] [PubMed]
- Woeller, C.F.; O’Loughlin, C.W.; Pollock, S.J.; Thatcher, T.H.; Feldon, S.E.; Phipps, R.P. Thy1 (Cd90) Controls Adipogenesis by Regulating Activity of the Src Family Kinase, Fyn. FASEB J. 2015, 29, 920–931. [Google Scholar] [CrossRef]
- Barker, T.H.; Grenett, H.E.; MacEwen, M.W.; Tilden, S.G.; Fuller, G.M.; Settleman, J.; Woods, A.; Murphy-Ullrich, J.; Hagood, J.S. Thy-1 Regulates Fibroblast Focal Adhesions, Cytoskeletal Organization and Migration through Modulation of P190 Rhogap and Rho Gtpase Activity. Exp. Cell Res. 2004, 295, 488–496. [Google Scholar] [CrossRef]
- Zur Hausen, J.D.; Burn, P.; Amrein, K.E. Co-Localization of Fyn with Cd3 Complex, Cd45 or Cd28 Depends on Different Mechanisms. Eur. J. Immunol. 1997, 27, 2643–2649. [Google Scholar] [CrossRef]
- Amanchy, R.; Zhong, J.; Hong, R.; Kim, J.H.; Gucek, M.; Cole, R.N.; Molina, H.; Pandey, A. Identification of C-Src Tyrosine Kinase Substrates in Platelet-Derived Growth Factor Receptor Signaling. Mol. Oncol. 2009, 3, 439–450. [Google Scholar] [CrossRef]
- Denny, M.F.; Kaufman, H.C.; Chan, A.C.; Straus, D.B. The Lck Sh3 Domain Is Required for Activation of the Mitogen-Activated Protein Kinase Pathway but Not the Initiation of T-Cell Antigen Receptor Signaling. J. Biol. Chem. 1999, 274, 5146–5152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roskoski, R., Jr. Src Protein-Tyrosine Kinase Structure, Mechanism, and Small Molecule Inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef]
- Salmond, R.J.; Filby, A.; Qureshi, I.; Caserta, S.; Zamoyska, R. T-Cell Receptor Proximal Signaling Via the Src-Family Kinases, Lck and Fyn, Influences T-Cell Activation, Differentiation, and Tolerance. Immunol. Rev. 2009, 228, 9–22. [Google Scholar] [CrossRef]
- Wadhawan, A.; Smith, C.; Nicholson, R.I.; Barrett-Lee, P.; Hiscox, S. Src-Mediated Regulation of Homotypic Cell Adhesion: Implications for Cancer Progression and Opportunities for Therapeutic Intervention. Cancer Treat Rev. 2011, 37, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Xiang, Y.; Guo, X.; Lu, J.; Xia, W.; Yu, Y.; Peng, Y.; Wang, L.; Wang, G.; Ye, Y.; et al. C-Src Activation Promotes Nasopharyngeal Carcinoma Metastasis by Inducing the Epithelial-Mesenchymal Transition Via Pi3k/Akt Signaling Pathway: A New and Promising Target for Npc. Oncotarget 2016, 7, 28340–28355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Lung, H.L.; Huang, T.; Lan, R.; Zha, S.; Chan, L.S.; Thor, W.; Tsoi, T.H.; Chau, H.F.; Borestrom, C.; et al. Reactivation of Epstein-Barr Virus by a Dual-Responsive Fluorescent Ebna1-Targeting Agent with Zn(2+)-Chelating Function. Proc. Natl. Acad. Sci. USA 2019, 116, 26614–26624. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Stanbridge, E.J.; Kong, H.; Bengtsson, U.; Lerman, M.I.; Lung, M.L. A Functional Investigation of Tumor Suppressor Gene Activities in a Nasopharyngeal Carcinoma Cell Line Hone1 Using a Monochromosome Transfer Approach. Genes Chromosom. Cancer 2000, 28, 82–91. [Google Scholar] [CrossRef]
- Shuen, W.H.; Kan, R.; Yu, Z.; Lung, H.L.; Lung, M.L. Novel Lentiviral-Inducible Transgene Expression Systems and Versatile Single-Plasmid Reporters for in Vitro and in Vivo Cancer Biology Studies. Cancer Gene Ther. 2015, 22, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Ip, J.C.; Chu, A.C.; Cheng, Y.; Leong, M.M.; Ko, J.M.; Shuen, W.H.; Lung, H.L.; Lung, M.L. Ptprg Suppresses Tumor Growth and Invasion Via Inhibition of Akt Signaling in Nasopharyngeal Carcinoma. Oncotarget 2015, 6, 13434–13447. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chiang, Y.C.; Chan, L.S.; Chau, W.Y.; Lung, M.L.; Kahn, M.; Lo, K.W.; Mak, N.K.; Lung, H.L. The Cbp/Beta-Catenin Antagonist, Icg-001, Inhibits Tumor Metastasis Via Blocking of the Mir-134/Itgb1 Axis-Mediated Cell Adhesion in Nasopharyngeal Carcinoma. Cancers 2022, 14, 3125. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, M.; Ali, A.; Jones, R.K.; Marsden, C.G.; Sheng, M.; Carrier, L.; Bu, Y.; Hangauer, D.; Rowan, B.G. Peptidomimetic Src/Pretubulin Inhibitor Kx-01 Alone and in Combination with Paclitaxel Suppresses Growth, Metastasis in Human Er/Pr/Her2-Negative Tumor Xenografts. Mol. Cancer Ther. 2012, 11, 1936–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Xiao, X.; Zhang, Y.; Zhang, B.; Li, D.; Liu, M.; Xie, X.; Liu, C.; Liu, P.; Ren, R. A Dual Inhibitor Overcomes Drug-Resistant Flt3-Itd Acute Myeloid Leukemia. J. Hematol. Oncol. 2021, 14, 105. [Google Scholar] [CrossRef]
- Au, F.K.C.; Hau, B.K.T.; Qi, R.Z. Nek2-Mediated Gas2l1 Phosphorylation and Centrosome-Linker Disassembly Induce Centrosome Disjunction. J. Cell Biol. 2020, 219, e201909094. [Google Scholar] [CrossRef] [Green Version]
- Narisawa-Saito, M.; Yamanashi, Y.; Morioka, T.; Oite, T.; Shimizu, F. Thy-1 Molecule Associates with Protein Tyrosine Kinase(S) in Rat Mesangial Cells. Clin. Exp. Immunol. 1996, 106, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.M.; Samelson, L.E. The Glycophosphatidylinositol-Anchored Thy-1 Molecule Interacts with the P60fyn Protein Tyrosine Kinase in T Cells. J. Biol. Chem. 1992, 267, 12317–12322. [Google Scholar] [CrossRef] [PubMed]
- Bottini, N.; Peterson, E.J. Tyrosine Phosphatase Ptpn22: Multifunctional Regulator of Immune Signaling, Development, and Disease. Annu. Rev. Immunol. 2014, 32, 83–119. [Google Scholar] [CrossRef]
- Cloutier, J.F.; Veillette, A. Cooperative Inhibition of T-Cell Antigen Receptor Signaling by a Complex between a Kinase and a Phosphatase. J. Exp. Med. 1999, 189, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Gjorloff-Wingren, A.; Saxena, M.; Williams, S.; Hammi, D.; Mustelin, T. Characterization of Tcr-Induced Receptor-Proximal Signaling Events Negatively Regulated by the Protein Tyrosine Phosphatase Pep. Eur. J. Immunol. 1999, 29, 3845–3854. [Google Scholar] [CrossRef]
- Vang, T.; Liu, W.H.; Delacroix, L.; Wu, S.; Vasile, S.; Dahl, R.; Yang, L.; Musumeci, L.; Francis, D.; Landskron, J.; et al. Lyp Inhibits T-Cell Activation When Dissociated from Csk. Nat. Chem. Biol. 2012, 8, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldin, C.H.; Lennartsson, J.; Westermark, B. Involvement of Platelet-Derived Growth Factor Ligands and Receptors in Tumorigenesis. J. Intern. Med. 2018, 283, 16–44. [Google Scholar]
- Kumar, S.; Lu, B.; Davra, V.; Hornbeck, P.; Machida, K.; Birge, R.B. Crk Tyrosine Phosphorylation Regulates Pdgf-Bb-Inducible Src Activation and Breast Tumorigenicity and Metastasis. Mol. Cancer Res. 2018, 16, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Puls, L.N.; Eadens, M.; Messersmith, W. Current Status of Src Inhibitors in Solid Tumor Malignancies. Oncologist 2011, 16, 566–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedov, E.; Koren, E.; Chopra, S.; Ankawa, R.; Yosefzon, Y.; Yusupova, M.; Weiss, L.E.; Mahly, A.; Soffer, A.; Feldman, A.; et al. Thy1-Mediated Mechanisms Converge to Drive Yap Activation in Skin Homeostasis and Repair. Nat. Cell Biol. 2022, 24, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, H.R.; Cao, Q.; Xu, J.; Pollock, S.; Veyberman, Y.; Guckert, N.L.; Keng, P.; Wang, N. Thy1 Expression Is Associated with Tumor Suppression of Human Ovarian Cancer. Cancer Genet. Cytogenet. 2003, 143, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Gabra, H.; Watson, J.E.; Taylor, K.J.; Mackay, J.; Leonard, R.C.; Steel, C.M.; Porteous, D.J.; Smyth, J.F. Definition and Refinement of a Region of Loss of Heterozygosity at 11q23.3-Q24.3 in Epithelial Ovarian Cancer Associated with Poor Prognosis. Cancer Res. 1996, 56, 950–954. [Google Scholar] [PubMed]
- Fiegel, H.C.; Kaifi, J.T.; Quaas, A.; Varol, E.; Krickhahn, A.; Metzger, R.; Sauter, G.; Till, H.; Izbicki, J.R.; Erttmann, R.; et al. Lack of Thy1 (Cd90) Expression in Neuroblastomas Is Correlated with Impaired Survival. Pediatr. Surg. Int. 2008, 24, 101–105. [Google Scholar] [CrossRef]
- Weinstein, J.L.; Katzenstein, H.M.; Cohn, S.L. Advances in the Diagnosis and Treatment of Neuroblastoma. Oncologist 2003, 8, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.; Dai, Y.D.; Tong, M.; Chan, Y.P.; Kwan, P.S.; Fu, L.; Qin, Y.R.; Tsao, S.W.; Lung, H.L.; Lung, M.L.; et al. A Cd90(+) Tumor-Initiating Cell Population with an Aggressive Signature and Metastatic Capacity in Esophageal Cancer. Cancer Res. 2013, 73, 2322–2332. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.F.; Zhang, L.J.; Zhao, A.L.; Wang, Y.; Wu, N.; Xiong, H.C.; Liang, Z.; Li, J.Y.; Huang, X.F.; Yang, Y. Abnormal Expression of Thy-1 as a Novel Tumor Marker in Lung Cancer and Its Prognostic Significance. Zhonghua Yi Xue Za Zhi 2005, 85, 1921–1925. [Google Scholar] [PubMed]
- Yan, X.; Luo, H.; Zhou, X.; Zhu, B.; Wang, Y.; Bian, X. Identification of Cd90 as a Marker for Lung Cancer Stem Cells in A549 and H446 Cell Lines. Oncol. Rep. 2013, 30, 2733–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingala, S.; Cui, Y.Y.; Chen, X.; Ruebner, B.H.; Qian, X.F.; Zern, M.A.; Wu, J. Immunohistochemical Staining of Cancer Stem Cell Markers in Hepatocellular Carcinoma. Exp. Mol. Pathol. 2010, 89, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.W.; Chang, J.G.; Yeh, K.T.; Chen, R.M.; Tsai, J.J.; Hu, R.M. Overexpression of Thy1/Cd90 in Human Hepatocellular Carcinoma Is Associated with Hbv Infection and Poor Prognosis. Acta Histochem. 2011, 113, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Xu, L.B.; Liu, C.; Zhang, R.; Wang, J. Clinicopathological Characteristics of 20 Cases of Hepatocellular Carcinoma with Bile Duct Tumor Thrombi. Dig. Dis. Sci. 2011, 56, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Hagood, J.S. Getting a Grip on Thy-1 Signaling. Biochim. Biophys. Acta 2009, 1793, 921–923. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, H.; Calderon, C.; Burgos-Bravo, F.; Kobler, O.; Zuschratter, W.; Ramirez, O.; Hartel, S.; Schneider, P.; Quest, A.F.; Herrera-Molina, R.; et al. Astrocyte-to-Neuron Communication through Integrin-Engaged Thy-1/Cbp/Csk/Src Complex Triggers Neurite Retraction Via the Rhoa/Rock Pathway. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Rege, T.A.; Pallero, M.A.; Gomez, C.; Grenett, H.E.; Murphy-Ullrich, J.E.; Hagood, J.S. Thy-1, Via Its Gpi Anchor, Modulates Src Family Kinase and Focal Adhesion Kinase Phosphorylation and Subcellular Localization, and Fibroblast Migration, in Response to Thrombospondin-1/Hep I. Exp. Cell Res. 2006, 312, 3752–3767. [Google Scholar] [CrossRef]
- Barker, T.H.; Pallero, M.A.; MacEwen, M.W.; Tilden, S.G.; Woods, A.; Murphy-Ullrich, J.E.; Hagood, J.S. Thrombospondin-1-Induced Focal Adhesion Disassembly in Fibroblasts Requires Thy-1 Surface Expression, Lipid Raft Integrity, and Src Activation. J. Biol. Chem. 2004, 279, 23510–23516. [Google Scholar] [CrossRef] [Green Version]
- Reif, A.E.; Allen, J.M. The Akr Thymic Antigen and Its Distribution in Leukemias and Nervous Tissues. J. Exp. Med. 1964, 120, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Sireci, F.; Dispenza, F.; Lorusso, F.; Immordino, A.; Immordino, P.; Gallina, S.; Peretti, G.; Canevari, F.R. Tumours of Nasal Septum: A Retrospective Study of 32 Patients. Int. J. Environ. Res. Public Health 2022, 19, 1713. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.; Sadri, S.; Eskazan, A.E. Dasatinib for the Treatment of Chronic Myeloid Leukemia: Patient Selection and Special Considerations. Drug Des. Devel. Ther. 2016, 10, 3355–3361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, R.; Hsyu, P.H. Clinical Pharmacokinetics and Pharmacodynamics of Bosutinib. Clin. Pharmacokinet. 2016, 55, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Duggan, S. Tirbanibulin: First Approval. Drugs 2021, 81, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Ripretinib: First Approval. Drugs 2020, 80, 1133–1138. [Google Scholar] [CrossRef]
- Kane, R.C.; Farrell, A.T.; Saber, H.; Tang, S.; Williams, G.; Jee, J.M.; Liang, C.; Booth, B.; Chidambaram, N.; Morse, D.; et al. Sorafenib for the Treatment of Advanced Renal Cell Carcinoma. Clin. Cancer Res. 2006, 12, 7271–7278. [Google Scholar] [CrossRef] [Green Version]
| Antibody | Manufacture | Catalog Number | Application | Host Species |
|---|---|---|---|---|
| Calpain 1 | Santa Cruz | sc-58323 | WB | Mouse |
| E-cadherin | Cell Signaling | 14472 | WB | Mouse |
| EGFR | Cell Signaling | 2232 | WB | Rabbit |
| FOXM1 | Cell Signaling | 5436 | WB | Rabbit |
| GAPDH | GeneTex | GTX627408 | WB | Mouse |
| IgG | Cell Signaling | 3900S | IP | Rabbit |
| IgG | Cell Signaling | 5415 | IP | Mouse |
| p84 | Abcam | ab131268 | WB | Rabbit |
| PDGF-Rβ | Cell Signaling | 3169 | WB | Rabbit |
| PTPN22 | Cell Signaling | 14693 | WB, IP | Rabbit |
| p-Tyr | Cell Signaling | 9411 | IP | Mouse |
| SRC | Cell Signaling | 2123 | WB, IP | Rabbit |
| phospho-SRC (Y416/419) | Cell Signaling | 6943 | WB | Rabbit |
| Survivin | Cell Signaling | 2808 | WB | Rabbit |
| THY1 | Cell Signaling | 13801 | WB, IHC | Rabbit |
| THY1 | Abcam | ab133350 | WB, IHC, IF | Rabbit |
| THY1 | Cell Signaling | 9798 | WB, IP | Rabbit |
| Vimentin | BD Bioscience | 550513 | WB | Mouse |
| β-actin | Sigma | A5441 | WB | Mouse |
| β-catenin | Cell signaling | 8480 | WB, IF, IP | Rabbit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Chau, W.Y.; Yuen, H.T.; Liu, X.H.; Qi, R.Z.; Lung, M.L.; Lung, H.L. THY1 (CD90) Maintains the Adherens Junctions in Nasopharyngeal Carcinoma via Inhibition of SRC Activation. Cancers 2023, 15, 2189. https://doi.org/10.3390/cancers15072189
Chen L, Chau WY, Yuen HT, Liu XH, Qi RZ, Lung ML, Lung HL. THY1 (CD90) Maintains the Adherens Junctions in Nasopharyngeal Carcinoma via Inhibition of SRC Activation. Cancers. 2023; 15(7):2189. https://doi.org/10.3390/cancers15072189
Chicago/Turabian StyleChen, Luo, Wai Yin Chau, Hei Tung Yuen, Xiao Han Liu, Robert Zhong Qi, Maria Li Lung, and Hong Lok Lung. 2023. "THY1 (CD90) Maintains the Adherens Junctions in Nasopharyngeal Carcinoma via Inhibition of SRC Activation" Cancers 15, no. 7: 2189. https://doi.org/10.3390/cancers15072189
APA StyleChen, L., Chau, W. Y., Yuen, H. T., Liu, X. H., Qi, R. Z., Lung, M. L., & Lung, H. L. (2023). THY1 (CD90) Maintains the Adherens Junctions in Nasopharyngeal Carcinoma via Inhibition of SRC Activation. Cancers, 15(7), 2189. https://doi.org/10.3390/cancers15072189







