Precancerous Lesions of the Head and Neck Region and Their Stromal Aberrations: Piecemeal Data
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy to Review the Topic of Stromal Changes in HNSCCs
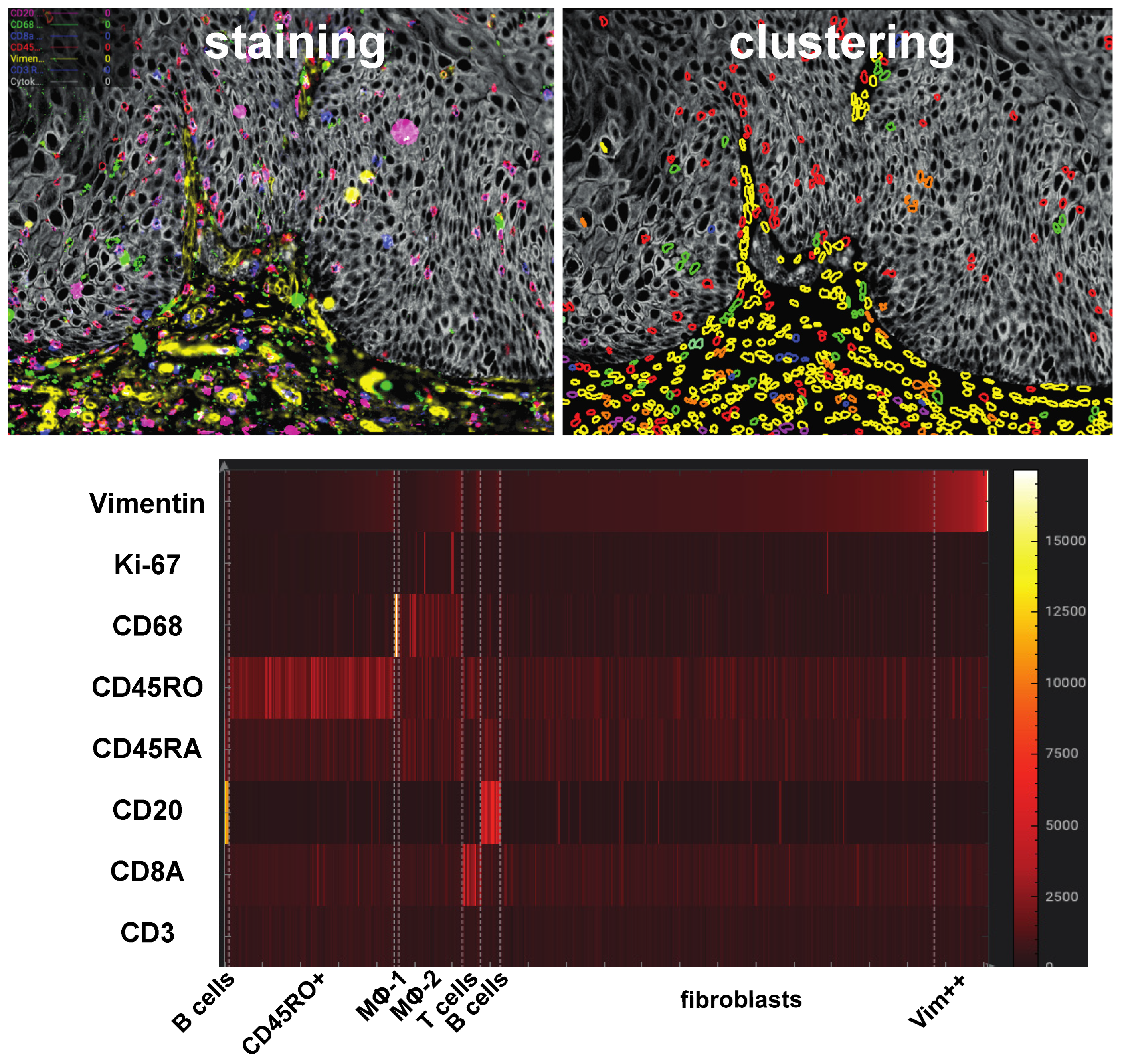
2.2. Ultraplex Immunofluorescence Stainings and Analysis
3. The Stroma
4. Activated Fibroblasts and Myofibroblasts
5. T Cells
6. NK Cells
7. Macrophages
8. B Cells
9. Langerhans Cells and Dendritic Cells
10. Granulocytes: Neutrophils, Eosinophils
10.1. Neutrophils
10.2. Eosinophils
11. Mast Cells
12. The Endothelium
12.1. The Conundrum of Neovascularization in Precancer
12.2. VEGF and Hypoxia
12.3. Hypoxia and Metabolic Reprogramming
12.4. The Angiogenic Switch as an Opportunity for Diagnosis
13. Immune Checkpoint Markers
14. Crossing the Border: The Critical Step of Invasion into the Stroma and the Emergence of the “True” Tumor Stroma
The Paradox of Hemidesmosomal Protein Upregulation in Precancer and Cancer
15. Epithelial Changes That May Drive Cancerous Stromal Remodeling: Which Genetic Drivers May Run the Show of Stromal Invasion and Remodeling?
Invasive Behavior as a Key Cancer Therapy Target
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maman, S.; Witz, I.P. A history of exploring cancer in context. Nat. Rev. Cancer 2018, 18, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI—Atheromatous affection of arteries. 1858. Nutr. Rev. 1989, 47, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Weber, O.F. In memoriam of Rudolf virchow: A historical retrospective including aspects of inflammation, infection and neoplasia. Contrib. Microbiol. 2006, 13, 1–15. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Merler, E.; Abernathy, C.; Williams, G. Isolation of a tumor factor responsible for angiogenesis. J. Exp. Med. 1971, 133, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Langer, R.; Conn, H.; Vacanti, J.; Haudenschild, C.; Folkman, J. Control of tumor growth in animals by infusion of an angiogenesis inhibitor. Proc. Natl. Acad. Sci. USA 1980, 77, 4331–4335. [Google Scholar] [CrossRef] [Green Version]
- Gimbrone, M.A., Jr.; Leapman, S.B.; Cotran, R.S.; Folkman, J. Tumor dormancy in vivo by prevention of neovascularization. J. Exp. Med. 1972, 136, 261–276. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Gospodarowicz, D. Respective roles of laminin and fibronectin in adhesion of human carcinoma and sarcoma cells. Nature 1981, 289, 304–306. [Google Scholar] [CrossRef]
- Schurch, W.; Seemayer, T.A.; Lagace, R. Stromal myofibroblasts in primary invasive and metastatic carcinomas. A combined immunological, light and electron microscopic study. Virchows Arch. A Pathol. Anat. Histol. 1981, 391, 125–139. [Google Scholar] [CrossRef]
- Delinassios, J.G.; Kottaridis, S.D.; Garas, J. Uncontrolled growth of tumour stromal fibroblasts in vitro. Exp. Cell Biol. 1983, 51, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Fukabori, Y.; McBride, G.; Nikolaropolous, S.; McKeehan, W.L. Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol. Cell. Biol. 1993, 13, 4513–4522. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Gabusi, A.; Morandi, L.; Asioli, S.; Foschini, M.P. Oral field cancerization: History and future perspectives. Pathologica 2017, 109, 60–65. [Google Scholar]
- Aguirre-Urizar, J.M.; Lafuente-Ibanez de Mendoza, I.; Warnakulasuriya, S. Malignant transformation of oral leukoplakia: Systematic review and meta-analysis of the last 5 years. Oral Dis. 2021, 27, 1881–1895. [Google Scholar] [CrossRef]
- Centuori, S.M.; Caulin, C.; Bauman, J.E. Precision and Immunoprevention Strategies for Tobacco-Related Head and Neck Cancer Chemoprevention. Curr. Treat. Options Oncol. 2021, 22, 52. [Google Scholar] [CrossRef]
- DeCosse, J.J. Potential for chemoprevention. Cancer 1982, 50, 2550–2553. [Google Scholar] [PubMed]
- Siemianowicz, K.; Likus, W.; Dorecka, M.; Wilk, R.; Dziubdziela, W.; Markowski, J. Chemoprevention of Head and Neck Cancers: Does It Have Only One Face? BioMed Res. Int. 2018, 2018, 9051854. [Google Scholar] [CrossRef] [Green Version]
- Migliorati, C.A.; Migliorati, E.K.; Silverman, S., Jr.; Greenspan, D.; Greenspan, J.S. Phenotypic identification of mononuclear cells in oral premalignant lesions and cancer by monoclonal antibodies. J. Oral Pathol. 1986, 15, 352–358. [Google Scholar] [CrossRef]
- Munzel, M.; Meister, P. Subepithelial changes in simple leukoplakic hyperplasia of the laryngeal mucosa (author’s transl). Laryngol. Rhinol. Otol. 1976, 55, 96–99. [Google Scholar]
- Stampe, H.; Jakobsen, K.K.; Bendtsen, S.K.; Gronhoj, C.; von Buchwald, C. Systematic review on the current knowledge and use of single-cell RNA sequencing in head and neck cancer. APMIS 2021, 129, 619–625. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Xiao, L.; Rong, H.; Ou, Z.; Cai, T.; Liu, N.; Li, B.; Zhang, L.; Wu, F.; Lan, T.; et al. Single-cell profiling of tumor-infiltrating TCF1/TCF7(+) T cells reveals a T lymphocyte subset associated with tertiary lymphoid structures/organs and a superior prognosis in oral cancer. Oral Oncol. 2021, 119, 105348. [Google Scholar] [CrossRef] [PubMed]
- Schurch, W.; Seemayer, T.A.; Gabbiani, G. The myofibroblast: A quarter century after its discovery. Am. J. Surg. Pathol. 1998, 22, 141–147. [Google Scholar] [CrossRef]
- Majno, G.; Gabbiani, G.; Hirschel, B.J.; Ryan, G.B.; Statkov, P.R. Contraction of granulation tissue in vitro: Similarity to smooth muscle. Science 1971, 173, 548–550. [Google Scholar] [CrossRef]
- De Wever, O.; Mareel, M. Role of tissue stroma in cancer cell invasion. J. Pathol. 2003, 200, 429–447. [Google Scholar] [CrossRef]
- Gabbiani, G.; Ryan, G.B.; Lamelin, J.P.; Vassalli, P.; Majno, G.; Bouvier, C.A.; Cruchaud, A.; Luscher, E.F. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to “nonmuscular” cells. Am. J. Pathol. 1973, 72, 473–488. [Google Scholar] [PubMed]
- Coletta, R.D.; Salo, T. Myofibroblasts in oral potentially malignant disorders: Is it related to malignant transformation? Oral Dis. 2018, 24, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Custodio, M.; Biddle, A.; Tavassoli, M. Portrait of a CAF: The story of cancer-associated fibroblasts in head and neck cancer. Oral Oncol. 2020, 110, 104972. [Google Scholar] [CrossRef] [PubMed]
- Zidar, N.; Gale, N.; Kambic, V.; Fischinger, J. Proliferation of myofibroblasts in the stroma of epithelial hyperplastic lesions and squamous carcinoma of the larynx. Oncology 2002, 62, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.; Scutt, A.; Meghji, S.; Canniff, J.P. Stimulation of human buccal mucosa fibroblasts in vitro by betel-nut alkaloids. Arch. Oral Biol. 1986, 31, 45–49. [Google Scholar] [CrossRef]
- Tilakaratne, W.M.; Klinikowski, M.F.; Saku, T.; Peters, T.J.; Warnakulasuriya, S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006, 42, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Angadi, P.V.; Kale, A.D.; Hallikerimath, S. Evaluation of myofibroblasts in oral submucous fibrosis: Correlation with disease severity. J. Oral Pathol. Med. 2011, 40, 208–213. [Google Scholar] [CrossRef]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwe, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Costea, D.E.; Hills, A.; Osman, A.H.; Thurlow, J.; Kalna, G.; Huang, X.; Pena Murillo, C.; Parajuli, H.; Suliman, S.; Kulasekara, K.K.; et al. Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer Res. 2013, 73, 3888–3901. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Chen, Y.L.; Lai, T.Y.; Ko, Y.C.; Chou, Y.F.; Chen, P.R.; Hsiao, J.R.; Chang, J.Y.; Shiah, S.G.; Lee, J.W.; et al. Identification of Prognostic Biomarkers Originating From the Tumor Stroma of Betel Quid-Associated Oral Cancer Tissues. Front. Oncol. 2021, 11, 769665. [Google Scholar] [CrossRef]
- Kojc, N.; Zidar, N.; Vodopivec, B.; Gale, N. Expression of CD34, alpha-smooth muscle actin, and transforming growth factor beta1 in squamous intraepithelial lesions and squamous cell carcinoma of the larynx and hypopharynx. Hum. Pathol. 2005, 36, 16–21. [Google Scholar] [CrossRef]
- Tong, C.C.L.; Koptyra, M.; Raman, P.; Rathi, K.S.; Choudhari, N.; Lin, X.; Seckar, T.; Wei, Z.; Kohanski, M.A.; O’Malley, B.W.; et al. Targeted gene expression profiling of inverted papilloma and squamous cell carcinoma. Int. Forum Allergy Rhinol. 2022, 12, 200–209. [Google Scholar] [CrossRef]
- Chang, M.C.; Chiang, C.P.; Lin, C.L.; Lee, J.J.; Hahn, L.J.; Jeng, J.H. Cell-mediated immunity and head and neck cancer: With special emphasis on betel quid chewing habit. Oral Oncol. 2005, 41, 757–775. [Google Scholar] [CrossRef]
- Foy, J.P.; Bertolus, C.; Ortiz-Cuaran, S.; Albaret, M.A.; Williams, W.N.; Lang, W.; Destandau, S.; Souza, G.; Sohier, E.; Kielbassa, J.; et al. Immunological and classical subtypes of oral premalignant lesions. Oncoimmunology 2018, 7, e1496880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorens, C.; Soriano, B.; Trilla-Fuertes, L.; Bagan, L.; Ramos-Ruiz, R.; Gamez-Pozo, A.; Pena, C.; Bagan, J.V. Immune expression profile identification in a group of proliferative verrucous leukoplakia patients: A pre-cancer niche for oral squamous cell carcinoma development. Clin. Oral Investig. 2021, 25, 2645–2657. [Google Scholar] [CrossRef] [PubMed]
- Lahav, Y.; Shats, M.; Huszar, M.; Haimovich, Y.; Warman, M.; Halperin, D.; Shoffel-Havakuk, H. Local inflammatory reaction to benign, pre-malignant and malignant glottic lesions: A matched case-control study. Clin. Otolaryngol. 2019, 44, 628–638. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Mao, F.; Cai, L.; Dan, H.; Jiang, L.; Zeng, X.; Li, T.; Zhou, Y.; Chen, Q. PD-1 blockade prevents the progression of oral carcinogenesis. Carcinogenesis 2021, 42, 891–902. [Google Scholar] [CrossRef]
- Kujan, O.; Agag, M.; Smaga, M.; Vaishnaw, Y.; Idrees, M.; Shearston, K.; Farah, C.S. PD-1/PD-L1, Treg-related proteins, and tumour-infiltrating lymphocytes are associated with the development of oral squamous cell carcinoma. Pathology 2022, 54, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Palmario, G.G. Histological and immunochemical studies of oral leukoplakia: Phenotype and distribution of immunocompetent cells. J. Philipp. Dent. Assoc. 1995, 47, 3–18. [Google Scholar] [CrossRef]
- Ohman, J.; Magnusson, B.; Telemo, E.; Jontell, M.; Hasseus, B. Langerhans cells and T cells sense cell dysplasia in oral leukoplakias and oral squamous cell carcinomas--evidence for immunosurveillance. Scand. J. Immunol. 2012, 76, 39–48. [Google Scholar] [CrossRef]
- Gannot, G.; Gannot, I.; Vered, H.; Buchner, A.; Keisari, Y. Increase in immune cell infiltration with progression of oral epithelium from hyperkeratosis to dysplasia and carcinoma. Br. J. Cancer 2002, 86, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Yagyuu, T.; Hatakeyama, K.; Imada, M.; Kurihara, M.; Matsusue, Y.; Yamamoto, K.; Obayashi, C.; Kirita, T. Programmed death ligand 1 (PD-L1) expression and tumor microenvironment: Implications for patients with oral precancerous lesions. Oral Oncol. 2017, 68, 36–43. [Google Scholar] [CrossRef]
- Kindt, N.; Descamps, G.; Seminerio, I.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Delvenne, P.; Journe, F.; Saussez, S. High stromal Foxp3-positive T cell number combined to tumor stage improved prognosis in head and neck squamous cell carcinoma. Oral Oncol. 2017, 67, 183–191. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Guan, X.; Wu, H.; Sun, Z.; Zeng, H. Immunosuppression Induced by Chronic Inflammation and the Progression to Oral Squamous Cell Carcinoma. Mediat. Inflamm. 2016, 2016, 5715719. [Google Scholar] [CrossRef] [Green Version]
- Kouketsu, A.; Sato, I.; Oikawa, M.; Shimizu, Y.; Saito, H.; Tashiro, K.; Yamashita, Y.; Takahashi, T.; Kumamoto, H. Regulatory T cells and M2-polarized tumour-associated macrophages are associated with the oncogenesis and progression of oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2019, 48, 1279–1288. [Google Scholar] [CrossRef]
- Kambic, V.; Gale, N.; Fischinger, J. Local immune response in hyperplastic lesions of the larynx. ORL J. Otorhinolaryngol. Relat. Spec. 1994, 56, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Stasikowska-Kanicka, O.; Wagrowska-Danilewicz, M.; Danilewicz, M. CD8+ and CD163+ infiltrating cells and PD-L1 immunoexpression in oral leukoplakia and oral carcinoma. APMIS 2018, 126, 732–738. [Google Scholar] [CrossRef]
- Piva, M.R.; De Souza, L.B.; Martins-Filho, P.R.S.; Nonaka, C.F.W.; De Santana Santos, T.; De Souza Andrade, E.S.; Piva, D. Role of inflammation in oral carcinogenesis (Part II): CD8, FOXP3, TNF-alpha, TGF-beta and NF-kappaB expression. Oncol. Lett. 2013, 5, 1909–1914. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, C. The Role of Human gammadelta T Cells in Anti-Tumor Immunity and Their Potential for Cancer Immunotherapy. Cells 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Tognarelli, S.; Jacobs, B.; Staiger, N.; Ullrich, E. Flow Cytometry-based Assay for the Monitoring of NK Cell Functions. J. Vis. Exp. 2016, 116, 54615. [Google Scholar] [CrossRef]
- De Paula, A.M.; Gomez, R.S. Immunolocalization of p53, glutathione S-tranferase pi and CD57 antigens in oral leukoplakia. Anticancer Res. 2001, 21, 379–385. [Google Scholar]
- Kalogirou, E.M.; Tosios, K.I.; Christopoulos, P.F. The Role of Macrophages in Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 611115. [Google Scholar] [CrossRef]
- Goswami, K.K.; Bose, A.; Baral, R. Macrophages in tumor: An inflammatory perspective. Clin. Immunol. 2021, 232, 108875. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.M.; Diel, L.F.; Lamers, M.L. Macrophages and prognosis of oral squamous cell carcinoma: A systematic review. J. Oral Pathol. Med. 2018, 47, 460–467. [Google Scholar] [CrossRef]
- Loning, T.; Burkhardt, A. Dyskeratosis in human and experimental oral precancer and cancer. An immunohistochemical and ultrastructural study in men, mice and rats. Arch. Oral Biol. 1982, 27, 361–366. [Google Scholar] [CrossRef]
- Seminerio, I.; Kindt, N.; Descamps, G.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Journe, F.; Saussez, S. High infiltration of CD68+ macrophages is associated with poor prognoses of head and neck squamous cell carcinoma patients and is influenced by human papillomavirus. Oncotarget 2018, 9, 11046–11059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Zhang, J.; Lu, R.; Zhou, G. Signal regulatory protein alpha associated with the progression of oral leukoplakia and oral squamous cell carcinoma regulates phenotype switch of macrophages. Oncotarget 2016, 7, 81305–81321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Shintani, S.; Terakado, N.; Nakashiro, K.; Hamakawa, H. Infiltration of tumor-associated macrophages in human oral squamous cell carcinoma. Oncol. Rep. 2002, 9, 1219–1223. [Google Scholar] [CrossRef]
- Lu, C.F.; Huang, C.S.; Tjiu, J.W.; Chiang, C.P. Infiltrating macrophage count: A significant predictor for the progression and prognosis of oral squamous cell carcinomas in Taiwan. Head Neck 2010, 32, 18–25. [Google Scholar] [CrossRef]
- Wang, S.; Sun, M.; Gu, C.; Wang, X.; Chen, D.; Zhao, E.; Jiao, X.; Zheng, J. Expression of CD163, interleukin-10, and interferon-gamma in oral squamous cell carcinoma: Mutual relationships and prognostic implications. Eur. J. Oral Sci. 2014, 122, 202–209. [Google Scholar] [CrossRef]
- Mori, K.; Haraguchi, S.; Hiori, M.; Shimada, J.; Ohmori, Y. Tumor-associated macrophages in oral premalignant lesions coexpress CD163 and STAT1 in a Th1-dominated microenvironment. BMC Cancer 2015, 15, 573. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, J.S.; Fuentes-Duculan, J.; Suarez-Farinas, M.; Pierson, K.C.; Pitts-Kiefer, A.; Fan, L.; Belkin, D.A.; Wang, C.Q.; Bhuvanendran, S.; Johnson-Huang, L.M.; et al. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J. Investig. Dermatol. 2011, 131, 1322–1330. [Google Scholar] [CrossRef] [Green Version]
- Lao, X.M.; Liang, Y.J.; Su, Y.X.; Zhang, S.E.; Zhou, X.I.; Liao, G.Q. Distribution and significance of interstitial fibrosis and stroma-infiltrating B cells in tongue squamous cell carcinoma. Oncol. Lett. 2016, 11, 2027–2034. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.M.; Appelman, H.D. Crohn’s-like lymphoid reaction and colorectal carcinoma: A potential histologic prognosticator. Mod. Pathol. 1990, 3, 332–335. [Google Scholar] [PubMed]
- Kindt, N.; Descamps, G.; Seminerio, I.; Bellier, J.; Lechien, J.R.; Pottier, C.; Larsimont, D.; Journe, F.; Delvenne, P.; Saussez, S. Langerhans cell number is a strong and independent prognostic factor for head and neck squamous cell carcinomas. Oral Oncol. 2016, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, J.; Rao, N.N.; Upadhyay, R.B. A comparative analysis of langerhans cell in oral epithelial dysplasia and oral squamous cell carcinoma using antibody CD-1a. J. Cancer Res. Ther. 2012, 8, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Lasisi, T.J.; Oluwasola, A.O.; Lasisi, O.A.; Akang, E.E. Association between langerhans cells population and histological grade of oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2013, 17, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Costa, N.L.; Goncalves, A.S.; Martins, A.F.; Arantes, D.A.; Silva, T.A.; Batista, A.C. Characterization of dendritic cells in lip and oral cavity squamous cell carcinoma. J. Oral Pathol. Med. 2016, 45, 418–424. [Google Scholar] [CrossRef]
- Reichert, T.E.; Scheuer, C.; Day, R.; Wagner, W.; Whiteside, T.L. The number of intratumoral dendritic cells and zeta-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer 2001, 91, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A.; Baker, E.; Weyant, R.J.; Clarke, M.R.; Myers, J.N.; Lotze, M.T. Peritumoral CD1a-positive dendritic cells are associated with improved survival in patients with tongue carcinoma. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 641–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellicioli, A.C.A.; Bingle, L.; Farthing, P.; Lopes, M.A.; Martins, M.D.; Vargas, P.A. Immunosurveillance profile of oral squamous cell carcinoma and oral epithelial dysplasia through dendritic and T-cell analysis. J. Oral Pathol. Med. 2017, 46, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Trellakis, S.; Bruderek, K.; Dumitru, C.A.; Gholaman, H.; Gu, X.; Bankfalvi, A.; Scherag, A.; Hutte, J.; Dominas, N.; Lehnerdt, G.F.; et al. Polymorphonuclear granulocytes in human head and neck cancer: Enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int. J. Cancer 2011, 129, 2183–2193. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Eruslanov, E.B.; Singhal, S.; Albelda, S.M. Mouse versus Human Neutrophils in Cancer: A Major Knowledge Gap. Trends Cancer 2017, 3, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandau, S.; Moses, K.; Lang, S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: Cousins, siblings or twins? Semin. Cancer Biol. 2013, 23, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Vanhaver, C.; van der Bruggen, P.; Bruger, A.M. MDSC in Mice and Men: Mechanisms of Immunosuppression in Cancer. J. Clin. Med. 2021, 10, 2872. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przeworski, E. Ueber die locale eosinophilie beim krebs nebst bemerkungen uber die bedeutung der eosinopbilen zellen im allgemeinen. In Zentralblatt fur Allgemeine Pathologie; Nabu Press: Charleston, SC, USA, 1896; Volume 5, pp. 177–191. [Google Scholar]
- Grisaru-Tal, S.; Itan, M.; Klion, A.D.; Munitz, A. A new dawn for eosinophils in the tumour microenvironment. Nat. Rev. Cancer 2020, 20, 594–607. [Google Scholar] [CrossRef]
- Hu, G.; Wang, S.; Zhong, K.; Xu, F.; Huang, L.; Chen, W.; Cheng, P. Tumor-associated tissue eosinophilia predicts favorable clinical outcome in solid tumors: A meta-analysis. BMC Cancer 2020, 20, 454. [Google Scholar] [CrossRef]
- Jain, M.; Kasetty, S.; Khan, S.; Jain, N.K. Tissue eosinophilia in head and neck squamous neoplasia: An update. Exp. Oncol. 2014, 36, 157–161. [Google Scholar] [PubMed]
- Deepthi, G.; Kulkarni, P.G.; Nandan, S.K.K. Eosinophils: An imperative histopathological prognostic indicator for oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2019, 23, 307. [Google Scholar] [CrossRef]
- Madhura, M.G.; Gajalakshmi, S.; Kumar, B.V.; Suma, S.; Sarita, Y.; Shweta, R.D. Role of tissue eosinophils in oral Leukoplakia: A pilot study. J. Oral Maxillofac. Pathol. 2015, 19, 286–290. [Google Scholar] [CrossRef]
- Jain, M.; Kasetty, S.; Sudheendra, U.S.; Tijare, M.; Khan, S.; Desai, A. Assessment of tissue eosinophilia as a prognosticator in oral epithelial dysplasia and oral squamous cell carcinoma-an image analysis study. Patholog. Res. Int. 2014, 2014, 507512. [Google Scholar] [CrossRef] [Green Version]
- Saito, H.; Matsumoto, K.; Okumura, S.; Kashiwakura, J.; Oboki, K.; Yokoi, H.; Kambe, N.; Ohta, K.; Okayama, Y. Gene expression profiling of human mast cell subtypes: An in silico study. Allergol. Int. 2006, 55, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majorini, M.T.; Colombo, M.P.; Lecis, D. Few, but Efficient: The Role of Mast Cells in Breast Cancer and Other Solid Tumors. Cancer Res. 2022, 82, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, E.Z.; Markopoulos, A.K.; Antoniades, D.Z. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent. J. 2008, 2, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohtasham, N.; Babakoohi, S.; Salehinejad, J.; Montaser-Kouhsari, L.; Shakeri, M.T.; Shojaee, S.; Sistani, N.S.; Firooz, A. Mast cell density and angiogenesis in oral dysplastic epithelium and low- and high-grade oral squamous cell carcinoma. Acta Odontol. Scand. 2010, 68, 300–304. [Google Scholar] [CrossRef]
- Telagi, N.; Ahmed Mujib, B.R.; Kulkarni, P.G.; Naik, R. The master switch: Comparative study of mast cell in oral epithelial dysplasia, oral submucous fibrosis and oral squamous cells carcinoma and their association with inflammation and angiogenesis. J. Oral Maxillofac. Pathol. 2015, 19, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jyothsna, M.; Rammanohar, M.; Kumar, K. Histomorphometric Analysis of Angiogenesis using CD31 Immunomarker and Mast Cell Density in Oral Premalignant and Malignant Lesions: A Pilot Study. J. Clin. Diagn. Res. 2017, 11, ZC37–ZC40. [Google Scholar] [CrossRef] [PubMed]
- Laishram, D.; Rao, K.; Devi, H.S.U.; Priya, N.S.; Smitha, T.; Sheethal, H.S. Mast cells and angiogenesis in malignant and premalignant oral lesions: An immunohistochemical study. J. Oral Maxillofac. Pathol. 2017, 21, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Iamaroon, A.; Pongsiriwet, S.; Jittidecharaks, S.; Pattanaporn, K.; Prapayasatok, S.; Wanachantararak, S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J. Oral Pathol. Med. 2003, 32, 195–199. [Google Scholar] [CrossRef]
- Sundararajan, A.; Muthusamy, R.; Gopal Siva, K.; Harikrishnan, P.; Kumar, S.C.K.; Rathinasamy, S.K. Correlation of Mast Cell and Angiogenesis in Oral Lichen Planus, Dysplasia (Leukoplakia), and Oral Squamous Cell Carcinoma. Rambam Maimonides Med. J. 2021, 12, e0016. [Google Scholar] [CrossRef] [PubMed]
- Muniz, J.M.; Bibiano Borges, C.R.; Beghini, M.; de Araujo, M.S.; Miranda Alves, P.; de Lima, L.M.; Pereira, S.A.; Nogueira, R.D.; Napimoga, M.H.; Rodrigues, V., Jr.; et al. Galectin-9 as an important marker in the differential diagnosis between oral squamous cell carcinoma, oral leukoplakia and oral lichen planus. Immunobiology 2015, 220, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Neto, H.H.; Leite, A.F.; Costa, N.L.; Alencar, R.C.; Lara, V.S.; Silva, T.A.; Leles, C.R.; Mendonca, F.E.; Batista, A.C. Decrease in mast cells in oral squamous cell carcinoma: Possible failure in the migration of these cells. Oral Oncol. 2007, 43, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, V.; Vij, R.; Aggarwal, R.; Sharma, B.; Nagpal, M. Evaluation of mast cells in oral premalignant and malignant lesions: A histochemical study. Natl. J. Maxillofac. Surg. 2018, 9, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Teofilo, C.R.; Ferreira Junior, A.E.C.; Batista, A.C.; Fechini Jamacaru, F.V.; Sousa, F.B.; Lima Mota, M.R.; Silva, M.F.E.; Barros Silva, P.G.; Alves, A. Mast Cells and Blood Vessels Profile in Oral Carcinogenesis: An Immunohistochemistry Study. Asian Pac. J. Cancer Prev. 2020, 21, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Franchi, A.; Gallo, O.; Paglierani, M.; Sardi, I.; Magnelli, L.; Masini, E.; Santucci, M. Inducible nitric oxide synthase expression in laryngeal neoplasia: Correlation with angiogenesis. Head Neck 2002, 24, 16–23. [Google Scholar] [CrossRef]
- de Cicco, R.L.; Watson, J.C.; Bassi, D.E.; Litwin, S.; Klein-Szanto, A.J. Simultaneous expression of furin and vascular endothelial growth factor in human oral tongue squamous cell carcinoma progression. Clin. Cancer Res. 2004, 10, 4480–4488. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, G.; Labriola, A.; Achille, G.; Florio, G.; Zito, A.F.; Grammatica, L.; Paradiso, A. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. Int. J. Oncol. 2002, 21, 1317–1323. [Google Scholar] [CrossRef]
- Arora, S.; Kaur, J.; Sharma, C.; Mathur, M.; Bahadur, S.; Shukla, N.K.; Deo, S.V.; Ralhan, R. Stromelysin 3, Ets-1, and vascular endothelial growth factor expression in oral precancerous and cancerous lesions: Correlation with microvessel density, progression, and prognosis. Clin. Cancer Res. 2005, 11, 2272–2284. [Google Scholar] [CrossRef] [Green Version]
- Forster, J.C.; Harriss-Phillips, W.M.; Douglass, M.J.; Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 2017, 5, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Thiem, D.G.E.; Schneider, S.; Venkatraman, N.T.; Kumar, V.V.; Brieger, J.; Frerich, B.; Kammerer, P.W. Semiquantifiable angiogenesis parameters in association with the malignant transformation of oral leukoplakia. J. Oral Pathol. Med. 2017, 46, 710–716. [Google Scholar] [CrossRef]
- Sheelam, S.; Reddy, S.P.; Kulkarni, P.G.; Nandan, S.; Keerthi, M.; Raj, G.S. Role of cell proliferation and vascularity in malignant transformation of potentially malignant disorders. J. Oral Maxillofac. Pathol. 2018, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Szafarowski, T.; Sierdzinski, J.; Szczepanski, M.J.; Whiteside, T.L.; Ludwig, N.; Krzeski, A. Microvessel density in head and neck squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2018, 275, 1845–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.N.F.; Dallarmi, L.B.; Araujo, A.K.C.; Alencar, R.C.G.; Mendonca, E.F.; Silva, T.A.; Batista, A.C.; Costa, N.L. Immunohistochemical analysis of neutrophils, interleukin-17, matrix metalloproteinase-9, and neoformed vessels in oral squamous cell carcinoma. J. Oral Pathol. Med. 2018, 47, 856–863. [Google Scholar] [CrossRef]
- Rzepakowska, A.; Zurek, M.; Grzybowski, J.; Pihowicz, P.; Gornicka, B.; Osuch-Wojcikiewicz, E.; Niemczyk, K. Correlation of narrow band imaging vascular patterns with immunohistological microvessel density in vocal fold lesions. Braz. J. Otorhinolaryngol. 2021, 87, 137–144. [Google Scholar] [CrossRef]
- Chawla, H.; Urs, A.B.; Augustine, J. Association of Macrophages with Angiogenesis in Oral Epithelial Dysplasia, Oral Verrucous Carcinoma, and Oral Squamous Cell Carcinoma: An Immunohistochemical Study. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denhart, B.C.; Guidi, A.J.; Tognazzi, K.; Dvorak, H.F.; Brown, L.F. Vascular permeability factor/vascular endothelial growth factor and its receptors in oral and laryngeal squamous cell carcinoma and dysplasia. Lab. Investig. 1997, 77, 659–664. [Google Scholar]
- Sauter, E.R.; Nesbit, M.; Watson, J.C.; Klein-Szanto, A.; Litwin, S.; Herlyn, M. Vascular endothelial growth factor is a marker of tumor invasion and metastasis in squamous cell carcinomas of the head and neck. Clin. Cancer Res. 1999, 5, 775–782. [Google Scholar]
- Sharada, P.; Swaminathan, U.; Nagamalini, B.R.; Kumar, K.V.; Ashwini, B.K.; Lavanya, V. Coalition of E-cadherin and vascular endothelial growth factor expression in predicting malignant transformation in common oral potentially malignant disorders. J. Oral Maxillofac. Pathol. 2018, 22, 40–47. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Crivellato, E.; Roccaro, A.M.; Vacca, A. The history of the angiogenic switch concept. Leukemia 2007, 21, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Pouso, A.I.; Gallas-Torreira, M.; Perez-Sayans, M.; Chamorro-Petronacci, C.M.; Alvarez-Calderon, O.; Takkouche, B.; Supuran, C.T.; Garcia-Garcia, A. Prognostic value of CAIX expression in oral squamous cell carcinoma: A systematic review and meta-analysis. J. Enzyme Inhib. Med. Chem. 2020, 35, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sayans, M.; Suarez-Penaranda, J.M.; Torres-Lopez, M.; Supuran, C.T.; Gandara-Vila, P.; Gayoso-Diz, P.; Barros-Angueira, F.; Gallas-Torreira, M.; Garcia-Garcia, A. The use of CA-IX as a diagnostic method for oral leukoplakia. Biotech. Histochem. 2015, 90, 124–131. [Google Scholar] [CrossRef]
- Perez-Sayans, M.; Suarez-Penaranda, J.M.; Pilar, G.D.; Supuran, C.T.; Pastorekova, S.; Barros-Angueira, F.; Gandara-Rey, J.M.; Garcia-Garcia, A. Expression of CA-IX is associated with advanced stage tumors and poor survival in oral squamous cell carcinoma patients. J. Oral Pathol. Med. 2012, 41, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sayans, M.; Suarez-Penaranda, J.M.; Torres-Lopez, M.; Supuran, C.T.; Gandara-Vila, P.; Gayoso-Diz, P.; Barros-Angueira, F.; Blanco-Carrion, A.; Gandara-Rey, J.M.; Garcia-Garcia, A. Expression of CA IX in dysplasia adjacent to surgical resection margins of oral squamous cell carcinoma. Biotech. Histochem. 2014, 89, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kim, K.Y.; Zheng, Z.; Bazarsad, S.; Kim, J. Nomogram for risk prediction of malignant transformation in oral leukoplakia patients using combined biomarkers. Oral Oncol. 2017, 72, 132–139. [Google Scholar] [CrossRef]
- Zhang, X.; Han, S.; Han, H.Y.; Ryu, M.H.; Kim, K.Y.; Choi, E.J.; Cha, I.H.; Kim, J. Risk prediction for malignant conversion of oral epithelial dysplasia by hypoxia related protein expression. Pathology 2013, 45, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yi, C.; Yang, M.J.; Sun, X.; Liu, X.; Ma, H.; Li, Y.; Li, H.; Wang, C.; He, Y.; et al. Metabolomics study reveals the potential evidence of metabolic reprogramming towards the Warburg effect in precancerous lesions. J. Cancer 2021, 12, 1563–1574. [Google Scholar] [CrossRef]
- Debta, P.; Sarode, G.; Siddhartha, S.; Sarode, S.; Debta, F.M.; Swain, S.K.; Sahu, M.C.; Patro, S.; Patil, S. GLUT-1 Expression: An Aid in Complementing the WHO Oral Epithelial Dysplasia Grading System. J. Contemp. Dent. Pract. 2020, 21, 951–955. [Google Scholar]
- Reisser, C.; Eichhorn, K.; Herold-Mende, C.; Born, A.I.; Bannasch, P. Expression of facilitative glucose transport proteins during development of squamous cell carcinomas of the head and neck. Int. J. Cancer 1999, 80, 194–198. [Google Scholar] [CrossRef]
- Lin, P.Y.; Yu, C.H.; Wang, J.T.; Chen, H.H.; Cheng, S.J.; Kuo, M.Y.; Chiang, C.P. Expression of hypoxia-inducible factor-1 alpha is significantly associated with the progression and prognosis of oral squamous cell carcinomas in Taiwan. J. Oral Pathol. Med. 2008, 37, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Ghosh, B.; Mandal, M.; Nawn, D.; Banerjee, S.; Pal, M.; Paul, R.R.; Banerjee, S.; Chatterjee, J. Pathophysiological relationship between hypoxia associated oxidative stress, Epithelial-mesenchymal transition, stemness acquisition and alteration of Shh/Gli-1 axis during oral sub-mucous fibrosis and oral squamous cell carcinoma. Eur. J. Cell Biol. 2021, 100, 151146. [Google Scholar] [CrossRef]
- Larghi, A.; Lecca, P.G.; Costamagna, G. High-resolution narrow band imaging endoscopy. Gut 2008, 57, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Gono, K.; Obi, T.; Yamaguchi, M.; Ohyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; Hamamoto, Y.; Endo, T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J. Biomed. Opt. 2004, 9, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Kunder, S.; Chatterjee, A.; Manna, S.; Mahimkar, M.; Patil, A.; Rangarajan, V.; Budrukkar, A.; Ghosh-Laskar, S.; Agarwal, J.P.; Gupta, T. Correlation between imaging and tissue biomarkers of hypoxia in squamous cell cancer of the head and neck. World J. Nucl. Med. 2021, 20, 228–236. [Google Scholar] [CrossRef]
- Bittner, M.I.; Wiedenmann, N.; Bucher, S.; Hentschel, M.; Mix, M.; Rucker, G.; Weber, W.A.; Meyer, P.T.; Werner, M.; Grosu, A.L.; et al. Analysis of relation between hypoxia PET imaging and tissue-based biomarkers during head and neck radiochemotherapy. Acta Oncol. 2016, 55, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norikane, T.; Yamamoto, Y.; Maeda, Y.; Kudomi, N.; Matsunaga, T.; Haba, R.; Iwasaki, A.; Hoshikawa, H.; Nishiyama, Y. Correlation of (18)F-fluoromisonidazole PET findings with HIF-1alpha and p53 expressions in head and neck cancer: Comparison with (18)F-FDG PET. Nucl. Med. Commun. 2014, 35, 30–35. [Google Scholar] [CrossRef]
- Ahn, P.H.; Finlay, J.C.; Gallagher-Colombo, S.M.; Quon, H.; O’Malley, B.W., Jr.; Weinstein, G.S.; Chalian, A.; Malloy, K.; Sollecito, T.; Greenberg, M.; et al. Lesion oxygenation associates with clinical outcomes in premalignant and early stage head and neck tumors treated on a phase 1 trial of photodynamic therapy. Photodiagnosis Photodyn. Ther. 2018, 21, 28–35. [Google Scholar] [CrossRef]
- Rangel, R.; Pickering, C.R.; Sikora, A.G.; Spiotto, M.T. Genetic Changes Driving Immunosuppressive Microenvironments in Oral Premalignancy. Front. Immunol. 2022, 13, 840923. [Google Scholar] [CrossRef]
- Malaspina, T.S.; Gasparoto, T.H.; Costa, M.R.; de Melo, E.F., Jr.; Ikoma, M.R.; Damante, J.H.; Cavassani, K.A.; Garlet, G.P.; da Silva, J.S.; Campanelli, A.P. Enhanced programmed death 1 (PD-1) and PD-1 ligand (PD-L1) expression in patients with actinic cheilitis and oral squamous cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 965–974. [Google Scholar] [CrossRef]
- Troiano, G.; Caponio, V.C.A.; Zhurakivska, K.; Arena, C.; Pannone, G.; Mascitti, M.; Santarelli, A.; Lo Muzio, L. High PD-L1 expression in the tumour cells did not correlate with poor prognosis of patients suffering for oral squamous cells carcinoma: A meta-analysis of the literature. Cell Prolif. 2019, 52, e12537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girolami, I.; Pantanowitz, L.; Munari, E.; Martini, M.; Nocini, R.; Bisi, N.; Molteni, G.; Marchioni, D.; Ghimenton, C.; Brunelli, M.; et al. Prevalence of PD-L1 expression in head and neck squamous precancerous lesions: A systematic review and meta-analysis. Head Neck 2020, 42, 3018–3030. [Google Scholar] [CrossRef]
- Dave, K.; Ali, A.; Magalhaes, M. Increased expression of PD-1 and PD-L1 in oral lesions progressing to oral squamous cell carcinoma: A pilot study. Sci. Rep. 2020, 10, 9705. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Huang, J.; Mei, Z.; Lam, A.K.; Zhao, J.; Ying, L. Analysis of Immune Microenvironment by Multiplex Immunohistochemistry Staining in Different Oral Diseases and Oral Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 555757. [Google Scholar] [CrossRef] [PubMed]
- Ries, J.; Agaimy, A.; Wehrhan, F.; Baran, C.; Bolze, S.; Danzer, E.; Frey, S.; Jantsch, J.; Most, T.; Buttner-Herold, M.; et al. Importance of the PD-1/PD-L1 Axis for Malignant Transformation and Risk Assessment of Oral Leukoplakia. Biomedicines 2021, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Zerdes, I.; Matikas, A.; Bergh, J.; Rassidakis, G.Z.; Foukakis, T. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: Biology and clinical correlations. Oncogene 2018, 37, 4639–4661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruns, I.B.; Beltman, J.B. Quantifying the contribution of transcription factor activity, mutations and microRNAs to CD274 expression in cancer patients. Sci. Rep. 2022, 12, 4374. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Sato, Y.; Nakano, M. KPNB1 Inhibitor Importazole Reduces Ionizing Radiation-Increased Cell Surface PD-L1 Expression by Modulating Expression and Nuclear Import of IRF1. Curr. Issues Mol. Biol. 2021, 43, 153–162. [Google Scholar] [CrossRef]
- Yang, L.S.; Shi, C.Y.; Liang, Y.H.; Liu, T.; Hou, X.R.; Tian, X.D.; Wang, X.Y. Bioinformatics analysis of programmed cell death ligand 1 co-expression genes and their regulatory network in head and neck squamous cell carcinoma. Hua Xi Kou Qiang Yi Xue Za Zhi 2019, 37, 516–520. [Google Scholar] [CrossRef]
- Kouketsu, A.; Sato, I.; Oikawa, M.; Shimizu, Y.; Saito, H.; Takahashi, T.; Kumamoto, H. Expression of immunoregulatory molecules PD-L1 and PD-1 in oral cancer and precancerous lesions: A cohort study of Japanese patients. J. Craniomaxillofac. Surg. 2019, 47, 33–40. [Google Scholar] [CrossRef]
- Nicolai, P.; Cappiello, J.; Peretti, G.; Antonelli, A.R.; Parolini, S.; Rosa, D.; Favret, M.; Maroccolo, D.; Molinari Tosatti, M.P. Distribution of laminin, type IV collagen and fibronectin in normal, dysplastic and neoplastic laryngeal tissue. Acta Otorhinolaryngol. Ital. 1990, 10, 139–149. [Google Scholar] [PubMed]
- Cheng, L.H.; Hudson, J. Ultrastructural changes in malignant transformation of oral mucosa. Br. J. Oral Maxillofac. Surg. 2002, 40, 207–212. [Google Scholar] [CrossRef]
- Kannan, S.; Balaram, P.; Pillai, M.R.; Chandran, G.J.; Nair, M.K.; Kartha, C.C.; Augustine, J.; Sudha, L.; Mangalam, M.K. Ultrastructural variations and assessment of malignant transformation risk in oral leukoplakia. Pathol. Res. Pract. 1993, 189, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.R.; Nicolai, P.; Cappiello, J.; Peretti, G.; Molinari Tosatti, M.P.; Rosa, D.; Grigolato, P.G.; Favret, M.; Maroccolo, D. Basement membrane components in normal, dysplastic, neoplastic laryngeal tissue and metastatic lymph nodes. Acta Otolaryngol. 1991, 111, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Sakr, W.A.; Zarbo, R.J.; Jacobs, J.R.; Crissman, J.D. Distribution of basement membrane in squamous cell carcinoma of the head and neck. Hum. Pathol. 1987, 18, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, H.; Schreiner, M.; Wiest, I.; Tubel, J.; Schleicher, E.D.; Nerlich, A.G. Defective basement membrane in laryngeal carcinomas with heterogeneous loss of distinct components. Hum. Pathol. 1998, 29, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, R.; Nagatsuka, H.; Siar, C.H.; Katase, N.; Naito, I.; Sado, Y.; Nagai, N. Comparative analysis of basal lamina type IV collagen alpha chains, matrix metalloproteinases-2 and -9 expressions in oral dysplasia and invasive carcinoma. Acta Histochem. 2013, 115, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Chandavarkar, V.; Naik, V.V.; Kale, A.D. An immunohistochemical study of basement membrane heparan sulfate proteoglycan (perlecan) in oral epithelial dysplasia and squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2013, 17, 31–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagedorn, H.G.; Sauer, U.; Schleicher, E.D.; Nerlich, A.G. Divergence in distribution and prognostic significance of major basement components in laryngeal carcinomas. Int. J. Oncol. 2001, 18, 1045–1051. [Google Scholar] [CrossRef]
- Parikka, M.; Kainulainen, T.; Tasanen, K.; Vaananen, A.; Bruckner-Tuderman, L.; Salo, T. Alterations of collagen XVII expression during transformation of oral epithelium to dysplasia and carcinoma. J. Histochem. Cytochem. 2003, 51, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Xin, Z.; Yamaguchi, A.; Sakamoto, K. Aberrant expression and altered cellular localization of desmosomal and hemidesmosomal proteins are associated with aggressive clinicopathological features of oral squamous cell carcinoma. Virchows Arch. 2014, 465, 35–47. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Okamura, T.; Morita, K.I.; Yamaguchi, S.; Harada, H.; Miki, Y.; Izumo, T.; Kayamori, K.; Yamaguchi, A.; Sakamoto, K. LAMC2 is a predictive marker for the malignant progression of leukoplakia. J. Oral Pathol. Med. 2017, 46, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Nordemar, S.; Hogmo, A.; Lindholm, J.; Auer, G.; Munck-Wikland, E. Laminin-5 gamma 2: A marker to identify oral mucosal lesions at risk for tumor development? Anticancer Res. 2003, 23, 4985–4989. [Google Scholar] [PubMed]
- Rousselle, P.; Scoazec, J.Y. Laminin 332 in cancer: When the extracellular matrix turns signals from cell anchorage to cell movement. Semin. Cancer Biol. 2020, 62, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Oakford, M.E.; Dixon, S.V.; August, S.; Pickard, C.; Ardern-Jones, M.; Lackie, P.; Friedmann, P.S.; Healy, E. Migration of immunocytes across the basement membrane in skin: The role of basement membrane pores. J. Investig. Dermatol. 2011, 131, 1950–1953. [Google Scholar] [CrossRef] [Green Version]
- Scott, R.A.; Lauweryns, B.; Snead, D.M.; Haynes, R.J.; Mahida, Y.; Dua, H.S. E-cadherin distribution and epithelial basement membrane characteristics of the normal human conjunctiva and cornea. Eye 1997, 11 Pt 5, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Howat, W.J.; Holmes, J.A.; Holgate, S.T.; Lackie, P.M. Basement membrane pores in human bronchial epithelium: A conduit for infiltrating cells? Am. J. Pathol. 2001, 158, 673–680. [Google Scholar] [CrossRef]
- Toner, P.G.; Carr, K.E.; Ferguson, A.; Mackay, C. Scanning and transmission electron microscopic studies of human intestinal mucosa. Gut 1970, 11, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Nikitakis, N.G.; Pentenero, M.; Georgaki, M.; Poh, C.F.; Peterson, D.E.; Edwards, P.; Lingen, M.; Sauk, J.J. Molecular markers associated with development and progression of potentially premalignant oral epithelial lesions: Current knowledge and future implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 650–669. [Google Scholar] [CrossRef] [Green Version]
- Salahshourifar, I.; Vincent-Chong, V.K.; Kallarakkal, T.G.; Zain, R.B. Genomic DNA copy number alterations from precursor oral lesions to oral squamous cell carcinoma. Oral Oncol. 2014, 50, 404–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasgupta, S.; Dash, R.; Das, S.K.; Sarkar, D.; Fisher, P.B. Emerging strategies for the early detection and prevention of head and neck squamous cell cancer. J. Cell. Physiol. 2012, 227, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noutomi, Y.; Oga, A.; Uchida, K.; Okafuji, M.; Ita, M.; Kawauchi, S.; Furuya, T.; Ueyama, Y.; Sasaki, K. Comparative genomic hybridization reveals genetic progression of oral squamous cell carcinoma from dysplasia via two different tumourigenic pathways. J. Pathol. 2006, 210, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.J.; Cho, S.H.; Lee, Y.S.; Park, G.S.; Kim, M.S.; Kim, B.K.; Park, W.S.; Lee, J.Y.; Kang, C.S. Loss of heterozygosity on chromosomes 3p, 8p, 9p and 17p in the progression of squamous cell carcinoma of the larynx. J. Korean Med. Sci. 2004, 19, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhosale, P.G.; Cristea, S.; Ambatipudi, S.; Desai, R.S.; Kumar, R.; Patil, A.; Kane, S.; Borges, A.M.; Schaffer, A.A.; Beerenwinkel, N.; et al. Chromosomal Alterations and Gene Expression Changes Associated with the Progression of Leukoplakia to Advanced Gingivobuccal Cancer. Transl. Oncol. 2017, 10, 396–409. [Google Scholar] [CrossRef] [Green Version]
- Ginzel, J.D.; Acharya, C.R.; Lubkov, V.; Mori, H.; Boone, P.G.; Rochelle, L.K.; Roberts, W.L.; Everitt, J.I.; Hartman, Z.C.; Crosby, E.J.; et al. HER2 Isoforms Uniquely Program Intratumor Heterogeneity and Predetermine Breast Cancer Trajectories During the Occult Tumorigenic Phase. Mol. Cancer Res. 2021, 19, 1699–1711. [Google Scholar] [CrossRef]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Glentis, A.; Oertle, P.; Mariani, P.; Chikina, A.; El Marjou, F.; Attieh, Y.; Zaccarini, F.; Lae, M.; Loew, D.; Dingli, F.; et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 2017, 8, 924. [Google Scholar] [CrossRef] [Green Version]
- Kelley, L.C.; Chi, Q.; Caceres, R.; Hastie, E.; Schindler, A.J.; Jiang, Y.; Matus, D.Q.; Plastino, J.; Sherwood, D.R. Adaptive F-Actin Polymerization and Localized ATP Production Drive Basement Membrane Invasion in the Absence of MMPs. Dev. Cell 2019, 48, 313–328.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.; Sim, Y.; Kim, J.; Kim, H.; Cho, I.; Nam, H.; Yoon, Y.G.; Chang, J.B. PICASSO allows ultra-multiplexed fluorescence imaging of spatially overlapping proteins without reference spectra measurements. Nat. Commun. 2022, 13, 2475. [Google Scholar] [CrossRef]
- Reddy, R.; Yang, L.; Liu, J.; Liu, Z.; Wang, J. Spatial Multiplex In Situ Tagging (MIST) Technology for Rapid, Highly Multiplexed Detection of Protein Distribution on Brain Tissue. Anal. Chem. 2022, 94, 3922–3929. [Google Scholar] [CrossRef]
- Lin, J.R.; Izar, B.; Wang, S.; Yapp, C.; Mei, S.; Shah, P.M.; Santagata, S.; Sorger, P.K. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. eLife 2018, 7, e31657. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; McMahon, N.P.; Zheng, T.; Eng, J.; Chin, K.; Kwon, S.; Nederlof, M.A.; Gray, J.W.; Gibbs, S.L. Oligonucleotide conjugated antibody strategies for cyclic immunostaining. Sci. Rep. 2021, 11, 23844. [Google Scholar] [CrossRef] [PubMed]
- Goltsev, Y.; Samusik, N.; Kennedy-Darling, J.; Bhate, S.; Hale, M.; Vazquez, G.; Black, S.; Nolan, G.P. Deep Profiling of Mouse Splenic Architecture with CODEX Multiplexed Imaging. Cell 2018, 174, 968–981.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickey, J.W.; Neumann, E.K.; Radtke, A.J.; Camarillo, J.M.; Beuschel, R.T.; Albanese, A.; McDonough, E.; Hatler, J.; Wiblin, A.E.; Fisher, J.; et al. Spatial mapping of protein composition and tissue organization: A primer for multiplexed antibody-based imaging. Nat. Methods 2022, 19, 284–295. [Google Scholar] [CrossRef]
- Kinkhabwala, A.; Herbel, C.; Pankratz, J.; Yushchenko, D.A.; Ruberg, S.; Praveen, P.; Reiss, S.; Rodriguez, F.C.; Schafer, D.; Kollet, J.; et al. MACSima imaging cyclic staining (MICS) technology reveals combinatorial target pairs for CAR T cell treatment of solid tumors. Sci. Rep. 2022, 12, 1911. [Google Scholar] [CrossRef]
| Cell Type | Markers | Trend | Conclusion |
|---|---|---|---|
| Fibroblasts/CAFs | ACTA2, CD34 | Abrupt appearance in SCC | Marker for SCC |
| T Cells | CD4, CD8, FOXP3, CD43, CD45RO, CD25, PDCD1 | Increase from normal to dysplasia to cancer | Already abundant in precancerous lesions |
| NK Cells | CD57, NCAM1 | Little evidence for association with progression from normal to cancer | More detailed study needed to better define NK cells |
| Macrophages | CD68, CD86, CD80, CD163, CD204, CD14 | CD68, CD86, CD163, CD204: Significant increase from normal to dysplasia CD14: Findings not statistically significant | Missing detailed study applying comprehensive set of markers |
| B Cells | CD19, CD20 | Rarely detected in PMD lesions | Understudied. May remain uninvolved in tumorigenic process |
| Langerhans Cells | CD1A, CD83 | Reduced presence of intra-epithelial LCs in tumors compared to dysplasia/normal | Changes in cell numbers/location complex during progression from normal to HNSCC |
| Neutrophils | CEACAM8, MPO | Increase in HNSCC tissues | Contribution to HNSCC formation/progression unclear |
| Eosinophils | Congo Red and histological stains | Steady increase from normal to severe dysplasia | Contribution during tumorigenesis unclear. Specific molecular markers (EPX or PRG2) should be studied |
| Mast Cells | Tryptase, histological stains | Gradual increase from normal to dysplasia to cancer | Role during progression from normal to tumor unclear |
| Endothelium and Hypoxia | PECAM1, VWF, VEGF, CA9, HIF1A, NBI endoscopy | Significant increase in vascularization already in dysplasia; elevated expression of angiogenic factors in PMD lesions | Hypoxia present in precancerous lesions |
| Immune Checkpoint | CD274, PDCD1 | Induction of epithelial CD274 expression and PD1 expressed in lymphocytes in PMD lesions | Immunohistological levels of PD-1 indication for immunosuppression in PMD lesions |
| Basal Lamina | Ultrastructure, COL4A1, COL17A1, HSPG2, laminins, ITGB4, ITGA6 | Already disturbed in PMD lesions | Unclear if cancer cells, CAFs, or inflammatory cells initiate breakdown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, A.; Andl, T. Precancerous Lesions of the Head and Neck Region and Their Stromal Aberrations: Piecemeal Data. Cancers 2023, 15, 2192. https://doi.org/10.3390/cancers15082192
Harris A, Andl T. Precancerous Lesions of the Head and Neck Region and Their Stromal Aberrations: Piecemeal Data. Cancers. 2023; 15(8):2192. https://doi.org/10.3390/cancers15082192
Chicago/Turabian StyleHarris, Ashlee, and Thomas Andl. 2023. "Precancerous Lesions of the Head and Neck Region and Their Stromal Aberrations: Piecemeal Data" Cancers 15, no. 8: 2192. https://doi.org/10.3390/cancers15082192
APA StyleHarris, A., & Andl, T. (2023). Precancerous Lesions of the Head and Neck Region and Their Stromal Aberrations: Piecemeal Data. Cancers, 15(8), 2192. https://doi.org/10.3390/cancers15082192






