Total Metabolic Tumor Volume on 18F-FDG PET/CT Is a Useful Prognostic Biomarker for Patients with Extensive Small-Cell Lung Cancer Undergoing First-Line Chemo-Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
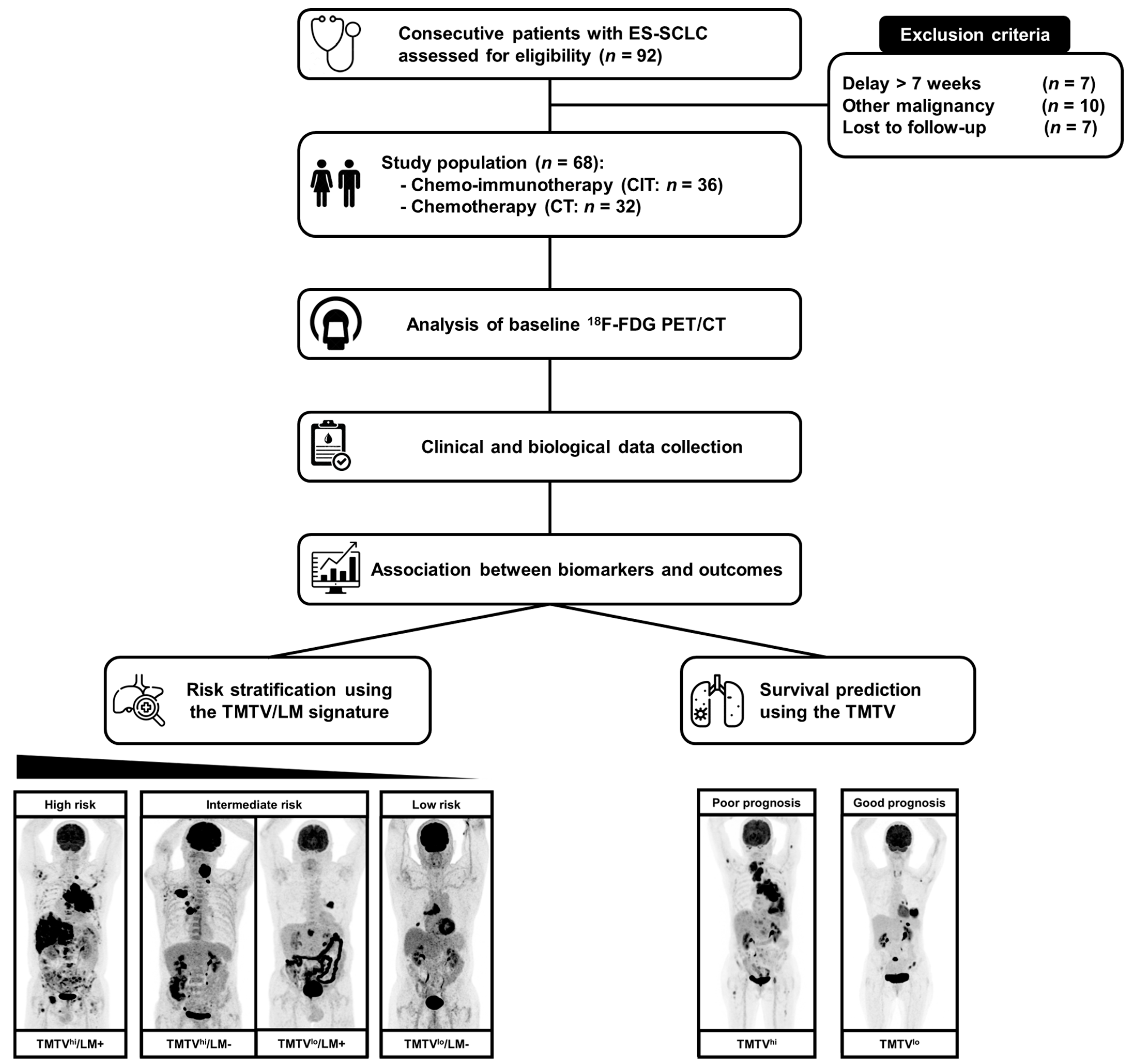
2. Materials and Methods
2.1. Patients
2.2. FDG PET/CT Acquisitions
2.3. Measurement of Biological and Imaging Biomarkers
2.4. Outcomes: Progression-Free Survival and Overall Survival
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlation between Biomarkers
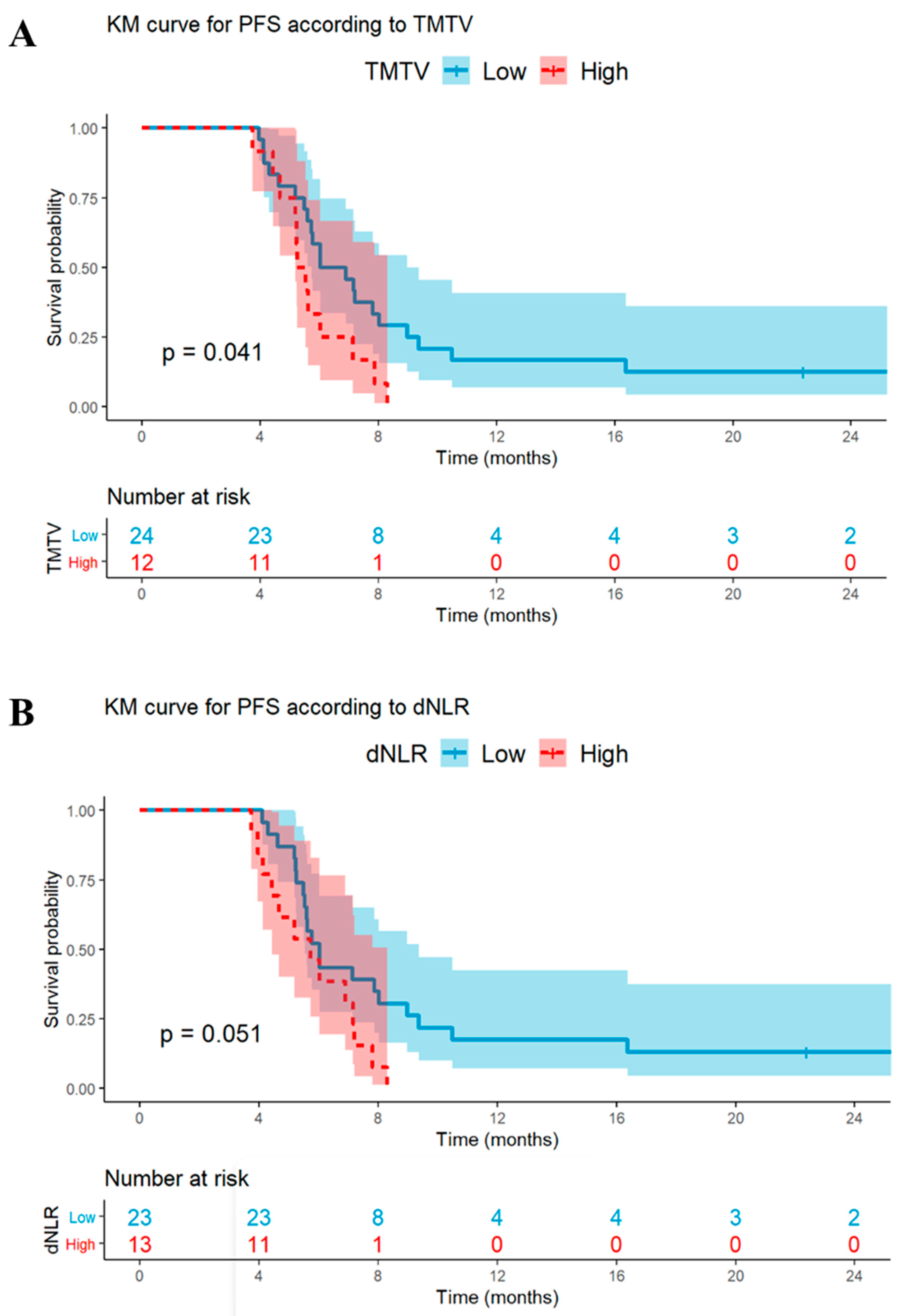
3.3. Univariable and Multivariable Analyses: PFS
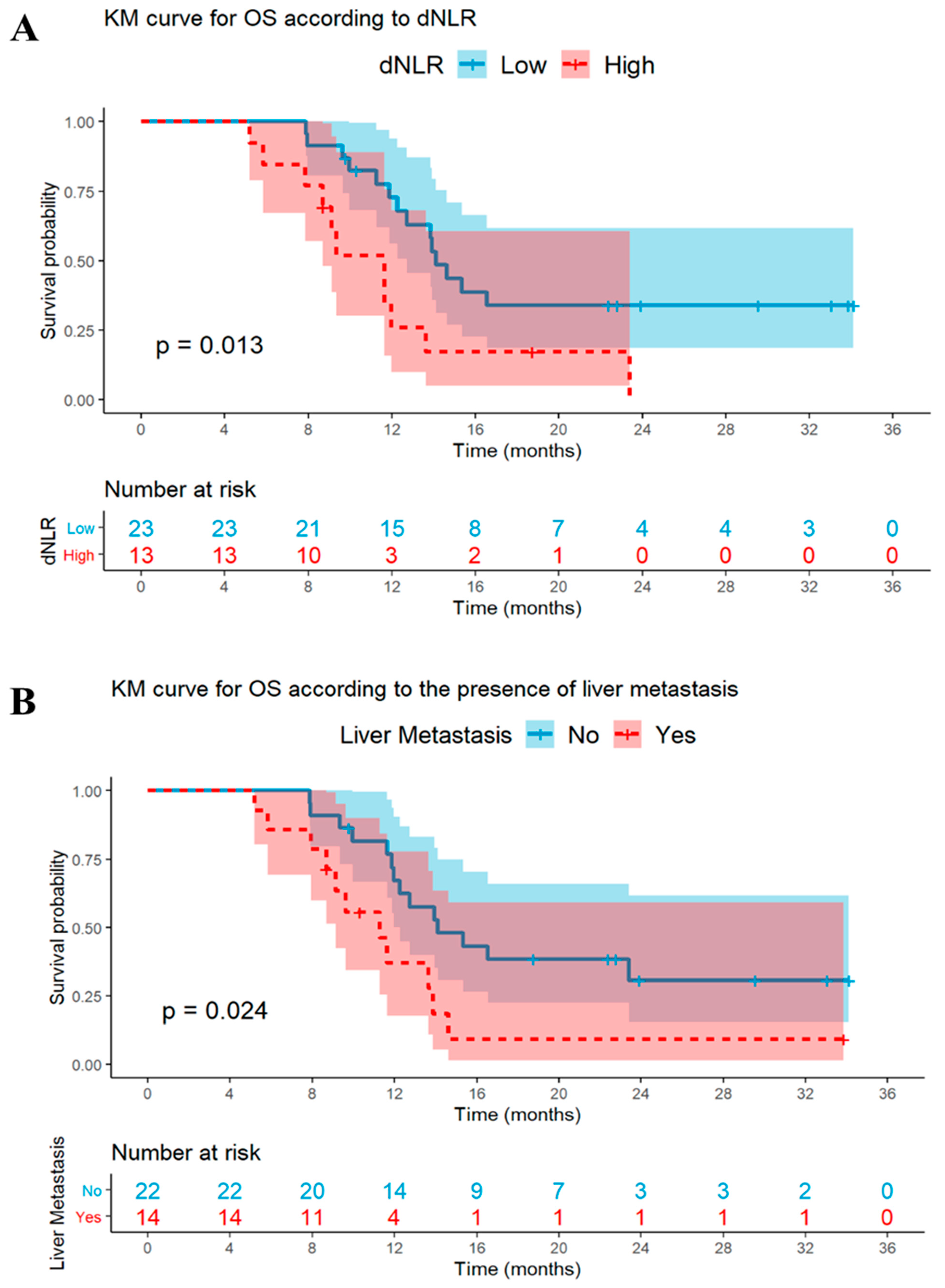
3.4. Univariable and Multivariable Analyses: OS
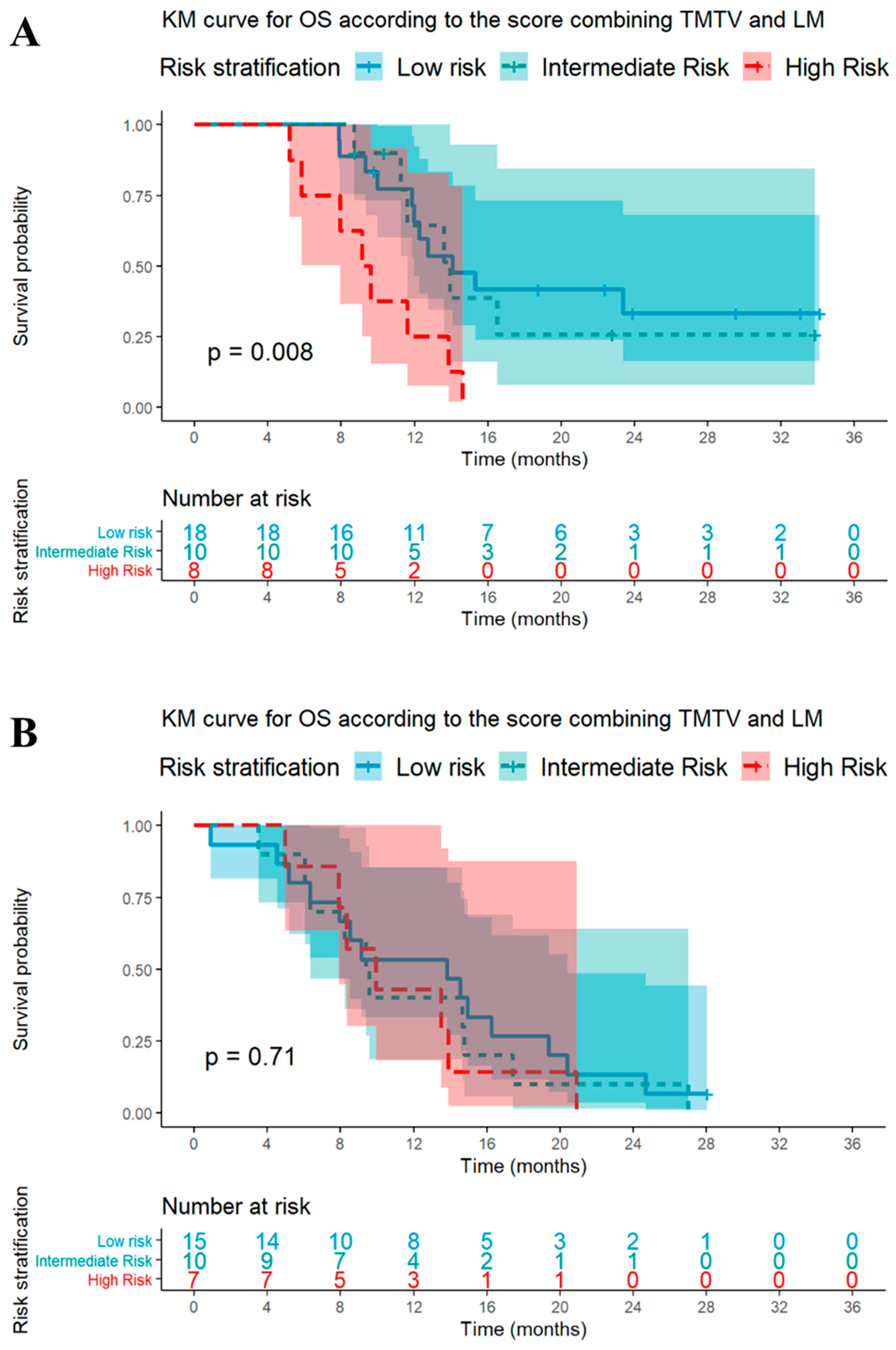
3.5. Signature Using TMTV and LM Status for the Prediction of Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernhardt, E.B.; Jalal, S.I. Small Cell Lung Cancer. Cancer Treat. Res. 2016, 170, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.R.; Renfro, L.A.; Schild, S.E.; Redman, M.W.; Wang, X.F.; Dahlberg, S.E.; Ding, K.; Bradbury, P.A.; Ramalingam, S.S.; Gandara, D.R.; et al. Multitrial Evaluation of Progression-Free Survival as a Surrogate End Point for Overall Survival in First-Line Extensive-Stage Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1099–1106. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus Platinum-Etoposide versus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Paz-Ares, L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J. Clin. Oncol. 2022, 40, 671–680. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Marabelle, A.; Caramella, C.; Garcia, C.; Aldea, M.; Chaput, N.; Robert, C.; Besse, B. Tumour Burden and Efficacy of Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2022, 19, 75–90. [Google Scholar] [CrossRef]
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.W.J.M.; et al. Joint EANM/SNMMI/ANZSNM Practice Guidelines/Procedure Standards on Recommended Use of [18F]FDG PET/CT Imaging during Immunomodulatory Treatments in Patients with Solid Tumors Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2323–2341. [Google Scholar] [CrossRef]
- Christensen, T.N.; Andersen, P.K.; Langer, S.W.; Fischer, B.M.B. Prognostic Value of 18F-FDG-PET Parameters in Patients with Small Cell Lung Cancer: A Meta-Analysis and Review of Current Literature. Diagnostics 2021, 11, 174. [Google Scholar] [CrossRef]
- Sharma, A.; Mohan, A.; Bhalla, A.S.; Sharma, M.C.; Vishnubhatla, S.; Das, C.J.; Pandey, A.K.; Sekhar Bal, C.; Patel, C.D.; Sharma, P.; et al. Role of Various Metabolic Parameters Derived from Baseline 18F-FDG PET/CT as Prognostic Markers in Non-Small Cell Lung Cancer Patients Undergoing Platinum-Based Chemotherapy. Clin. Nucl. Med. 2018, 43, e8–e17. [Google Scholar] [CrossRef] [PubMed]
- Prelaj, A.; Tay, R.; Ferrara, R.; Chaput, N.; Besse, B.; Califano, R. Predictive Biomarkers of Response for Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer. Eur. J. Cancer 2019, 106, 144–159. [Google Scholar] [CrossRef]
- Seban, R.-D.; Mezquita, L.; Berenbaum, A.; Dercle, L.; Botticella, A.; Le Pechoux, C.; Caramella, C.; Deutsch, E.; Grimaldi, S.; Adam, J.; et al. Baseline Metabolic Tumor Burden on FDG PET/CT Scans Predicts Outcome in Advanced NSCLC Patients Treated with Immune Checkpoint Inhibitors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wroblewski, K.; Appelbaum, D.; Pu, Y. Independent Prognostic Value of Whole-Body Metabolic Tumor Burden from FDG-PET in Non-Small Cell Lung Cancer. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Yip, D.; Harper, P.G. Predictive and Prognostic Factors in Small Cell Lung Cancer: Current Status. Lung Cancer 2000, 28, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Meignan, M.; Sasanelli, M.; Casasnovas, R.O.; Luminari, S.; Fioroni, F.; Coriani, C.; Masset, H.; Itti, E.; Gobbi, P.G.; Merli, F.; et al. Metabolic Tumour Volumes Measured at Staging in Lymphoma: Methodological Evaluation on Phantom Experiments and Patients. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1113–1122. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Empereur-Mot, C.; Guillemain, H.; Latouche, A.; Zagury, J.-F.; Viallon, V.; Montes, M. Predictiveness Curves in Virtual Screening. J. Cheminform. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Viallon, V.; Latouche, A. Discrimination Measures for Survival Outcomes: Connection between the AUC and the Predictiveness Curve. Biom. J. 2011, 53, 217–236. [Google Scholar] [CrossRef]
- Andrini, E.; Lamberti, G.; Argalia, G.; Fortunati, E.; Ambrosini, V.; Fanti, S.; Ardizzoni, A.; Campana, D. 149P Total Metabolic Tumor Volume: A New Potential Prognostic Factor in SCLC. Ann. Oncol. 2022, 33, S101. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Calabrò, D.; Conci, N.; Argalia, G.; Marchese, P.V.; Fabbri, F.; Fragomeno, B.; Ricci, D.; Fanti, S.; Ambrosini, V.; et al. Baseline Total Metabolic Tumour Volume on 2-Deoxy-2-[18F]Fluoro-d-Glucose Positron Emission Tomography-Computed Tomography as a Promising Biomarker in Patients with Advanced Non–Small Cell Lung Cancer Treated with First-Line Pembrolizumab. Eur. J. Cancer 2021, 150, 99–107. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Hellmann, M.D.; Hamid, O.; Tsai, K.K.; Loo, K.L.; Gubens, M.A.; Rosenblum, M.; Harview, C.L.; Taube, J.M.; Handley, N.; et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017, 5, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver Metastasis Restrains Immunotherapy Efficacy via Macrophage-Mediated T Cell Elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef]
- Cabibbo, G.; Aghemo, A.; Lai, Q.; Masarone, M.; Montagnese, S.; Ponziani, F.R.; Italian Association for the Study of the Liver (AISF). Optimizing Systemic Therapy for Advanced Hepatocellular Carcinoma: The Key Role of Liver Function. Dig. Liver Dis. 2022, 54, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Seban, R.-D.; Assié, J.-B.; Giroux-Leprieur, E.; Massiani, M.-A.; Soussan, M.; Bonardel, G.; Chouaid, C.; Playe, M.; Goldfarb, L.; Duchemann, B.; et al. Association of the Metabolic Score Using Baseline FDG-PET/CT and DNLR with Immunotherapy Outcomes in Advanced NSCLC Patients Treated with First-Line Pembrolizumab. Cancers 2020, 12, 2234. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Toschi, L.; Rossi, S.; Mazziotti, E.; Lopci, E. The Immune-Metabolic-Prognostic Index and Clinical Outcomes in Patients with Non-Small Cell Lung Carcinoma under Checkpoint Inhibitors. J. Cancer Res. Clin. Oncol. 2020, 146, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. The Systemic Inflammation-Based Glasgow Prognostic Score: A Decade of Experience in Patients with Cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Dercle, L.; Ammari, S.; Champiat, S.; Massard, C.; Ferté, C.; Taihi, L.; Seban, R.-D.; Aspeslagh, S.; Mahjoubi, L.; Kamsu-Kom, N.; et al. Rapid and Objective CT Scan Prognostic Scoring Identifies Metastatic Patients with Long-Term Clinical Benefit on Anti-PD-1/-L1 Therapy. Eur. J. Cancer 2016, 65, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Rossi, S.; Mazziotti, E.; Toschi, L.; Lopci, E. Hyperprogressive Disease in Patients with Non-Small Cell Lung Cancer Treated with Checkpoint Inhibitors: The Role of 18F-FDG PET/CT. J. Nucl. Med. 2020, 61, 821–826. [Google Scholar] [CrossRef]
- Cassidy, M.R.; Wolchok, R.E.; Zheng, J.; Panageas, K.S.; Wolchok, J.D.; Coit, D.; Postow, M.A.; Ariyan, C. Neutrophil to Lymphocyte Ratio Is Associated with Outcome During Ipilimumab Treatment. EBioMedicine 2017, 18, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Sorich, M.J.; Rowland, A.; Karapetis, C.S.; Hopkins, A.M. Evaluation of the Lung Immune Prognostic Index for Prediction of Survival and Response in Patients Treated with Atezolizumab for NSCLC: Pooled Analysis of Clinical Trials. J. Thorac. Oncol. 2019, 14, 1440–1446. [Google Scholar] [CrossRef]
| All Patients (n = 68) | CIT Group (n = 36) | CT Group (n = 32) | |
|---|---|---|---|
| Median [Range], n (%) | Median [Range], n (%) | Median [Range], n (%) | |
| CLINICAL CHARACTERISTICS | |||
| Demographic parameters | |||
| Age (years) | 67 (50–84) | 67 (51–84) | 66 (50–84) |
| Gender (Male/Female) | 50 (74)/18 (26) | 25 (69)/11 (31) | 25 (78)/7 (22) |
| Performance Status (ECOG) | 1 (0–3) | 1 (0–2) | 1 (0–3) |
| Smoking history (current/former/no/unknown) | 31 (46)/33 (49)/3 (4)/1 (1) | 13 (36)/20 (55)/2 (6)/1 (3) | 18 (56)/13 (41)/1 (3)/0 (0) |
| Biology | |||
| Leukocytes (G/L) | 8.9 (5.8–19.6) | 8.7 (6.1–19.6) | 9.6 (5.8–16.4) |
| Neutrophils (G/L) | 6.5 (2.0–15.0) | 5.9 (2.6–15.0) | 6.7 (2.0–12.1) |
| dNLR | 2.5 (0.3–10.1) | 2.6 (0.7–9.0) | 2.4 (0.3–10.1) |
| Lymphocytes (G/L) | 1.7 (0.3–3.7) | 1.7 (0.6–3.0) | 1.7 (0.3–3.7) |
| Staging | |||
| Number of metastatic sites | 2 (1–8) | 2 (1–8) | 2 (1–6) |
| Liver involvement | 26 (38) | 14 (39) | 12 (37) |
| Brain involvement | 22 (32) | 10 (28) | 12 (37) |
| PET IMAGING PARAMETERS | |||
| Tumor glucose uptake | |||
| Tumor SUVmax | 12.7 (3.7–44.0) | 13.4 (6.0–44.0) | 12.3 (3.7–29.3) |
| Metabolic Tumor Burden | |||
| TMTV | 182.9 (1.7–2257.2) | 178.5 (3.1–2257.2) | 196.3 (1.7–880.6) |
| TREATMENT | |||
| First-line | |||
| Carboplatin—Etoposide—Atezolizumab | 34 (50) | 34 (94) | NA |
| Carboplatin—Etoposide—Durvalumab | 2 (3) | 2 (6) | NA |
| Carboplatin—Etoposide | 32 (47) | NA | 32 (100) |
| SURVIVAL | |||
| Progression | 65 (96) | 34 (94) | 31 (97) |
| Death | 56 (82) | 25 (69) | 31 (97) |
| N = 36 pts | OVERALL SURVIVAL (Events: n = 25) | PROGRESSION-FREE SURVIVAL (Events: n = 34) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| Variable | p | HR (95CI) | p | HR (95CI) | p | HR (95CI) | p | HR (95CI) |
| DEMOGRAPHICS | ||||||||
| Age ≥ 70 years (vs. <70 years) | 0.08 | 2.1 (0.9–4.8) | 0.41 | 1.5 (0.6–3.8) | 0.14 | 1.8 (0.8–3.7) | - | - |
| Male (vs. female) | 0.15 | 0.5 (0.2–1.2) | - | - | 0.45 | 1.3 (0.4–1.6) | - | - |
| PS ≥ 2 (vs. <2) | 0.63 | 1.3 (0.4–3.8) | - | - | 0.78 | 1.1 (0.4–2.1) | - | - |
| BIOLOGY | ||||||||
| High dNLR | 0.02 | 2.7 (1.2–6.0) | 0.03 | 2.7 (1.1–6.5) | 0.05 | 2.1 (0.9–4.3) | <0.01 | 3.1 (1.3–7.2) |
| IMAGING | ||||||||
| 18F-FDG PET/CT | ||||||||
| High TMTV | 0.06 | 2.2 (1.0–4.8) | 0.07 | 2.3 (0.9–5.8) | 0.04 | 2.2 (1.1–4.6) | 0.03 | 2.5 (1.1–5.9) |
| High tumor SUVmax | 0.75 | 1.1 (0.4–1.9) | - | - | 0.53 | 1.2 (0.4–1.6) | - | - |
| Liver metastases (vs. no) | 0.03 | 2.5 (1.1–5.6) | 0.26 | 1.7 (0.9–5.8) | 0.57 | 1.2 (0.6–2.5) | - | - |
| BRAIN MRI and/or CT | ||||||||
| Brain metastases (vs. no) | 0.68 | 0.8 (0.3–2.0) | - | - | 0.48 | 0.8 (0.4–1.6) | - | - |
| N = 32 pts | OVERALL SURVIVAL (Events: n = 31) | PROGRESSION-FREE SURVIVAL (Events: n = 31) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| Variable | p | HR (95CI) | p | HR (95CI) | p | HR (95CI) | p | HR (95CI) |
| DEMOGRAPHICS | ||||||||
| Age ≥ 70 years (vs. <70 years) | 0.09 | 1.8 (0.9–3.8) | 0.83 | 1.1 (0.5–2.7) | 0.38 | 1.4 (0.7–3.0) | ||
| Male (vs. female) | 0.37 | 0.7 (0.3–1.6) | - | - | 0.10 | 0.5 (0.2–1.2) | 0.58 | 0.8 (0.3–1.9) |
| PS ≥ 2 (vs. <2) | 0.15 | 1.9 (0.8–4.7) | - | - | 0.90 | 1.1 (0.4–2.5) | - | - |
| BIOLOGY | ||||||||
| High dNLR | <0.01 | 3.4 (1.5–7.9) | 0.02 | 3.2 (1.2–8.7) | 0.05 | 2.2 (1.0–4.8) | <0.01 | 3.3 (1.4–7.7) |
| IMAGING | ||||||||
| 18F-FDG PET/CT | ||||||||
| High TMTV | 0.18 | 1.7 (0.8–3.6) | - | - | 0.28 | 1.5 (0.7–3.3) | - | - |
| High tumor SUVmax | 0.12 | 0.6 (0.3–1.2) | - | - | 0.68 | 0.9 (0.4–1.8) | - | - |
| Liver metastases (vs. no) | 0.94 | 1.0 (0.5–2.1) | - | - | 0.28 | 0.6 (0.3–1.4) | - | - |
| BRAIN MRI and/or CT | ||||||||
| Brain metastases (vs. no) | 0.34 | 1.4 (0.7–3.0) | - | - | 0.29 | 1.5 (0.7–3.2) | - | - |
| Cohort and Strata | Patients (n) | Median OS [95% CI] | Events (n) | p-Value | Median PFS [95% CI] | Events (n) | p-Value * |
|---|---|---|---|---|---|---|---|
| CIT cohort | 36 | <0.01 | 0.33 | ||||
| High risk | 8 | 9.4 [7.9–NA] | 8 | 5.4 [5.2–NA] | 8 | ||
| Intermediate risk | 10 | 13.9 [11.6–NA] | 6 | 5.8 [4.7–NA] | 10 | ||
| Low risk | 18 | 14.1 [12.0–NA] | 11 | 6.6 [5.6–10.5] | 16 | ||
| CT cohort | 32 | 0.71 | 0.38 | ||||
| High risk | 7 | 9.9 [7.9–NA] | 7 | 6.5 [5.5–NA] | 7 | ||
| Intermediate risk | 10 | 9.5 [6.4–NA] | 10 | 7.3 [3.5–NA] | 9 | ||
| Low risk | 15 | 13.8 [8.0–20.4] | 14 | 6.1 [5.0–8.5] | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grambow-Velilla, J.; Seban, R.-D.; Chouahnia, K.; Assié, J.-B.; Champion, L.; Girard, N.; Bonardel, G.; Matton, L.; Soussan, M.; Chouaïd, C.; et al. Total Metabolic Tumor Volume on 18F-FDG PET/CT Is a Useful Prognostic Biomarker for Patients with Extensive Small-Cell Lung Cancer Undergoing First-Line Chemo-Immunotherapy. Cancers 2023, 15, 2223. https://doi.org/10.3390/cancers15082223
Grambow-Velilla J, Seban R-D, Chouahnia K, Assié J-B, Champion L, Girard N, Bonardel G, Matton L, Soussan M, Chouaïd C, et al. Total Metabolic Tumor Volume on 18F-FDG PET/CT Is a Useful Prognostic Biomarker for Patients with Extensive Small-Cell Lung Cancer Undergoing First-Line Chemo-Immunotherapy. Cancers. 2023; 15(8):2223. https://doi.org/10.3390/cancers15082223
Chicago/Turabian StyleGrambow-Velilla, Julia, Romain-David Seban, Kader Chouahnia, Jean-Baptiste Assié, Laurence Champion, Nicolas Girard, Gerald Bonardel, Lise Matton, Michael Soussan, Christos Chouaïd, and et al. 2023. "Total Metabolic Tumor Volume on 18F-FDG PET/CT Is a Useful Prognostic Biomarker for Patients with Extensive Small-Cell Lung Cancer Undergoing First-Line Chemo-Immunotherapy" Cancers 15, no. 8: 2223. https://doi.org/10.3390/cancers15082223
APA StyleGrambow-Velilla, J., Seban, R.-D., Chouahnia, K., Assié, J.-B., Champion, L., Girard, N., Bonardel, G., Matton, L., Soussan, M., Chouaïd, C., & Duchemann, B. (2023). Total Metabolic Tumor Volume on 18F-FDG PET/CT Is a Useful Prognostic Biomarker for Patients with Extensive Small-Cell Lung Cancer Undergoing First-Line Chemo-Immunotherapy. Cancers, 15(8), 2223. https://doi.org/10.3390/cancers15082223








