The Significance of SPP1 in Lung Cancers and Its Impact as a Marker for Protumor Tumor-Associated Macrophages
Abstract
:Simple Summary
Abstract
1. Introduction
2. The M1/M2 Concept of TAMs in Lung Cancers
3. Function and Significance of TAMs in Lung Cancers
4. Significance of SPP1 in Lung Cancer
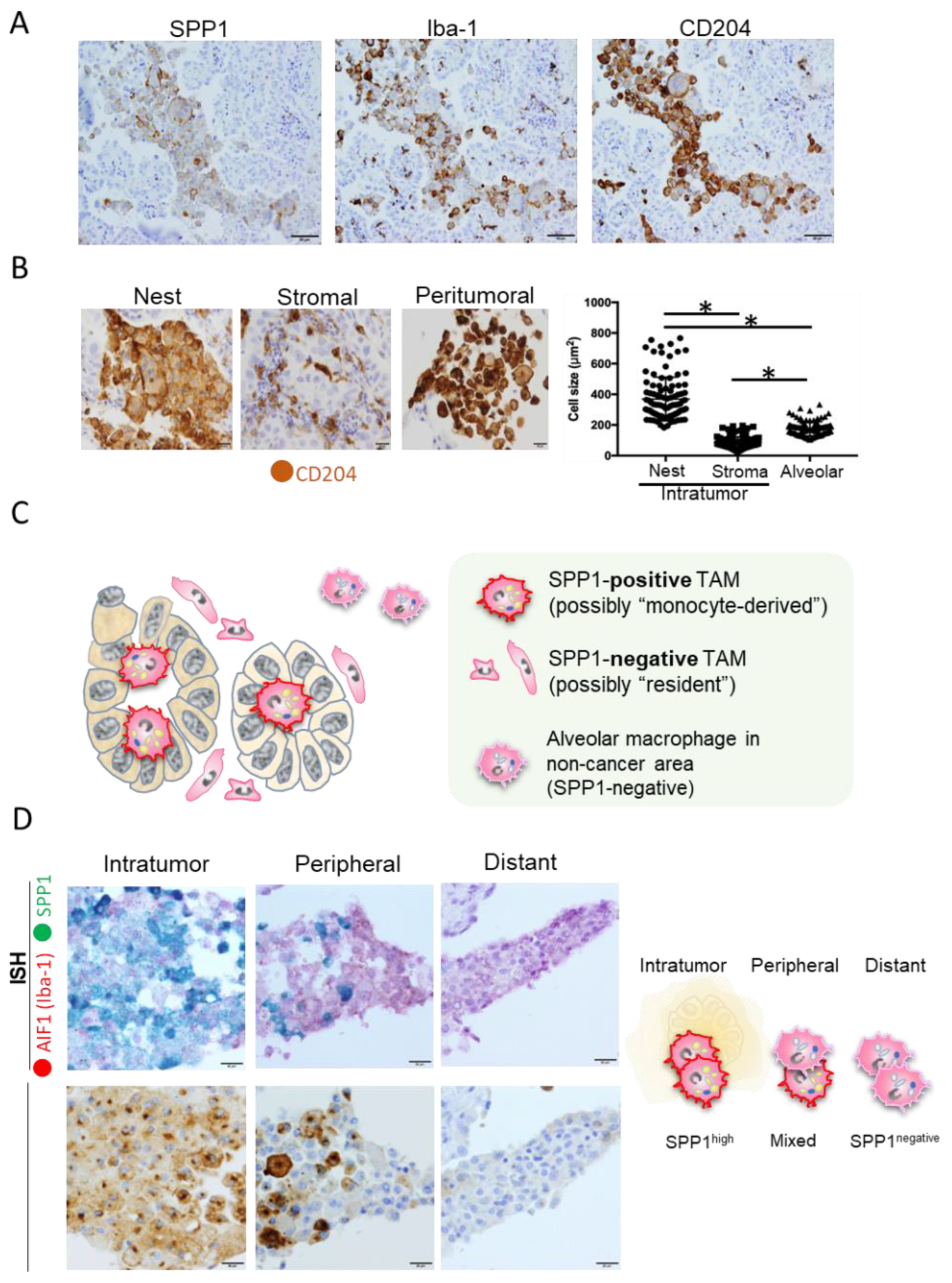
5. Significance of SPP1 in TAMs
6. Significance of SPP1 in TAMs: A New Perspective from Single-Cell Analysis
7. Possible Involvement of the GM-CSF-SPP1 Loop in Chemoresistance
8. Potential Immunotherapy against GM-CSF
9. Significance of TAM-Derived SPP1 in Immune Suppression
10. Significance of SPP1 Expression in TAMs in Malignant Tumors Other Than Lung Cancer
11. Limitations
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, P.C.; Mauer, A.M.; Vokes, E.E. Lung cancer. Lancet 2000, 355, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Motira, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomized phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [Green Version]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Hellmann, M.D.; Paz-Ares, L.; Caro, R.B.; Zurawski, B.; Kim, S.W.; Costa, E.C.; Park, K.; Alexandru, A.; Lupinacci, L.; Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1 Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Sun, Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016, 380, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Wei, R.; Liu, S.; Zhang, S.; Min, L.; Zhu, S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal. Cell. Pathol. 2020, 2020, 6283796. [Google Scholar] [CrossRef] [Green Version]
- Remark, R.; Becker, C.; Gomez, J.E.; Damotte, D.; Dieu-Nosjean, M.C.; Sautès-Fridman, C.; Fridman, W.H.; Powell, C.A.; Altorki, N.K.; Merad, M.; et al. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am. J. Respir. Crit. Care Med. 2015, 191, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Eenoo, F.V.; Rouas, G.; Francis, P.; Crown, J.; Hitre, A.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Wang, K.; Huang, X.J. Myeloid-derived suppressor cells in hematological malignancies: Friend or foes. J. Hematol. Oncol. 2019, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Komohara, Y.; Fujiwara, Y.; Ohnishi, K.; Takeya, M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv. Drug Deliv. Rev. 2016, 99, 180–185. [Google Scholar] [CrossRef]
- Komohara, Y.; Jinushi, M.; Takeya, M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014, 105, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, E.; Komohara, Y.; Esumi, S.; Shinchi, Y.; Ishizuka, S.; Mito, R.; Pan, C.; Yano, H.; Kobayashi, D.; Fujiwara, Y.; et al. SPP1 Derived from Macrophages Is Associated with a Worse Clinical Course and Chemo-Resistance in Lung Adenocarcinoma. Cancers 2022, 14, 4374. [Google Scholar] [CrossRef] [PubMed]
- Lamort, A.-S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Inoue, M.; Shinohara, M. Intracellular osteopontin (iOPN) and immunity. Immunol. Res. 2011, 49, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Kariya, Y.; Kariya, Y. Osteopontin in Cancer: Mechanisms and Therapeutic Targets. Int. J. Transl. Med. 2022, 2, 419–447. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X. Role of osteopontin in lung cancer evolution and heterogeneity. Semin. Cell Dev. Biol. 2017, 64, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Lane, J.; Jiang, W.G. Osteopontin and Cancer: Insights into Its Role in Drug Resistance. Biomedicines 2023, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Komohara, Y.; Oshiumi, H. The role of macrophages in anti-tumor immune responses: Pathological significance and potential as therapeutic targets. Hum. Cell 2021, 34, 1031–1039. [Google Scholar] [CrossRef]
- Li, Z.; Maeda, D.; Yoshida, M.; Umakoshi, M.; Nanjo, H.; Shiraishi, K.; Saito, M.; Kohno, T.; Konno, H.; Saito, H.; et al. The intratumoral distribution influences the prognostic impact of CD68- and CD204-positive macrophages in non-small cell lung cancer. Lung Cancer 2018, 123, 127–135. [Google Scholar] [CrossRef]
- Wu, P.; Wu, D.; Zhao, L.; Huang, L.; Chen, G.; Shen, G.; Huang, G.; Chai, Y. Inverse role of distinct subsets and distribution of macrophage in lung cancer prognosis: A meta-analysis. Oncotarget 2016, 7, 40451–40460. [Google Scholar] [CrossRef] [Green Version]
- Ohtaki, Y.; Ishii, G.; Nagai, K.; Ashimine, S.; Kuwata, T.; Hishida, T.; Nishimura, M.; Yoshida, J.; Takeyoshi, I.; Ochiai, A. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J. Thorac. Oncol. 2010, 5, 1507–1515. [Google Scholar] [CrossRef] [Green Version]
- Kaseda, K.; Ishii, G.; Aokage, K.; Takahashi, A.; Kuwata, T.; Hishida, T.; Yoshida, J.; Kohno, M.; Nagai, K.; Ochiai, A. Identification of intravascular tumor microenviroment features predicting the recurrence of pathological stage I lung adenocarcinoma. Cancer Sci. 2013, 104, 1262–1269. [Google Scholar] [CrossRef]
- Ito, M.; Ishii, G.; Nagai, K.; Maeda, R.; Nakano, Y.; Ochiai, A. Prognostic impact of cancer-associated stromal cells in patients with Stage I lung adenocarcinoma. Chest 2012, 142, 151–158. [Google Scholar] [CrossRef]
- Ohri, C.M.; Shikotra, A.; Green, R.H.; Waller, D.A.; Bradding, P. The tissue microlocalisation and cellular expression of CD163, VEGF, HLA-DR, iNOS, and MRP 8/14 is correlated to clinical outcome in NSCLC. PLoS ONE 2011, 6, e21874. [Google Scholar] [CrossRef]
- Chung, F.T.; Lee, K.Y.; Wang, C.W.; Heh, C.C.; Chan, Y.F.; Chen, H.W.; Kuo, C.H.; Feng, P.H.; Lin, T.Y.; Wang, C.H.; et al. Tumor-associated macrophages correlate with response to epidermal growth factor receptor- tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int. J. Cancer 2012, 131, E227–E235. [Google Scholar] [CrossRef] [PubMed]
- Carus, A.; Ladekarl, M.; Hager, H.; Pilegaard, H.; Nielsen, P.S.; Donskov, F. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: No immediate impact on patient outcome. Lung Cancer 2013, 81, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, L.; Che, G.; Yu, N.; Dai, F.; You, Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 2010, 10, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirayama, S.; Ishii, G.; Nagai, K.; Ono, S.; Kojima, M.; Yamauchi, C.; Aokage, K.; Hishida, T.; Yoshida, J.; Suzuki, K.; et al. Prognostic impact of CD204-positive macrophages in lung squamous cell carcinoma. possible contribution of Cd204-positive macrophages to the tumor-promoting microenvironment. J. Thorac. Oncol. 2012, 7, 1790–1797. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, A.; Ishii, G.; Kinoshita, T.; Yoshida, T.; Umemura, S.; Hishida, T.; Yoh, K.; Niho, S.; Goto, K.; Ohmatsu, H.; et al. Identification of prognostic immunophenotypic features in cancer stromal cells of high-grade neuroendocrine carcinomas of the lung. J. Cancer Res. Clin. Oncol. 2013, 139, 1869–1878. [Google Scholar] [CrossRef]
- Iriki, T.; Ohnishi, K.; Fujiwara, Y.; Horlad, H.; Saito, Y.; Pan, C.; Ikeda, K.; Mori, T.; Suzuki, M.; Ichiyasu, H.; et al. The cell-cell interaction between tumor-associated macrophages and small cell lung cancer cells is involved in tumor progression via STAT3 activation. Lung Cancer 2017, 106, 22–32. [Google Scholar] [CrossRef]
- Matsubara, E.; Komohara, Y.; Shinchi, Y.; Mito, R.; Fujiwara, Y.; Ikeda, K.; Shima, T.; Shimoda, M.; Kanai, Y.; Sakagami, T.; et al. CD163-positive cancer cells are a predictor of a worse clinical course in lung adenocarcinoma. Pathol. Int. 2021, 71, 666–673. [Google Scholar] [CrossRef]
- Garvin, S.; Oda, H.; Arnesson, L.G.; Lindström, A.; Shabo, I. Tumor cell expression of CD163 is associated to postoperative radiotherapy and poor prognosis in patients with breast cancer treated with breast-conserving surgery. J. Cancer Res. Clin. Oncol. 2018, 44, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Shabo, I.; Olsson, H.; Sun, X.F.; Svanvik, J. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. Int. J. Cancer 2009, 125, 1826–1831. [Google Scholar] [CrossRef]
- Donati, V.; Boldrini, L.; Dell’Omodarme, M.; Prati, M.C.; Faviana, P.; Camacci, T.; Lucchi, M.; Mussi, A.; Santoro, M.; Basolo, F.; et al. Osteopontin expression and prognostic significance in non-small cell lung cancer. Clin. Cancer Res. 2005, 11, 6459–6465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Hou, X.; Lu, S.; Rao, H.; Hou, J.; Luo, R.; Huang, H.; Zhao, H.; Jian, H.; Chen, Z.; et al. Predictive significance of bone sialoprotein and osteopontin for bone metastases in resected Chinese non-small-cell lung cancer patients: A large cohort retrospective study. Lung Cancer 2010, 67, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.S.; Li, Y.; Zhang, Z.F.; You, J.; Wang, C.L. Osteopontin combined with CD44v6, a novel prognostic biomarker in non-small cell lung cancer undergoing curative resection. Ann. Thorac. Surg. 2013, 96, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Rud, A.K.; Boye, K.; Øijordsbakken, M.; Lund-Iversen, M.; Halvorsen, A.R.; Solberg, S.K.; Berge, G.; Helland, Å.; Brustugun, O.T.; Mælandsmo, G.M. Osteopontin is a prognostic biomarker in non-small cell lung cancer. BMC Cancer 2013, 13, 540. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhang, D.; Zhao, D.; Hou, X.; Yang, T. Osteopontin expression is associated with platinum-based chemotherapy response and prognosis of patients with advanced non small cell lung cancer. J BUON 2014, 19, 742–748. [Google Scholar]
- Yan, C.H.; Lv, M.; Li, H.; Song, X.; Yan, F.; Cao, S.; Ren, X. Osteopontin is a novel prognostic biomarker in early-stage non-small cell lung cancer after surgical resection. J. Cancer Res. Clin. Oncol. 2015, 141, 1371–1378. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, Y.; Jin, X.; Zhao, W.; Hu, T.; Wu, F.; Huang, J. Osteopontin promotes cancer cell drug resistance, invasion, and lactate production and is associated with poor outcome of patients with advanced non-small-cell lung cancer. OncoTargets Ther. 2018, 11, 5933–5941. [Google Scholar] [CrossRef] [Green Version]
- Mack, P.C.; Redman, M.W.; Chansky, K.; Willamson, S.K.; Farneth, N.C.; Lara, P.N., Jr.; Franklin, W.A.; Le, Q.T.; Crowley, J.J.; Gandara, D.R. Lower osteopontin plasma levels are associated with superior outcomes in advanced non-small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003. J. Clin. Oncol. 2008, 26, 4771–4776. [Google Scholar] [CrossRef] [Green Version]
- Isa, S.; Kawaguchi, T.; Teramukai, S.; Minato, K.; Ohsaki, Y.; Shibata, K.; Yonei, T.; Hayashibara, K.; Fukushima, M.; Kawahara, M.; et al. Serum osteopontin levels are highly prognostic for survival in advanced non-small cell lung cancer results from JMTO LC 0004. J. Thorac. Oncol. 2009, 4, 1104–1110. [Google Scholar] [CrossRef] [Green Version]
- Han, S.S.; Lee, S.J.; Kim, W.J.; Ryu, D.R.; Won, J.Y.; Park, S.; Cheon, M.J. Plasma osteopontin is a useful diagnostic biomarker for advanced non-small cell lung cancer. Tuberc. Respir. Dis. 2013, 75, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Ostheimer, C.; Bache, M.; Güttler, A.; Kotzsch, M.; Vordermark, D. A pilot study on potential plasma hypoxia markers in the radiotherapy of non-small cell lung cancer. Osteopontin, carbonic anhydrase IX and vascular endothelial growth factor. Strahlenther. Onkol. 2014, 190, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Hanagiri, T.; Shinohara, S.; Yasuda, M.; Chikaishi, Y.; Oka, S.; Shimokawa, H.; Nagata, Y.; Nakagawa, M.; Uramoto, H.; et al. Serum level of osteopontin as a prognostic factor in patients who underwent surgical resection for non-small-cell lung cancer. Clin. Lung Cancer 2013, 14, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Grossi, F.; Bonaventura, A.; Vecchiè, A.; Minetti, S.; Bardi, N.; Elia, E.; Ansaldo, A.M.; Ferrara, D.; Rijavec, E.; et al. Baseline serum levels of osteopontin predict clinical response to treatment with nivolumab in patients with non-small cell lung cancer. Clin. Exp. Metastasis 2019, 36, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Pass, H.I.; Lott, D.; Lonardo, F.; Harbut, M.; Liu, Z.; Tang, N.; Carbone, M.; Webb, C.; Wali, A. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N. Engl. J. Med. 2005, 353, 1564–1573. [Google Scholar] [CrossRef] [Green Version]
- Wheatley-Price, P.; Yang, B.; Patsios, D.; Patel, D.; Ma, C.; Xu, W.; Leighl, N.; Feld, R.; Cho, B.J.; O’Sullivan, B.; et al. Soluble mesothelin-related Peptide and osteopontin as markers of response in malignant mesothelioma. J. Clin. Oncol. 2010, 28, 3316–3322. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Marcar, L.; Black, J.; Liu, Q.; Li, X.; Nagulapalli, K.; Sequist, L.V.; Mak, R.H.; Benes, C.H.; et al. Radiation Resistance in KRAS-Mutated Lung Cancer Is Enabled by Stem-like Properties Mediated by an Osteopontin-EGFR Pathway. Cancer Res. 2017, 77, 2018–2028. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Marisetty, A.; Schrand, B.; Gabrusiewicz, K.; Hashimoto, Y.; Ott, M.; Grami, Z.; Kong, L.Y.; Ling, X.; Caruso, H.; et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J. Clin. Investig. 2019, 129, 137–149. [Google Scholar] [CrossRef]
- Choi, S.I.; Kim, S.Y.; Lee, J.H.; Kim, J.Y.; Cho, E.W.; Kim, I.G. Osteopontin production by TM4SF4 signaling drives a positive feedback autocrine loop with the STAT3 pathway to maintain cancer stem cell-like properties in lung cancer cells. Oncotarget 2017, 8, 101284–101297. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, G.; Jain, S.; Kundu, G.C. Osteopontin promotes vascular endothelial growth factor-dependent bresat tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008, 68, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, H.; Zhao, Y.; Yue, D.; Chen, C.; Li, C.; Zhang, Z.; Wang, C. Tumor-associated macrophages (TAMs)-derived osteopontin (OPN) upregulates PD-L1 expression and predicts poor prognosis in non-small cell lung cancer (NSCLC). Thorac. Cancer 2021, 12, 2698–2709. [Google Scholar] [CrossRef]
- Li, Y.; Sun, B.S.; Pei, B.; Li, C.G.; Zhang, Z.F.; Yin, Y.S.; Wang, C.L. Osteopontin-Expressing Macrophages in Non-Small Cell Lung Cancer Predict Survival. Ann. Thorac. Surg. 2015, 99, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, W.; Chen, Z.; Xiang, C. Upregulation of PD-L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma. Exp. Cell Res. 2017, 359, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, T.; Matsuda, K.; Uchibori, T.; Sugano, M.; Uehara, T.; Honda, T. Upregulation of osteopontin expression via the interaction of macrophages and fibroblasts under IL-1b stimulation. Cytokine 2018, 110, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yoshida, N.; Yamamoto, T.; Isobe, S.; Moriyama, H.; Goto, S.; Kitakata, H.; et al. IL (Interleukin)-10-STAT3-Galection-3 Axis Is Essential for Osteopontin-Producing Reparative Macrophage Polarization after Myocardial Infarction. Circulation 2018, 138, 2021–2035. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, K.; Morine, Y.; Miyazaki, K.; Yamada, S.; Saito, Y.; Nishi, M.; Tokunaga, T.; Ikemoto, T.; Imura, S.; Shimada, M. The interaction between cancer associated fibroblasts and tumor associated macrophages via the osteopontin pathway in the microenvironment of hepatocellular carcinoma. Oncotarget 2021, 12, 333–343. [Google Scholar] [CrossRef]
- Ozato, Y.; Kojima, Y.; Kobayashi, Y.; Hisamatsu, Y.; Toshima, T.; Yonemura, Y.; Masuda, T.; Kagawa, K.; Goto, Y.; Utou, M.; et al. Spatial and single-cell transcriptomics decipher the cellular environment containing HLA-G+ cancer cells and SPP1+ macrophages in colorectal cancer. Cell Res. 2023, 42, 111929. [Google Scholar] [CrossRef]
- Sathe, A.; Mason, K.; Grimes, S.M.; Zhou, Z.; Lau, B.T.; Bai, X.; Su, A.; Tan, X.; Lee, H.; Suarez, C.J.; et al. Colorectal Cancer Metastases in the Liver Establish Immunosuppressive Spatial Networking between Tumor-Associated SPP1+ Macrophages and Fibroblasts. Clin. Cancer Res. 2023, 29, 244–260. [Google Scholar] [CrossRef]
- Casanova-Acebes, M.; Dalla, E.; Leader, A.M.; Leberichel, J.; Nikolic, J.; Morales, B.M.; Brown, M.; Chang, C.; Troncoso, L.; Chen, S.T.; et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature 2021, 595, 578–584. [Google Scholar] [CrossRef]
- Sheng, W.; Yang, F.; Zhou, Y.; Yang, H.; Low, P.Y.; Kemeny, D.M.; Tan, P.; Moh, A.; Kaplan, M.H.; Zhang, Y.; et al. STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res. 2014, 24, 1387–1402. [Google Scholar] [CrossRef] [Green Version]
- Shinchi, Y.; Komohara, Y.; Yonemitsu, K.; Sato, K.; Ohnishi, K.; Saito, Y.; Fujiwara, Y.; Mori, T.; Shiraishi, K.; Ikeda, K.; et al. Accurate expression of PD-L1/L2 in lung adenocarcinoma cells: A retrospective study by double immunohistochemistry. Cancer Sci. 2019, 110, 2711–2721. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, S.; Baghdadi, M.; Tsuchikawa, T.; Wada, H.; Nakamura, T.; Abe, H.; Nakanishi, S.; Usui, Y.; Higuchi, K.; Takahashi, M.; et al. Chemotherapy-Derived Inflammatory Responses Accelerate the Formation of Immunosuppressive Myeloid Cells in the Tissue Microenvironment of Human Pancreatic Cancer. Cancer Res. 2015, 75, 2629–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Hou, J.; Wang, L.; Fu, H.; Zhang, Y.; Song, Y.; Wang, X. Regulatory roles of osteopontin in human lung cancer cell epithelial-to-mesenchymal transitions and responses. Clin. Transl. Med. 2021, 11, e486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, M.; Chen, J.; Wang, Z.; Du, X.; Liu, P.; Li, W. Osteopontin knockdown inhibits αv, β3 integrin-Induced cell migration and invasion and promotes apoptosis of breast cancer cells by inducing autophagy and inactivating the PI3K/Akt/mTOR pathway. Cell Physiol. Biochem. 2014, 33, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; LeSavage, B.L.; Hubka, K.M.; Ma, C.; Natarajan, S.; Eggold, J.T.; Xiao, Y.; Fuh, K.C.; Krishnan, V.; Enejder, A.; et al. Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling. J. Clin. Investig. 2021, 131, e146186. [Google Scholar] [CrossRef]
- Nakamura, T.; Shinriki, S.; Jono, H.; Ueda, M.; Nagata, M.; Guo, J.; Hayashi, M.; Yoshida, R.; Ota, T.; Ota, K.; et al. Osteopontin-integrin αv β3 axis is crucial for 5-fluorouracil resistance in oral squamous cell carcinoma. FEBS Lett. 2015, 89, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Ostheimer, C.; Bache, M.; Güttler, A.; Reese, T.; Vordermark, D. Prognostic information of serial plasma osteopontin measurement in radiotherapy of non-small-cell lung cancer. BMC Cancer 2014, 14, 858. [Google Scholar] [CrossRef] [Green Version]
- Moorman, H.R.; Poschel, D.; Klement, J.D.; Lu, C.; Redd, P.S.; Liu, K. Osteopontin: A Key Regulator of Tumor Progression and Immunomodulation. Cancers 2020, 12, 3379. [Google Scholar] [CrossRef]
- Hamilton, J.A. GM-CSF in inflammation. J. Exp. Med. 2020, 217, e20190945. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Nobs, S.P.; Kurrer, M.; Rehrauer, H.; Thiele, C.; Kopf, M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014, 15, 1026–1037. [Google Scholar] [CrossRef]
- McCarthy, C.; Lee, E.; Bridges, J.P.; Sallese, A.; Suzuki, T.; Woods, J.C.; Bartholmai, B.J.; Wang, T.; Chalk, C.; Carey, B.C.; et al. Statin as a novel pharmacotherapy of pulmonary alveolar proteinosis. Nat. Commun. 2018, 9, 3127. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.C.; Saurigny, D.; Vencovsky, J.; Takeuchi, T.; Nakamura, T.; Matsievskaia, G.; Hunt, B.; Wagner, T.; Souberbielle, B. Efficacy and safety of namilumab, a human monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an inadequate response or intolerance to an anti-TNF (tumour necrosis factor) biologic therapy: A randomized, controlled trial. Arthritis Res. Ther. 2019, 21, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.L.; Shen, K.Y.; Tien, C.Y.; Chen, Y.A.; Liu, S.J. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy 2017, 9, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Korc, M.; Kamath, S.D.; Munshi, H.G.; Rana, A. Trials and tribulations of pancreatic cancer immunotherapy. Cancer Lett. 2021, 504, 1–14. [Google Scholar] [CrossRef]
- Kumar, A.; Khani, A.T.; Ortiz, A.S.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef] [PubMed]
- Yonemitsu, K.; Pan, C.; Fujiwara, Y.; Miyasato, Y.; Shiota, T.; Yano, H.; Hosaka, S.; Tamada, K.; Yamamoto, Y.; Komohara, Y. GM-CSF derived from the inflammatory microenvironment potentially enhanced PD-L1 expression on tumor-associated macrophages in human breast cancer. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Klement, J.D.; Paschall, A.V.; Redd, P.S.; Ibrahim, M.L.; Lu, C.; Yang, D.; Celis, E.; Abrams, S.; Ozato, K.; Liu, K. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J. Clin. Investig. 2018, 28, 5549–5560. [Google Scholar] [CrossRef] [Green Version]
- Sangaletti, S.; Tripodo, C.; Sandri, S.; Torselli, I.; Vitali, C.; Ratti, C.; Botti, L.; Burocchi, A.; Porcasi, R.; Tomirotti, A.; et al. Osteopontin Shapes Immunosupression in the Metastatic Niche. Cancer Res. 2014, 74, 4706–4719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; Sun, H.; Zhang, Y.; Wang, Z.; Xun, Z.; Li, Z.; Ding, X.; Bao, R.; Hong, L.; Jia, W.; et al. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer. Nat. Commun. 2022, 13, 1742. [Google Scholar] [CrossRef]
- Suzuki, J.; Tsuboi, M.; Ishii, G. Cancer-associated fibroblasts and the tumor microenvironment in non-small cell lung cancer. Expert Rev. Anticancer Ther. 2022, 22, 169–182. [Google Scholar] [CrossRef]
- Komohara, Y.; Takeya, M. CAFs and TAMs: Maestros of the tumour microenvironment. J. Pathol. 2017, 241, 313–315. [Google Scholar] [CrossRef] [Green Version]
- Komohara, Y.; Kurotaki, D.; Tsukamoto, H.; Miyasato, Y.; Yano, H.; Pan, C.; Yamamoto, Y.; Fujiwara, Y. Involvement of protumor macrophages in breast cancer progression and characterization of macrophage phenotypes. Cancer Sci. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Liu, Y.; Xun, Z.; Ma, K.; Liang, S.; Li, X.; Zhou, S.; Sun, L.; Liu, Y.; Du, Y.; Guo, X.; et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J. Hepatol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Xie, W.; Cheng, J.; Hong, Z.; Cai, W.; Zhuo, H.; Hou, J.; Lin, L.; Wei, X.; Wang, K.; Chen, X.; et al. Multi-Transcriptomic Analysis Reveals the Heterogeneity and Tumor-Promoting Role of SPP1/CD44--Mediated Intratumoral Crosstalk in Gastric Cancer. Cancers 2022, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Sheng, L.; Pan, D.; Jiang, S.; Ding, L.; Ma, X.; Liu, Y.; Jia, D. Single-Cell Transcriptomic Analysis Revealed a Critical Role of SPP1/CD44-Mediated Crosstalk Between Macrophages and Cancer Cells in Glioma. Front. Cell Dev. Biol. 2021, 9, 779319. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, R.; Deng, J.; Dai, X.; Zhu, X.; Fu, Q.; Zhang, H.; Tong, Z.; Zhao, P.; Fang, W.; et al. Construction of TME and Identification of crosstalk between malignant cells and macrophages by SPP1 in hepatocellular carcinoma. Cancer Immunol. Immunother. 2022, 71, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Airoldi, I. HLA-G and Other Immune Checkpoint Molecules as Targets for Novel Combined Immunotherapies. Int. J. Mol. Sci. 2022, 23, 2925. [Google Scholar] [CrossRef]
- Nakajima, T.; Uehara, T.; Iwaya, M.; Matsuda, K.; Wada, M.; Nagaya, T.; Ehara, T.; Ota, H. Osteopontin expression in the invasive front stroma of colorectal adenocarcimoma is associated with tumor budding and prognosis. Pathol. Res. Pract. 2022, 240, 154190. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Z.; Li, B.; Li, J.; Ou, Y.; Yu, X.; Zhang, Z.; Liu, S.; Fu, X.; Jin, H.; et al. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. Oncoimmunology 2022, 11, 2085432. [Google Scholar] [CrossRef]
- Yang, S.; Qian, L.; Li, Z.; Li, Y.; Bai, J.; Zheng, B.; Chen, K.; Qiu, X.; Cai, G.; Wang, S.; et al. Integrated Multi-Omics Landscape of Liver Metastases. Gastroenterology 2022, 164, 407–423. [Google Scholar] [CrossRef]
- Luo, H.; Xia, X.; Huang, L.B.; An, H.; Cao, M.; Kim, G.D.; Chen, H.N.; Zhang, W.H.; Shu, Y.; Kong, X.; et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 2022, 13, 6619. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Q.; Liu, X.; Wang, F.; Ma, Y.; Zhang, S.; Shao, Z.; Yang, Y.; Tian, X. Single Cell RNA-Seq Identifies Immune-Related Prognostic Model and Key Signature-SPP1 in Pancreatic Ductal Adenocarcinoma. Genes 2022, 13, 1760. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubara, E.; Yano, H.; Pan, C.; Komohara, Y.; Fujiwara, Y.; Zhao, S.; Shinchi, Y.; Kurotaki, D.; Suzuki, M. The Significance of SPP1 in Lung Cancers and Its Impact as a Marker for Protumor Tumor-Associated Macrophages. Cancers 2023, 15, 2250. https://doi.org/10.3390/cancers15082250
Matsubara E, Yano H, Pan C, Komohara Y, Fujiwara Y, Zhao S, Shinchi Y, Kurotaki D, Suzuki M. The Significance of SPP1 in Lung Cancers and Its Impact as a Marker for Protumor Tumor-Associated Macrophages. Cancers. 2023; 15(8):2250. https://doi.org/10.3390/cancers15082250
Chicago/Turabian StyleMatsubara, Eri, Hiromu Yano, Cheng Pan, Yoshihiro Komohara, Yukio Fujiwara, Shukang Zhao, Yusuke Shinchi, Daisuke Kurotaki, and Makoto Suzuki. 2023. "The Significance of SPP1 in Lung Cancers and Its Impact as a Marker for Protumor Tumor-Associated Macrophages" Cancers 15, no. 8: 2250. https://doi.org/10.3390/cancers15082250
APA StyleMatsubara, E., Yano, H., Pan, C., Komohara, Y., Fujiwara, Y., Zhao, S., Shinchi, Y., Kurotaki, D., & Suzuki, M. (2023). The Significance of SPP1 in Lung Cancers and Its Impact as a Marker for Protumor Tumor-Associated Macrophages. Cancers, 15(8), 2250. https://doi.org/10.3390/cancers15082250





