Safety of Early Bevacizumab Administration after Central Venous Port Placement for Patients with Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
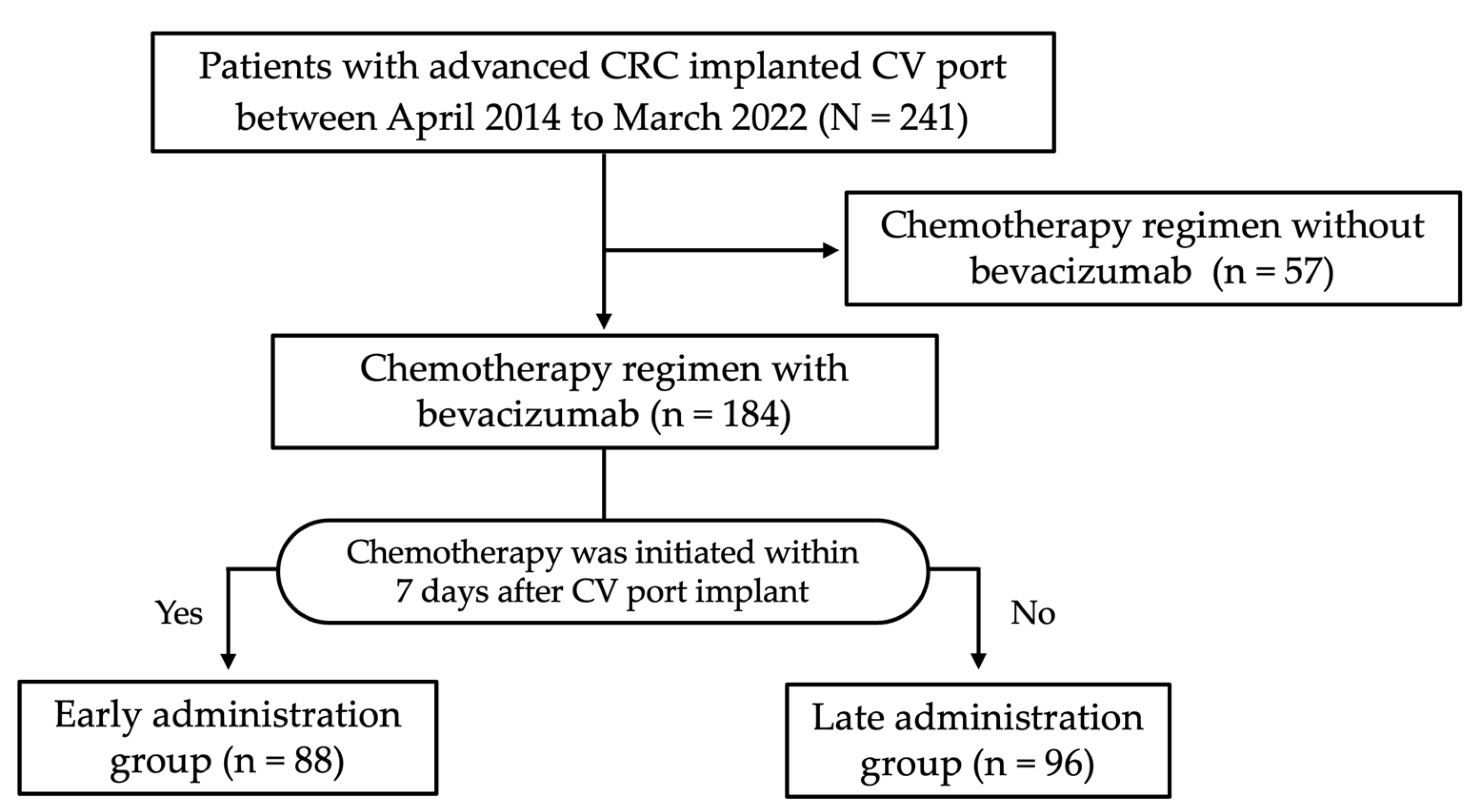
2.1. Study Design and Patients
2.2. Data Collection
2.3. CV Port Placement
2.4. Complications and Event-Free Survival
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. CV Port Characteristics and Outcomes
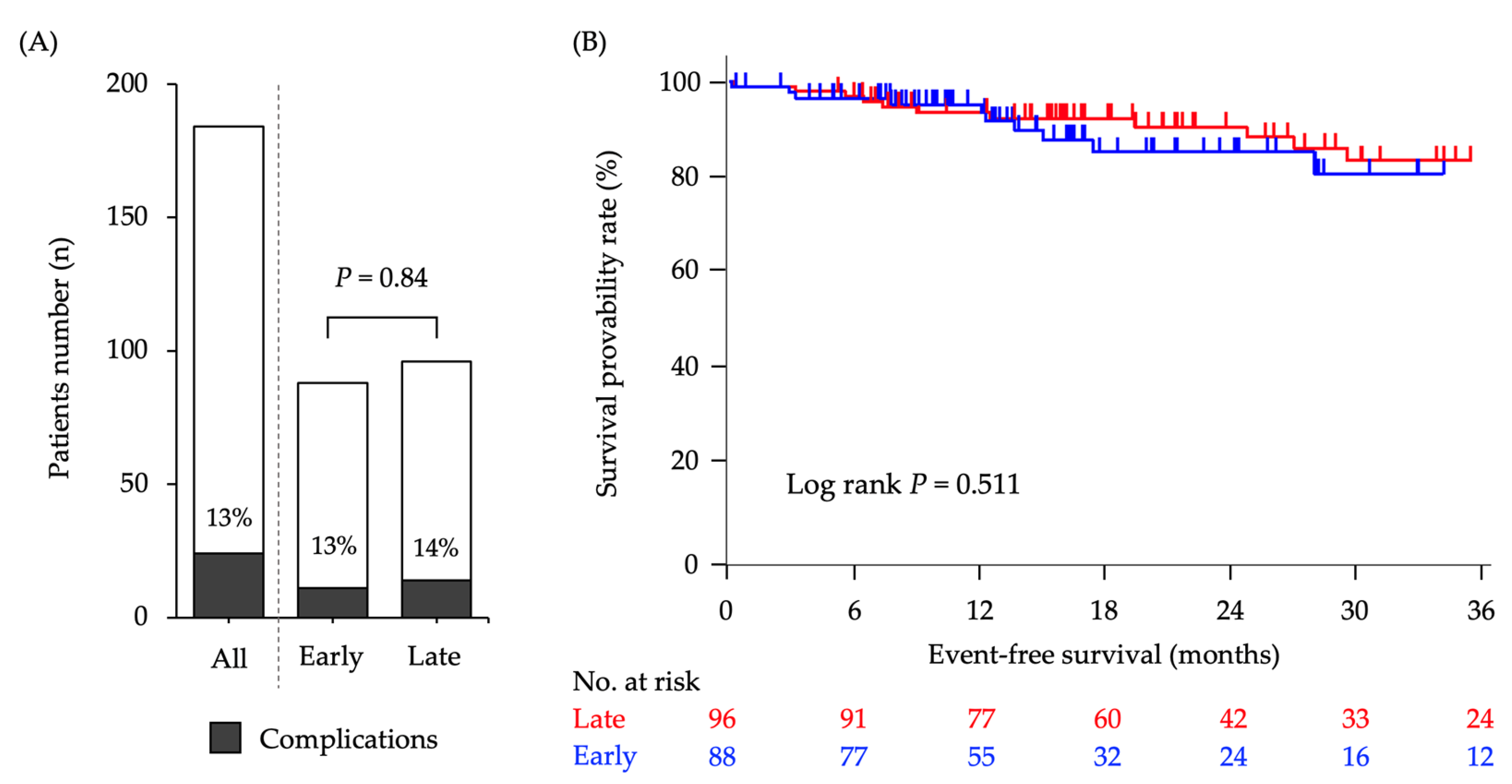
3.3. Complications during or after CV Port Implantation
3.4. Propensity Score Matching Analysis and Risk Factors of Complications
3.5. Comparision of Complication Rates with/without BEV-Containing Chemotherapy Regimen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Yamazaki, K.; Nagase, M.; Tamagawa, H.; Ueda, S.; Tamura, T.; Murata, K.; Eguchi-Nakajima, T.; Baba, E.; Tsuda, M.; Moriwaki, T.; et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann. Oncol. 2016, 27, 1539–1546. [Google Scholar] [CrossRef]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef]
- Niederhuber, J.E.; Ensminger, W.; Gyves, J.W.; Liepman, M.; Doan, K.; Cozzi, E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery 1982, 92, 706–712. [Google Scholar]
- Teichgräber, U.K.; Kausche, S.; Nagel, S.N.; Gebauer, B. Outcome analysis in 3,160 implantations of radiologically guided placements of totally implantable central venous port systems. Eur. Radiol. 2011, 21, 1224–1232. [Google Scholar] [CrossRef]
- Biffi, R.; De Braud, F.; Orsi, F.; Pozzi, S.; Arnaldi, P.; Goldhirsch, A.; Rotmensz, N.; Robertson, C.; Bellomi, M.; Andreoni, B. A randomized, prospective trial of central venous ports connected to standard open-ended or Groshong catheters in adult oncology patients. Cancer 2001, 92, 1204–1212. [Google Scholar] [CrossRef]
- Saif, M.W.; Mehra, R. Incidence and management of bevacizumab-related toxicities in colorectal cancer. Expert Opin. Drug Saf. 2006, 5, 553–566. [Google Scholar] [CrossRef]
- Zawacki, W.J.; Walker, T.G.; DeVasher, E.; Halpern, E.F.; Waltman, A.C.; Wicky, S.T.; Ryan, D.P.; Kalva, S.P. Wound dehiscence or failure to heal following venous access port placement in patients receiving bevacizumab therapy. J. Vasc. Interv. Radiol. 2009, 20, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Erinjeri, J.P.; Fong, A.J.; Kemeny, N.E.; Brown, K.T.; Getrajdman, G.I.; Solomon, S.B. Timing of administration of bevacizumab chemotherapy affects wound healing after chest wall port placement. Cancer 2011, 117, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Grenader, T.; Goldberg, A.; Verstandig, A.; Shavit, L. Indwelling central venous access port insertion during bevacizumab-based therapy. Anti Cancer Drugs 2010, 21, 704–707. [Google Scholar] [CrossRef]
- Japanese Society of Interventional Radiology. Off. J. Jpn. Soc. Interv. Radiol. 2021, 35, 359–397. [CrossRef]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese clinical practice guideline for diabetes 2019. J. Diabetes Investig. 2020, 11, 1020–1076. [Google Scholar] [CrossRef]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef]
- Lipsy, R.J. The National Cholesterol Education Program Adult Treatment Panel III guidelines. J. Manag. Care Pharm. 2003, 9, 2–5. [Google Scholar] [CrossRef]
- De Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef]
- Maindrault-Goebel, F.; Louvet, C.; André, T.; Carola, E.; Lotz, J.P.; Molitor, J.L.; Garcia, M.L.; Gilles-Amar, V.; Izrael, V.; Krulik, M.; et al. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur. J. Cancer 1999, 35, 1338–1342. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Cunningham, D.; Roth, A.D.; Navarro, M.; James, R.D.; Karasek, P.; Jandik, P.; Iveson, T.; Carmichael, J.; Alakl, M.; et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 2000, 355, 1041–1047. [Google Scholar] [CrossRef]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Shiono, M.; Takahashi, S.; Takahashi, M.; Yamaguchi, T.; Ishioka, C. Current situation regarding central venous port implantation procedures and complications: A questionnaire-based survey of 11,693 implantations in Japan. Int. J. Clin. Oncol. 2016, 21, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, S.; Goto, Y.; Miyake, H.; Nagai, H.; Yoshioka, Y.; Yuasa, N.; Takamizawa, J. Late complications associated with totally implantable venous access port implantation via the internal jugular vein. Support. Care Cancer 2020, 28, 2761–2768. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qian, S.; He, W.; Han, G.; Li, H.; Luo, R. Implanting totally implantable venous access port via the internal jugular vein guided by ultrasonography is feasible and safe in patients with breast cancer. World J. Surg. Oncol. 2014, 12, 378. [Google Scholar] [CrossRef]
- Charvát, J.; Linke, Z.; Horáèková, M.; Prausová, J. Implantation of central venous ports with catheter insertion via the right internal jugular vein in oncology patients: Single center experience. Support. Care Cancer 2006, 14, 1162–1165. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Monfardini, L.; Sozzi, G.; Armano, G.; Butera, D.; Scarpelli, E.; Barresi, G.; Benegiamo, A.; Berretta, R. Peripherally Inserted Central venous Catheters (PICC) versus totally implantable venous access device (PORT) for chemotherapy administration: A meta-analysis on gynecological cancer patients. Acta Biomed. 2021, 92, e2021257. [Google Scholar] [CrossRef]
- Dariushnia, S.R.; Wallace, M.J.; Siddiqi, N.H.; Towbin, R.B.; Wojak, J.C.; Kundu, S.; Cardella, J.F.; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for central venous access. J. Vasc. Interv. Radiol. 2010, 21, 976–981. [Google Scholar] [CrossRef]
- Kazazi-Hyseni, F.; Beijnen, J.H.; Schellens, J.H. Bevacizumab. Oncologist 2010, 15, 819–825. [Google Scholar] [CrossRef]
- Tanaka, T.; Suzuki, H.; Ushijima, T.; Nagasu, S.; Akagi, Y.; Kawaguchi, T.; Miwa, K. Case report: Changes in serum bevacizumab concentration in a hemodialysis patient with unresectable colorectal cancer treated with FOLFIRI plus bevacizumab. Front. Oncol. 2022, 12, 947013. [Google Scholar] [CrossRef]
- Biffi, R.; Toro, A.; Pozzi, S.; Di Carlo, I. Totally implantable vascular access devices 30 years after the first procedure. What has changed and what is still unsolved? Support. Care Cancer 2014, 22, 1705–1714. [Google Scholar] [CrossRef]
- Bonciarelli, G.; Batacchi, S.; Biffi, R.; Buononato, M.; Damascelli, B.; Ghibaudo, F.; Orsi, F.; Pittiruti, M.; Scoppettuolo, G.; Verzè, A.; et al. GAVeCeLT* consensus statement on the correct use of totally implantable venous access devices for diagnostic radiology procedures. J. Vasc. Access 2011, 12, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Wolosker, N.; Bernardi, C.V.; Yazbek, G. Totally implantable ports connected to valved catheters for chemotherapy: Experience from 350 Groshong devices. J. Vasc. Access 2010, 11, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Practice guidelines for central venous access 2020: An updated report by the American society of anesthesiologists task force on central venous access. Anesthesiology 2020, 132, 8–43. [CrossRef]
- Schreckenbach, T.; Münch, I.; El Youzouri, H.; Bechstein, W.O.; Habbe, N. The safety level of total central venous access port implantation performed by residents. J. Surg. Educ. 2019, 76, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Weng, H.H.; Huang, W.S.; Wang, W.K.; Kao, C.L.; Lu, M.S.; Wang, C.S. Analysis of risk factors for central venous port failure in cancer patients. World J. Gastroenterol. 2009, 15, 4709–4714. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.C.; Cutsem, E.V.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef]
- Cutsem, E.V.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef]
| Characteristics | Chemotherapy Regimen with BEV (n = 184) | Early Administration Group (n = 88) | Late Administration Group (n = 96) | p |
|---|---|---|---|---|
| Age, years | 67 [32–85] | 68 [34–85] | 66 [32–84] | 0.018 |
| Sex; female | 96 (52) | 46 (52) | 42 (44) | 0.25 |
| Male | 88 (48) | 42 (48) | 54 (56) | |
| Eastern Oncology Group Performance Status score | 0.77 | |||
| 0 | 106 (58) | 53 (60) | 53 (55) | |
| 1 | 66 (36) | 30 (34) | 35 (38) | |
| 2 | 12 (6) | 5 (6) | 7 (7) | |
| Tumor site | 0.03 | |||
| Colon | 117 (64) | 63 (72) | 54 (56) | |
| Rectum | 67 (34) | 25 (28) | 42 (44) | |
| Hypertension | 0.22 | |||
| Yes | 39 (21) | 22 (25) | 17 (18) | |
| No | 145 (79) | 66 (75) | 79 (82) | |
| Hyperlipidemia | 0.20 | |||
| Yes | 14 (8) | 9 (10) | 5 (5) | |
| No | 170 (93) | 79 (90) | 91 (95) | |
| Chronic kidney disease | 0.37 | |||
| Yes | 9 (5) | 3 (3) | 6 (6) | |
| No | 175 (95) | 85 (97) | 90 (94) | |
| Diabetes | 0.71 | |||
| Yes | 19 (10) | 10 (11) | 9 (10) | |
| No | 165 (90) | 78 (89) | 87 (90) | |
| Anticoagulant medication | 0.66 | |||
| Yes | 29 (16) | 10 (11) | 19 (20) | |
| No | 155 (84) | 78 (89) | 77 (80) | |
| Body mass index | 21.5 [16–42] | 21.5 [16–29] | 21.4 [16–42] | 0.72 |
| Characteristics | All (n = 184) | Early Administration Group (n = 88) | Late Administration Group (n = 96) | p |
|---|---|---|---|---|
| Insertion vein Right internal jugular Left internal jugular Right subclavian | 169 (92) 11 (6) 4 (2) | 83 (94) 5 (6) 0 (0) | 86 (90) 6 (6) 4 (4) | 0.04 |
| Procedure time (minutes) | 40 [16–65] | 39.5 [16–60] | 40 [21–65] | 0.10 |
| Catheter indwelling period (days) | 541 [2–3014] | 441.5 [2–2163] | 641 [5–3014] | <0.01 |
| Device type PowerPort® MRI PowerPort® ClearVUE® isp Safe Guide™ Micro Needle Port | 129 (70) 32 (17) 23 (13) | 67 (76) 15 (17) 6 (7) | 62 (64) 17 (18) 17 (18) | 0.66 |
| Subjects | All (n = 184) | Early Administration Group (n = 88) | Late Administration Group (n = 96) | p |
|---|---|---|---|---|
| Early complications (within 30 days) * | 2 (1.1) | 1 (1.1) | 1 (1.0) | 1.00 |
| Time to complication onset (days) | 367.5 [2–1467] | 364 [2–1467] | 371 [3–1349] | 0.62 |
| Catheter/port system-related complications | ||||
| Infection | 6 (3.3) | 3 (3.4) | 3 (3.1) | 1.00 |
| Catheter damage | 5 (2.7) | 0 (0) | 5 (5.2) | 0.06 |
| Catheter migration | 1 (0.5) | 1 (1.1) | 0 (0) | 0.48 |
| Catheter embolization | 2 (1.1) | 0 (0) | 2 (2.1) | 0.50 |
| Port site skin damage | 10 (5.4) | 7 (8.0) | 3 (3.1) | 0.20 |
| Bevacizumab-related complications | ||||
| Subcutaneous hematoma | 0 | 0 | 0 | 1.00 |
| Erosions or ulcers | 0 | 0 | 0 | 1.00 |
| Wound dehiscence | 0 | 0 | 0 | 1.00 |
| Delayed wound healing | 0 | 0 | 0 | 1.00 |
| GI perforation | 0 | 0 | 0 | 1.00 |
| Fistula | 0 | 0 | 0 | 1.00 |
| Method | n | Odds Ratio | 95% CI | p |
|---|---|---|---|---|
| Unadjusted | 184 | 0.912 | 0.386–2.157 | 0.834 |
| Matching | 138 | 1.185 | 0.377–3.727 | 0.771 |
| IPTW | 184 | 0.822 | 0.442–1.530 | 0.537 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigyo, H.; Suzuki, H.; Tanaka, T.; Moriyama, E.; Shimotsuura, Y.; Nagasu, S.; Iwamoto, H.; Akagi, Y.; Murotani, K.; Kawaguchi, T.; et al. Safety of Early Bevacizumab Administration after Central Venous Port Placement for Patients with Colorectal Cancer. Cancers 2023, 15, 2264. https://doi.org/10.3390/cancers15082264
Shigyo H, Suzuki H, Tanaka T, Moriyama E, Shimotsuura Y, Nagasu S, Iwamoto H, Akagi Y, Murotani K, Kawaguchi T, et al. Safety of Early Bevacizumab Administration after Central Venous Port Placement for Patients with Colorectal Cancer. Cancers. 2023; 15(8):2264. https://doi.org/10.3390/cancers15082264
Chicago/Turabian StyleShigyo, Hirona, Hiroyuki Suzuki, Toshimitsu Tanaka, Etsuko Moriyama, Yasutaka Shimotsuura, Sachiko Nagasu, Hideki Iwamoto, Yoshito Akagi, Kenta Murotani, Takumi Kawaguchi, and et al. 2023. "Safety of Early Bevacizumab Administration after Central Venous Port Placement for Patients with Colorectal Cancer" Cancers 15, no. 8: 2264. https://doi.org/10.3390/cancers15082264
APA StyleShigyo, H., Suzuki, H., Tanaka, T., Moriyama, E., Shimotsuura, Y., Nagasu, S., Iwamoto, H., Akagi, Y., Murotani, K., Kawaguchi, T., & Miwa, K. (2023). Safety of Early Bevacizumab Administration after Central Venous Port Placement for Patients with Colorectal Cancer. Cancers, 15(8), 2264. https://doi.org/10.3390/cancers15082264







