Comedications with Immune Checkpoint Inhibitors: Involvement of the Microbiota, Impact on Efficacy and Practical Implications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methodology of Literature Search and Selection
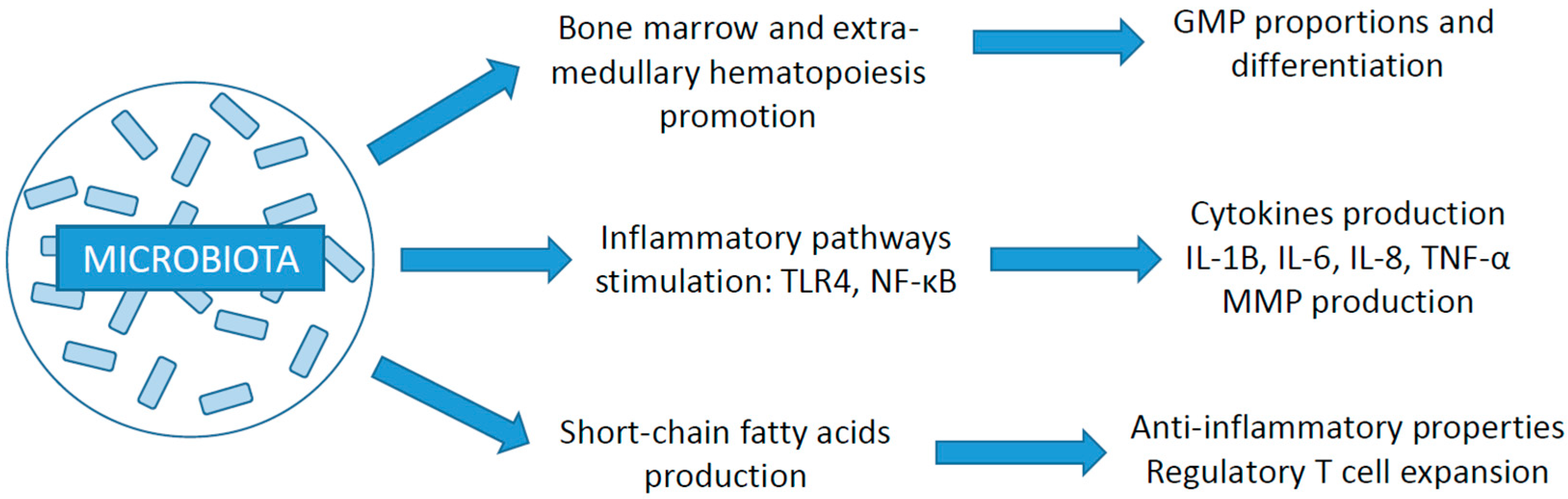
3. The Microbiota and the Immune System
3.1. Microbiota: Definition and Composition
3.2. Relation with the Immune System
3.3. Impact of Gut Microbiota on Cancer Development and ICI Efficacy
3.4. Focus on Akkermansia Muciniphila
3.5. Role of Other Organ-Specific Microbiomes
3.6. Treatment-Induced Microbiome Changes
4. Corticosteroids
4.1. Impact of Immunosuppression on Cancer Development and Microbiota Composition
4.2. Effect on ICI Treatment Efficacy
5. Antibiotics
5.1. Antibiotic-Induced Perturbations of the Microbiota
5.2. Impact of Antibiotics on ICI Response according to Histology
5.3. Specificities under Immunotherapy versus Chemotherapy
5.4. Importance of the Antibiotic Treatment Modality: Timing and Duration
5.5. Importance of the Antibiotic Treatment Modality: Molecule and Spectrum
6. Proton Pump Inhibitors
6.1. PPI-Induced Alterations of the Microbiota
6.2. Impact on ICI Efficacy
6.3. Differences in Histology
6.4. Importance of Timing
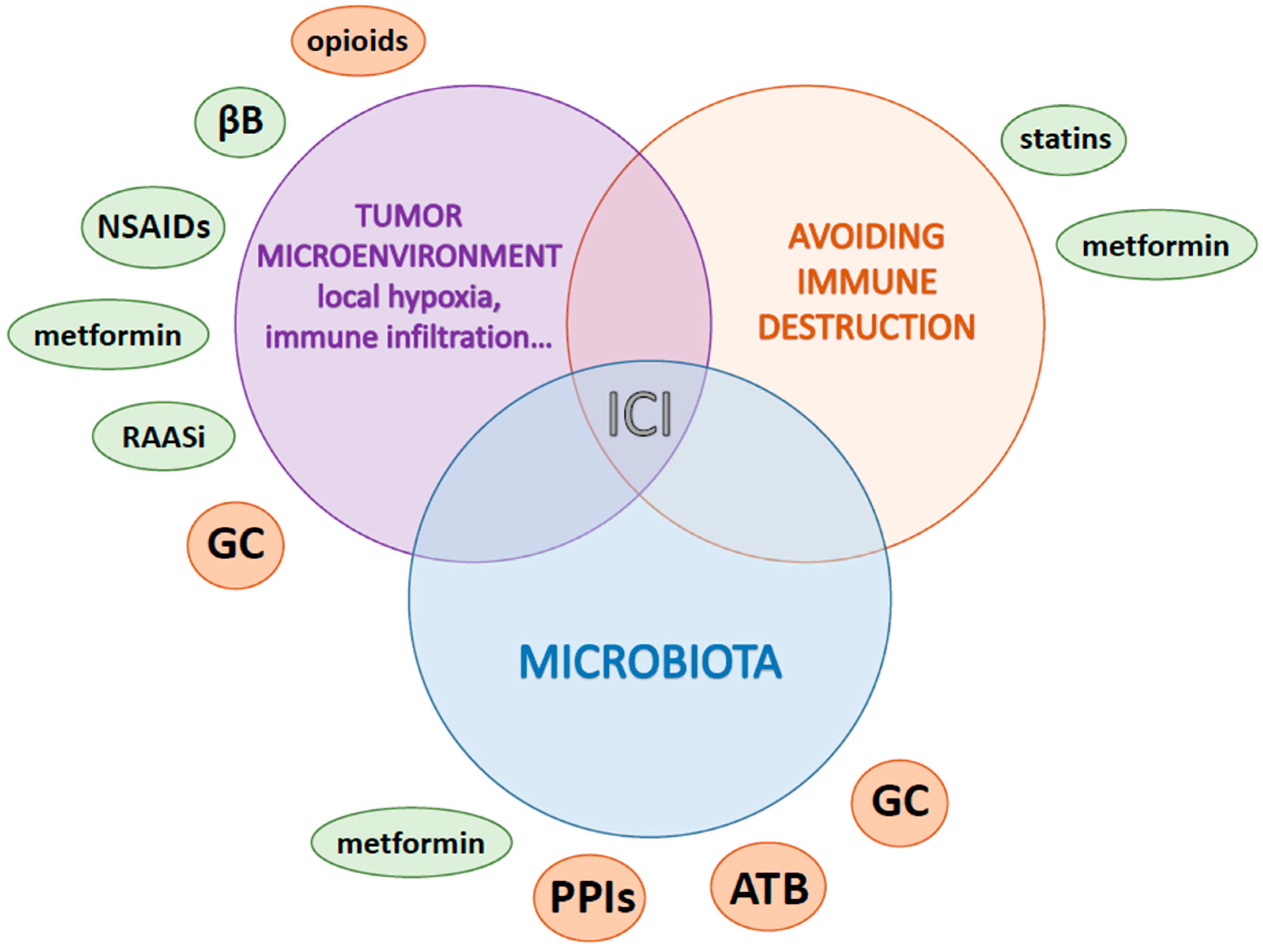
7. Other Medications according to Pathway Alterations
7.1. Metabolism and Hypoxia Lowering: Metformin
7.2. Local Inflammation: Aspirin and Nonsteroidal Anti-Inflammatory Drugs
7.3. Stress and Neuro-Oncology: Beta Blockers
7.4. Microenvironment Remodeling and Immune Modulation
7.4.1. Renin-Angiotensin-Aldosterone System Inhibitors
7.4.2. Opioids
7.4.3. Statins
8. Discussion
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune Checkpoint Inhibitors in Melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. In Regulation of Cancer Immune Checkpoints; Xu, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1248, pp. 33–59. ISBN 9789811532658. [Google Scholar]
- Kalfeist, L.; Galland, L.; Ledys, F.; Ghiringhelli, F.; Limagne, E.; Ladoire, S. Impact of Glucocorticoid Use in Oncology in the Immunotherapy Era. Cells 2022, 11, 770. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between Drugs and the Gut Microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Gaucher, L.; Adda, L.; Séjourné, A.; Joachim, C.; Guillaume, C.; Poulet, C.; Liabeuf, S.; Gras-Champel, V.; Masmoudi, K.; Houessinon, A.; et al. Associations between Dysbiosis-Inducing Drugs, Overall Survival and Tumor Response in Patients Treated with Immune Checkpoint Inhibitors. Ther. Adv. Med. Oncol. 2021, 13, 175883592110005. [Google Scholar] [CrossRef]
- D’Argenio, V.; Salvatore, F. The Role of the Gut Microbiome in the Healthy Adult Status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef] [Green Version]
- MetaHIT Consortium (Additional Members); Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut Microbiota and IBD: Causation or Correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Pavel, F.M.; Vesa, C.M.; Gheorghe, G.; Diaconu, C.C.; Stoicescu, M.; Munteanu, M.A.; Babes, E.E.; Tit, D.M.; Toma, M.M.; Bungau, S. Highlighting the Relevance of Gut Microbiota Manipulation in Inflammatory Bowel Disease. Diagnostics 2021, 11, 1090. [Google Scholar] [CrossRef]
- Imdad, A.; Nicholson, M.R.; Tanner-Smith, E.E.; Zackular, J.P.; Gomez-Duarte, O.G.; Beaulieu, D.B.; Acra, S. Fecal Transplantation for Treatment of Inflammatory Bowel Disease. Cochrane Database Syst. Rev. 2018, 2018, CD012774. [Google Scholar] [CrossRef]
- Olivares, M.; Neef, A.; Castillejo, G.; Palma, G.D.; Varea, V.; Capilla, A.; Palau, F.; Nova, E.; Marcos, A.; Polanco, I.; et al. The HLA-DQ2 Genotype Selects for Early Intestinal Microbiota Composition in Infants at High Risk of Developing Coeliac Disease. Gut 2015, 64, 406–417. [Google Scholar] [CrossRef]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.K.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced Diversity of the Intestinal Microbiota during Infancy Is Associated with Increased Risk of Allergic Disease at School Age. J. Allergy Clin. Immunol. 2011, 128, 646–652.e5. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, Inflammation, and the Gut Microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambring, C.B.; Siraj, S.; Patel, K.; Sankpal, U.T.; Mathew, S.; Basha, R. Impact of the Microbiome on the Immune System. Crit. Rev. Immunol. 2019, 39, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Yáñez, A.; Price, J.G.; Chow, A.; Merad, M.; Goodridge, H.S.; Mazmanian, S.K. Gut Microbiota Promote Hematopoiesis to Control Bacterial Infection. Cell Host Microbe 2014, 15, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Tabowei, G.; Gaddipati, G.N.; Mukhtar, M.; Alzubaidee, M.J.; Dwarampudi, R.S.; Mathew, S.; Bichenapally, S.; Khachatryan, V.; Muazzam, A.; Hamal, C.; et al. Microbiota Dysbiosis a Cause of Colorectal Cancer or Not? A Systematic Review. Cureus 2022, 14, e30893. [Google Scholar] [CrossRef]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef]
- Ting, N.L.-N.; Lau, H.C.-H.; Yu, J. Cancer Pharmacomicrobiomics: Targeting Microbiota to Optimise Cancer Therapy Outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti–PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A Defined Commensal Consortium Elicits CD8 T Cells and Anti-Cancer Immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal Microbiota Is Altered in Patients with Colon Cancer and Modified by Probiotic Intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, Y.; Ikeda, T.; Sakata, S.; Saruwatari, K.; Sato, R.; Iyama, S.; Jodai, T.; Akaike, K.; Ishizuka, S.; Saeki, S.; et al. Association of Probiotic Clostridium Butyricum Therapy with Survival and Response to Immune Checkpoint Blockade in Patients with Lung Cancer. Cancer Immunol. Res. 2020, 8, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia Muciniphila Predicts Clinical Response to PD-1 Blockade in Patients with Advanced Non-Small-Cell Lung Cancer. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Desilets, A.; Daillère, R.; Terrisse, S.; Kroemer, G.; Zitvogel, L. Microbiota-Centered Interventions: The Next Breakthrough in Immuno-Oncology? Cancer Discov. 2021, 11, 2396–2412. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia Muciniphila: Paradigm for next-Generation Beneficial Microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Yan, J.; Sheng, L.; Li, H. Akkermansia Muciniphila: Is It the Holy Grail for Ameliorating Metabolic Diseases? Gut Microbes 2021, 13, 1984104. [Google Scholar] [CrossRef]
- Ramírez-Labrada, A.G.; Isla, D.; Artal, A.; Arias, M.; Rezusta, A.; Pardo, J.; Gálvez, E.M. The Influence of Lung Microbiota on Lung Carcinogenesis, Immunity, and Immunotherapy. Trends Cancer 2020, 6, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, J.E.; Albrich, W.C.; Dmitrijeva, M.; Kahlert, C.R. The Effects of Corticosteroids on the Respiratory Microbiome: A Systematic Review. Front. Med. 2021, 8, 588584. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Santoni, M.; Ticinesi, A.; Buti, S. The Urinary Microbiome and Anticancer Immunotherapy: The Potentially Hidden Role of Unculturable Microbes. Target. Oncol. 2019, 14, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Burcher, K.M.; Burcher, J.T.; Inscore, L.; Bloomer, C.H.; Furdui, C.M.; Porosnicu, M. A Review of the Role of Oral Microbiome in the Development, Detection, and Management of Head and Neck Squamous Cell Cancers. Cancers 2022, 14, 4116. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Harrington, K.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Vokes, E.; Gillison, M.; Even, C.; et al. Abstract CT022: Evaluation of Oral Microbiome Profiling as a Response Biomarker in Squamous Cell Carcinoma of the Head and Neck: Analyses from CheckMate 141. Cancer Res. 2017, 77, CT022. [Google Scholar] [CrossRef]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The Influence of a Short-Term Gluten-Free Diet on the Human Gut Microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.A.; Verdi, S.; Maxan, M.-E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.; Williams, F.M.K.; Menni, C.; Bell, J.T.; et al. Gut Microbiota Associations with Common Diseases and Prescription Medications in a Population-Based Cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef] [Green Version]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-Level Analysis of Gut Microbiome Variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Ticinesi, A.; Milani, C.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut Microbiota Composition Is Associated with Polypharmacy in Elderly Hospitalized Patients. Sci. Rep. 2017, 7, 11102. [Google Scholar] [CrossRef] [Green Version]
- Gemikonakli, G.; Mach, J.; Zhang, F.; Bullock, M.; Tran, T.; El-Omar, E.; Hilmer, S.N. Polypharmacy with High Drug Burden Index (DBI) Alters the Gut Microbiome Overriding Aging Effects and Is Reversible with Deprescribing. J. Gerontol. Ser. A 2022, 78, 213–222. [Google Scholar] [CrossRef]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids Use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acuna, S.A. Etiology of Increased Cancer Incidence after Solid Organ Transplantation. Transplant. Rev. 2018, 32, 218–224. [Google Scholar] [CrossRef]
- Guba, M.; Graeb, C.; Jauch, K.-W.; Geissler, E.K. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 2004, 77, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.E.; Anver, M.R.; Schechter, S.L.; Bole, G.G. Prolonged Lifespan and High Incidence of Neoplasms in NZB/NZW Mice Treated with Hydrocortisone Sodium Succinate. Kidney Int. 1978, 14, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.P.; Koh, A.Y. The Gut Microbiota in Transplant Patients. Blood Rev. 2020, 39, 100614. [Google Scholar] [CrossRef]
- Tourret, J.; Willing, B.P.; Dion, S.; MacPherson, J.; Denamur, E.; Finlay, B.B. Immunosuppressive Treatment Alters Secretion of Ileal Antimicrobial Peptides and Gut Microbiota, and Favors Subsequent Colonization by Uropathogenic Escherichia coli. Transplantation 2017, 101, 74–82. [Google Scholar] [CrossRef]
- Wu, T.; Yang, L.; Jiang, J.; Ni, Y.; Zhu, J.; Zheng, X.; Wang, Q.; Lu, X.; Fu, Z. Chronic Glucocorticoid Treatment Induced Circadian Clock Disorder Leads to Lipid Metabolism and Gut Microbiota Alterations in Rats. Life Sci. 2018, 192, 173–182. [Google Scholar] [CrossRef]
- Huang, E.Y.; Inoue, T.; Leone, V.A.; Dalal, S.; Touw, K.; Wang, Y.; Musch, M.W.; Theriault, B.; Higuchi, K.; Donovan, S.; et al. Using Corticosteroids to Reshape the Gut Microbiome: Implications for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2015, 21, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Scott, S.C.; Pennell, N.A. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 1771–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciuti, B.; Dahlberg, S.E.; Adeni, A.; Sholl, L.M.; Nishino, M.; Awad, M.M. Immune Checkpoint Inhibitor Outcomes for Patients with Non–Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J. Clin. Oncol. 2019, 37, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in Patients with Melanoma and Brain Metastases: An Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef]
- Chasset, F.; Pages, C.; Biard, L.; Roux, J.; Sidina, I.; Madelaine, I.; Basset-Seguin, N.; Viguier, M.; Madjlessi-EzrA, N.; Schneider, P.; et al. Single-Center Study under a French Temporary Authorization for Use (TAU) Protocol for Ipilimumab in Metastatic Melanoma: Negative Impact of Baseline Corticosteroids. Eur. J. Dermatol. 2015, 25, 36–44. [Google Scholar] [CrossRef]
- Hendriks, L.E.L.; Henon, C.; Auclin, E.; Mezquita, L.; Ferrara, R.; Audigier-Valette, C.; Mazieres, J.; Lefebvre, C.; Rabeau, A.; Le Moulec, S.; et al. Outcome of Patients with Non–Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors. J. Thorac. Oncol. 2019, 14, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Kaseb, A.; Wang, Y.; Saeed, A.; Szafron, D.; Jun, T.; Dharmapuri, S.; Naqash, A.R.; Muzaffar, M.; Navaid, M.; et al. Impact of Corticosteroid Therapy on the Outcomes of Hepatocellular Carcinoma Treated with Immune Checkpoint Inhibitor Therapy. J. Immunother. Cancer 2020, 8, e000726. [Google Scholar] [CrossRef]
- Umehara, K.; Yama, K.; Goto, K.; Wakamoto, A.; Hatsuyama, T.; Honjo, O.; Saikai, T.; Fujita, A.; Sato, H. Effect of Systemic Corticosteroid Therapy on the Efficacy and Safety of Nivolumab in the Treatment of Non-Small-Cell Lung Cancer. Cancer Control 2021, 28, 107327482098579. [Google Scholar] [CrossRef]
- Gaucher, L.; Adda, L.; Séjourné, A.; Joachim, C.; Chaby, G.; Poulet, C.; Liabeuf, S.; Gras-Champel, V.; Masmoudi, K.; Moreira, A.; et al. Impact of the Corticosteroid Indication and Administration Route on Overall Survival and the Tumor Response after Immune Checkpoint Inhibitor Initiation. Ther. Adv. Med. Oncol. 2021, 13, 175883592199665. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Huang, X.; Li, J.; Ma, H.; Zeng, R. Impact of Corticosteroid Use on Outcomes of Non–Small-cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. J. Clin. Pharm. Ther. 2021, 46, 927–935. [Google Scholar] [CrossRef]
- Jessurun, C.A.C.; Hulsbergen, A.F.C.; de Wit, A.E.; Tewarie, I.A.; Snijders, T.J.; Verhoeff, J.J.C.; Phillips, J.G.; Reardon, D.A.; Mekary, R.A.; Broekman, M.L.D. The Combined Use of Steroids and Immune Checkpoint Inhibitors in Brain Metastasis Patients: A Systematic Review and Meta-Analysis. Neuro-Oncology 2021, 23, 1261–1272. [Google Scholar] [CrossRef]
- Korpela, K.; de Vos, W. Antibiotic Use in Childhood Alters the Gut Microbiota and Predisposes to Overweight. Microb. Cell 2016, 3, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early Infancy Microbial and Metabolic Alterations Affect Risk of Childhood Asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the Gut Microbiota. J. Clin. Investig. 2014, 124, 4212–4218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-Term Antibiotic Treatment Has Differing Long-Term Impacts on the Human Throat and Gut Microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simin, J.; Fornes, R.; Liu, Q.; Olsen, R.S.; Callens, S.; Engstrand, L.; Brusselaers, N. Antibiotic Use and Risk of Colorectal Cancer: A Systematic Review and Dose–Response Meta-Analysis. Br. J. Cancer 2020, 123, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Gagnière, J. Gut Microbiota Imbalance and Colorectal Cancer. World J. Gastroenterol. 2016, 22, 501. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative Association of Antibiotics on Clinical Activity of Immune Checkpoint Inhibitors in Patients with Advanced Renal Cell and Non-Small-Cell Lung Cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment with Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients with Cancer. JAMA Oncol. 2019, 5, 1774. [Google Scholar] [CrossRef]
- Tinsley, N.; Zhou, C.; Tan, G.; Rack, S.; Lorigan, P.; Blackhall, F.; Krebs, M.; Carter, L.; Thistlethwaite, F.; Graham, D.; et al. Cumulative Antibiotic Use Significantly Decreases Efficacy of Checkpoint Inhibitors in Patients with Advanced Cancer. Oncologist 2020, 25, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Cortellini, A.; Di Maio, M.; Nigro, O.; Leonetti, A.; Cortinovis, D.L.; Aerts, J.G.; Guaitoli, G.; Barbieri, F.; Giusti, R.; Ferrara, M.G.; et al. Differential Influence of Antibiotic Therapy and Other Medications on Oncological Outcomes of Patients with Non-Small Cell Lung Cancer Treated with First-Line Pembrolizumab versus Cytotoxic Chemotherapy. J. Immunother. Cancer 2021, 9, e002421. [Google Scholar] [CrossRef]
- Cortellini, A.; Ricciuti, B.; Facchinetti, F.; Alessi, J.V.M.; Venkatraman, D.; Dall’Olio, F.G.; Cravero, P.; Vaz, V.R.; Ottaviani, D.; Majem, M.; et al. Antibiotic-Exposed Patients with Non-Small-Cell Lung Cancer Preserve Efficacy Outcomes Following First-Line Chemo-Immunotherapy. Ann. Oncol. 2021, 32, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Naeem, M.; Pinato, D.J. Concomitant Medications and Immune Checkpoint Inhibitor Therapy for Cancer: Causation or Association? Hum. Vaccines Immunother. 2021, 17, 55–61. [Google Scholar] [CrossRef]
- Lurienne, L.; Cervesi, J.; Duhalde, L.; de Gunzburg, J.; Andremont, A.; Zalcman, G.; Buffet, R.; Bandinelli, P.-A. NSCLC Immunotherapy Efficacy and Antibiotic Use: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2020, 15, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, X.; Wang, H.; Ge, W.; Cao, D. The Association between Antibiotics Use and Outcome of Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2020, 149, 102909. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, J.; Wu, S.; Xie, X. The Impact of Antibiotics on Efficacy of Immune Checkpoint Inhibitors in Malignancies: A Study Based on 44 Cohorts. Int. Immunopharmacol. 2021, 92, 107303. [Google Scholar] [CrossRef]
- Luo, Z.; Hao, S.; Li, Y.; Cheng, L.; Zhou, X.; Gunes, E.G.; Liu, S.; Chen, J. The Negative Effect of Antibiotics on RCC Patients with Immunotherapy: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 1065004. [Google Scholar] [CrossRef]
- Ahmed, J.; Kumar, A.; Parikh, K.; Anwar, A.; Knoll, B.M.; Puccio, C.; Chun, H.; Fanucchi, M.; Lim, S.H. Use of Broad-Spectrum Antibiotics Impacts Outcome in Patients Treated with Immune Checkpoint Inhibitors. OncoImmunology 2018, 7, e1507670. [Google Scholar] [CrossRef] [Green Version]
- Chalabi, M.; Cardona, A.; Nagarkar, D.R.; Dhawahir Scala, A.; Gandara, D.R.; Rittmeyer, A.; Albert, M.L.; Powles, T.; Kok, M.; Herrera, F.G. Efficacy of Chemotherapy and Atezolizumab in Patients with Non-Small-Cell Lung Cancer Receiving Antibiotics and Proton Pump Inhibitors: Pooled Post Hoc Analyses of the OAK and POPLAR Trials. Ann. Oncol. 2020, 31, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Medik, Y.B.; Zhou, Y.; Kahn, L.M.; Patel, B.; Babcock, R.L.; Chrisikos, T.T.; Wan, X.; Dyevoich, A.; Ajami, N.J.; Wargo, J.A.; et al. Outcome of Concurrent Treatment with A-CTLA4 and Metronidazole in Murine Model of Colon Adenocarcinoma. J. Clin. Oncol. 2021, 39, e14566. [Google Scholar] [CrossRef]
- Cortellini, A.; Facchinetti, F.; Derosa, L.; Pinato, D.J. Antibiotic Exposure and Immune Checkpoint Inhibitors in Patients With NSCLC: The Backbone Matters. J. Thorac. Oncol. 2022, 17, 739–741. [Google Scholar] [CrossRef]
- Monge, B.M.C.; Xie, C.; Mabry-Hrones, D.; Wood, B.J.; Steinberg, S.M.; Kleiner, D.E.; Greten, T.F. Phase II Study of Nivolumab (Anti-PD1), Tadalafil, and Oral Vancomycin in Patients with Refractory Primary Hepatocellular Carcinoma or Liver Dominant Metastatic Cancer from Colorectal or Pancreatic Cancers. J. Clin. Oncol. 2020, 38, TPS4656. [Google Scholar] [CrossRef]
- Nehra, A.K.; Alexander, J.A.; Loftus, C.G.; Nehra, V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin. Proc. 2018, 93, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Freedberg, D.E.; Lebwohl, B.; Abrams, J.A. The Impact of Proton Pump Inhibitors on the Human Gastrointestinal Microbiome. Clin. Lab. Med. 2014, 34, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton Pump Inhibitors Alter the Composition of the Gut Microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Leonard, J.; Marshall, J.K.; Moayyedi, P. Systematic Review of the Risk of Enteric Infection in Patients Taking Acid Suppression. Am. J. Gastroenterol. 2007, 102, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Goto, Y.; Sakata, S.; Imamura, K.; Minemura, A.; Oka, K.; Hayashi, A.; Jodai, T.; Akaike, K.; Anai, M.; et al. Clostridium Butyricum Therapy Restores the Decreased Efficacy of Immune Checkpoint Blockade in Lung Cancer Patients Receiving Proton Pump Inhibitors. OncoImmunology 2022, 11, 2081010. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Kichenadasse, G.; Karapetis, C.S.; Rowland, A.; Sorich, M.J. Concomitant Proton Pump Inhibitor Use and Survival in Urothelial Carcinoma Treated with Atezolizumab. Clin. Cancer Res. 2020, 26, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Stokes, W.A.; Behera, M.; Jiang, R.; Gutman, D.; Giuste, F.; Burns, A.; Sebastian, N.; Ramalingam, S.; Sukhatme, V.; Lowe, M.C.; et al. Association of Proton Pump Inhibitors with Survival in Veterans with Non-Small Cell Lung Cancer Receiving Immunotherapy. J. Clin. Oncol. 2021, 39, e18729. [Google Scholar] [CrossRef]
- Baek, Y.; Kang, E.J.; Hong, S.; Park, S.; Kim, J.H.; Shin, J. Survival Outcomes of Patients with Nonsmall Cell Lung Cancer Concomitantly Receiving Proton Pump Inhibitors and Immune Checkpoint Inhibitors. Int. J. Cancer 2022, 150, 1291–1300. [Google Scholar] [CrossRef]
- Peng, K.; Chen, K.; Teply, B.A.; Yee, G.C.; Farazi, P.A.; Lyden, E.R. Impact of Proton Pump Inhibitor Use on the Effectiveness of Immune Checkpoint Inhibitors in Advanced Cancer Patients. Ann. Pharmacother. 2022, 56, 377–386. [Google Scholar] [CrossRef]
- Failing, J.J.; Finnes, H.D.; Kottschade, L.A.; Allred, J.B.; Markovic, S.N. Effects of Commonly Used Chronic Medications on the Outcomes of Ipilimumab Therapy in Patients with Metastatic Melanoma. Melanoma Res. 2016, 26, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeng, C.; Yao, J.; Ge, Y.; An, G. The Association between Proton Pump Inhibitors Use and Clinical Outcome of Patients Receiving Immune Checkpoint Inhibitors Therapy. Int. Immunopharmacol. 2020, 88, 106972. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, H.; Mao, H.; Tong, J.; Yang, M.; Yan, X. An Up-To-Date Investigation Into the Correlation Between Proton Pump Inhibitor Use and the Clinical Efficacy of Immune Checkpoint Inhibitors in Advanced Solid Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 753234. [Google Scholar] [CrossRef]
- Chen, B.; Yang, C.; Dragomir, M.P.; Chi, D.; Chen, W.; Horst, D.; Calin, G.A.; Li, Q. Association of Proton Pump Inhibitor Use with Survival Outcomes in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Ther. Adv. Med. Oncol. 2022, 14, 175883592211117. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.; Merza, N.; Rahim, M.; Qatani, A.; Varughese, T.; Mohammad, A.; Masood, F.; Reza, F.; Shucenwan; Almas, T.; et al. Impact of Proton-Pump Inhibitors on the Efficacy of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Ann. Med. Surg. 2022, 78, 103752. [Google Scholar] [CrossRef]
- Verdura, S.; Cuyàs, E.; Martin-Castillo, B.; Menendez, J.A. Metformin as an Archetype Immuno-Metabolic Adjuvant for Cancer Immunotherapy. OncoImmunology 2019, 8, e1633235. [Google Scholar] [CrossRef]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An Increase in the Akkermansia Spp. Population Induced by Metformin Treatment Improves Glucose Homeostasis in Diet-Induced Obese Mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Munoz, L.E.; Huang, L.; Bommireddy, R.; Sharma, R.; Monterroza, L.; Guin, R.N.; Samaranayake, S.G.; Pack, C.D.; Ramachandiran, S.; Reddy, S.J.C.; et al. Metformin Reduces PD-L1 on Tumor Cells and Enhances the Anti-Tumor Immune Response Generated by Vaccine Immunotherapy. J. Immunother. Cancer 2021, 9, e002614. [Google Scholar] [CrossRef]
- Cha, J.-H.; Yang, W.-H.; Xia, W.; Wei, Y.; Chan, L.-C.; Lim, S.-O.; Li, C.-W.; Kim, T.; Chang, S.-S.; Lee, H.-H.; et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol. Cell 2018, 71, 606–620.e7. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lu, C.; Zhang, K.; Lin, C.; Wu, F.; Tang, X.; Wu, D.; Dou, Y.; Han, R.; Wang, Y.; et al. Metformin Combining PD-1 Inhibitor Enhanced Anti-Tumor Efficacy in STK11 Mutant Lung Cancer Through AXIN-1-Dependent Inhibition of STING Ubiquitination. Front. Mol. Biosci. 2022, 9, 780200. [Google Scholar] [CrossRef]
- Scharping, N.E.; Menk, A.V.; Whetstone, R.D.; Zeng, X.; Delgoffe, G.M. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol. Res. 2017, 5, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Afzal, M.Z.; Mercado, R.R.; Shirai, K. Efficacy of Metformin in Combination with Immune Checkpoint Inhibitors (Anti-PD-1/Anti-CTLA-4) in Metastatic Malignant Melanoma. J. Immunother. Cancer 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Afzal, M.Z.; Dragnev, K.; Sarwar, T.; Shirai, K. Clinical Outcomes in Non-Small-Cell Lung Cancer Patients Receiving Concurrent Metformin and Immune Checkpoint Inhibitors. Lung Cancer Manag. 2019, 8, LMT11. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Kim, S.H.; Jung, E.H.; Kim, S.; Suh, K.J.; Lee, J.Y.; Kim, J.; Kim, J.W.; Lee, J.; Kim, Y.J.; et al. The Effect of Metformin or Dipeptidyl Peptidase 4 Inhibitors on Clinical Outcomes in Metastatic Non-small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Thorac. Cancer 2023, 14, 52–60. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Luo, J.; Liu, M.; Luo, Z. Pleiotropic Effects of Metformin on the Antitumor Efficiency of Immune Checkpoint Inhibitors. Front. Immunol. 2021, 11, 586760. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Ninomiya, T.; Hotta, K.; Kozuki, T.; Toyooka, S.; Okada, H.; Fujiwara, T.; Udono, H.; Kiura, K. Study Protocol: Phase-Ib Trial of Nivolumab Combined With Metformin for Refractory/Recurrent Solid Tumors. Clin. Lung Cancer 2018, 19, e861–e864. [Google Scholar] [CrossRef]
- Kunzmann, A.T.; Murray, L.J.; Cardwell, C.R.; McShane, C.M.; McMenamin, Ú.C.; Cantwell, M.M. PTGS2 (Cyclooxygenase-2) Expression and Survival among Colorectal Cancer Patients: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1490–1497. [Google Scholar] [CrossRef] [Green Version]
- Pi, C.; Jing, P.; Li, B.; Feng, Y.; Xu, L.; Xie, K.; Huang, T.; Xu, X.; Gu, H.; Fang, J. Reversing PD-1 Resistance in B16F10 Cells and Recovering Tumour Immunity Using a COX2 Inhibitor. Cancers 2022, 14, 4134. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, B.P.; Ansa-Addo, E.A.; Gutierrez, J.; Timmers, C.D.; Liu, B.; Li, Z. Cutting Edge: Targeting Thrombocytes to Rewire Anticancer Immunity in the Tumor Microenvironment and Potentiate Efficacy of PD-1 Blockade. J. Immunol. 2019, 203, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kumar, P.; Adashek, J.J.; Skelton, W.P.; Li, J.; Vosoughi, A.; Chahoud, J.; Manley, B.J.; Spiess, P.E. Adding Cyclooxygenase Inhibitors to Immune Checkpoint Inhibitors Did Not Improve Outcomes in Metastatic Renal Cell Carcinoma. Cells 2022, 11, 2505. [Google Scholar] [CrossRef]
- Aiad, M.; Tahir, A.; Fresco, K.; Prenatt, Z.; Ramos-Feliciano, K.; Walia, J.; Stoltzfus, J.; Albandar, H.J. Does the Combined Use of Aspirin and Immunotherapy Result in Better Outcomes in Non-Small Cell Lung Cancer Than Immunotherapy Alone? Cureus 2022, 14, e25891. [Google Scholar] [CrossRef] [PubMed]
- Kanai, O.; Ito, T.; Saito, Z.; Yamamoto, Y.; Fujita, K.; Okamura, M.; Hashimoto, M.; Nakatani, K.; Sawai, S.; Mio, T. Effect of Cyclooxygenase Inhibitor Use on Immunotherapy Efficacy in Non-small Cell Lung Cancer. Thorac. Cancer 2021, 12, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; Rastelli, F.; Bisonni, R.; Santini, D.; et al. Integrated Analysis of Concomitant Medications and Oncological Outcomes from PD-1/PD-L1 Checkpoint Inhibitors in Clinical Practice. J. Immunother. Cancer 2020, 8, e001361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Chen, S.; Li, Z.; Chen, J.; Li, W. The Effect of Concomitant Use of Statins, NSAIDs, Low-Dose Aspirin, Metformin and Beta-Blockers on Outcomes in Patients Receiving Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. OncoImmunology 2021, 10, 1957605. [Google Scholar] [CrossRef]
- Mravec, B.; Horvathova, L.; Hunakova, L. Neurobiology of Cancer: The Role of β-Adrenergic Receptor Signaling in Various Tumor Environments. Int. J. Mol. Sci. 2020, 21, 7958. [Google Scholar] [CrossRef]
- Yan, X.; Liu, P.; Li, D.; Hu, R.; Tao, M.; Zhu, S.; Wu, W.; Yang, M.; Qu, X. Novel Evidence for the Prognostic Impact of β-Blockers in Solid Cancer Patients Receiving Immune Checkpoint Inhibitors. Int. Immunopharmacol. 2022, 113, 109383. [Google Scholar] [CrossRef]
- Fjæstad, K.Y.; Rømer, A.M.A.; Goitea, V.; Johansen, A.Z.; Thorseth, M.-L.; Carretta, M.; Engelholm, L.H.; Grøntved, L.; Junker, N.; Madsen, D.H. Blockade of Beta-Adrenergic Receptors Reduces Cancer Growth and Enhances the Response to Anti-CTLA4 Therapy by Modulating the Tumor Microenvironment. Oncogene 2022, 41, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Kokolus, K.M.; Zhang, Y.; Sivik, J.M.; Schmeck, C.; Zhu, J.; Repasky, E.A.; Drabick, J.J.; Schell, T.D. Beta Blocker Use Correlates with Better Overall Survival in Metastatic Melanoma Patients and Improves the Efficacy of Immunotherapies in Mice. OncoImmunology 2018, 7, e1405205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellgard, G.; Patel, V.G.; Zhong, X.; Joshi, H.; Qin, Q.; Wang, B.; Parikh, A.; Jun, T.; Alerasool, P.; Garcia, P.; et al. Effect of Concurrent Beta-Blocker Use in Patients Receiving Immune Checkpoint Inhibitors for Advanced Solid Tumors. J. Cancer Res. Clin. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Guzner, A.; Wainwright, D.A.; Mohindra, N.A.; Chae, Y.K.; Behdad, A.; Villaflor, V.M. The Impact of Beta Blockers on Survival Outcomes in Patients With Non–Small-Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. Clin. Lung Cancer 2021, 22, e57–e62. [Google Scholar] [CrossRef]
- Gandhi, S.; Pandey, M.R.; Attwood, K.; Ji, W.; Witkiewicz, A.K.; Knudsen, E.S.; Allen, C.; Tario, J.D.; Wallace, P.K.; Cedeno, C.D.; et al. Phase I Clinical Trial of Combination Propranolol and Pembrolizumab in Locally Advanced and Metastatic Melanoma: Safety, Tolerability, and Preliminary Evidence of Antitumor Activity. Clin. Cancer Res. 2021, 27, 87–95. [Google Scholar] [CrossRef]
- Nakamura, K.; Yaguchi, T.; Ohmura, G.; Kobayashi, A.; Kawamura, N.; Iwata, T.; Kiniwa, Y.; Okuyama, R.; Kawakami, Y. Involvement of Local Renin-Angiotensin System in Immunosuppression of Tumor Microenvironment. Cancer Sci. 2018, 109, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, G.; Cheng, T.; Lin, J.; Zhang, L.; Zheng, J.; Liu, Y.; Xie, G.; Wang, B.; Yuan, Y. Local Angiotensin II Contributes to Tumor Resistance to Checkpoint Immunotherapy. J. Immunother. Cancer 2018, 6, 88. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, V.P.; Chen, I.X.; Tong, R.; Ng, M.R.; Martin, J.D.; Naxerova, K.; Wu, M.W.; Huang, P.; Boucher, Y.; Kohane, D.S.; et al. Reprogramming the Microenvironment with Tumor-Selective Angiotensin Blockers Enhances Cancer Immunotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 10674–10680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin Inhibition Enhances Drug Delivery and Potentiates Chemotherapy by Decompressing Tumour Blood Vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuzzo, P.V.; Adib, E.; Weise, N.; Curran, C.; Stewart, T.; Freeman, D.; Nassar, A.H.; Abou Alaiwi, S.; Bakouny, Z.; McGregor, B.A.; et al. Impact of Renin-Angiotensin System Inhibitors on Outcomes in Patients with Metastatic Renal Cell Carcinoma Treated with Immune-Checkpoint Inhibitors. Clin. Genitourin. Cancer 2022, 20, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Drobni, Z.D.; Michielin, O.; Quinaglia, T.; Zlotoff, D.A.; Zubiri, L.; Gilman, H.K.; Supraja, S.; Merkely, B.; Muller, V.; Sullivan, R.J.; et al. Renin–Angiotensin–Aldosterone System Inhibitors and Survival in Patients with Hypertension Treated with Immune Checkpoint Inhibitors. Eur. J. Cancer 2022, 163, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Medjebar, S.; Truntzer, C.; Perrichet, A.; Limagne, E.; Fumet, J.-D.; Richard, C.; Elkrief, A.; Routy, B.; Rébé, C.; Ghiringhelli, F. Angiotensin-Converting Enzyme (ACE) Inhibitor Prescription Affects Non-Small-Cell Lung Cancer (NSCLC) Patients Response to PD-1/PD-L1 Immune Checkpoint Blockers. OncoImmunology 2020, 9, 1836766. [Google Scholar] [CrossRef] [PubMed]
- Prasetya, R.A.; Metselaar-Albers, M.; Engels, F. Concomitant Use of Analgesics and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: A Pharmacodynamics Perspective. Eur. J. Pharmacol. 2021, 906, 174284. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, L.; Song, B. Impact of Opioid Analgesics on the Efficacy of Immune Checkpoint Inhibitors in a Lung Cancer Population. BMC Pulm. Med. 2022, 22, 431. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Tamiya, A.; Matsuda, Y.; Adachi, Y.; Enomoto, T.; Azuma, K.; Kouno, S.; Tokoro, A.; Atagi, S. Opioids Impair Nivolumab Outcomes: A Retrospective Propensity Score Analysis in Non-Small-Cell Lung Cancer. BMJ Support. Palliat. Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Cirillo, A.; Pomati, G.; Cerbelli, B.; Scagnoli, S.; Roberto, M.; Gelibter, A.; Mammone, G.; Calandrella, M.L.; Cerbelli, E.; et al. The Role of Opioids in Cancer Response to Immunotherapy. J. Transl. Med. 2021, 19, 119. [Google Scholar] [CrossRef]
- Mao, Z.; Jia, X.; Jiang, P.; Wang, Q.; Zhang, Y.; Li, Y.; Fu, X.; Jiao, M.; Jiang, L.; Liu, Z.; et al. Effect of Concomitant Use of Analgesics on Prognosis in Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 861723. [Google Scholar] [CrossRef] [PubMed]
- Shwe, T.H.; Pothacharoen, P.; Phitak, T.; Wudtiwai, B.; Kongtawelert, P. Atorvastatin Attenuates Programmed Death Ligand-1 (PD-L1) Induction in Human Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 8755. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.-J.; Lee, C.-H.; Bae, J.-H.; Park, J.-M.; Park, S.-S.; Baek, M.-C. Atorvastatin Enhances the Efficacy of Immune Checkpoint Therapy and Suppresses the Cellular and Extracellular Vesicle PD-L1. Pharmaceutics 2022, 14, 1660. [Google Scholar] [CrossRef]
- Lim, W.-J.; Lee, M.; Oh, Y.; Fang, X.-Q.; Lee, S.; Lim, C.-H.; Park, J.; Lim, J.-H. Statins Decrease Programmed Death-Ligand 1 (PD-L1) by Inhibiting AKT and β-Catenin Signaling. Cells 2021, 10, 2488. [Google Scholar] [CrossRef] [PubMed]
- Cantini, L.; Pecci, F.; Hurkmans, D.P.; Belderbos, R.A.; Lanese, A.; Copparoni, C.; Aerts, S.; Cornelissen, R.; Dumoulin, D.W.; Fiordoliva, I.; et al. High-Intensity Statins Are Associated with Improved Clinical Activity of PD-1 Inhibitors in Malignant Pleural Mesothelioma and Advanced Non-Small Cell Lung Cancer Patients. Eur. J. Cancer 2021, 144, 41–48. [Google Scholar] [CrossRef]
- Rossi, A.; Filetti, M.; Taurelli Salimbeni, B.; Piras, M.; Rizzo, F.; Giusti, R.; Marchetti, P. Statins and Immunotherapy: Togetherness Makes Strength The Potential Effect of Statins on Immunotherapy for NSCLC. Cancer Rep. 2021, 4, e1368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Tian, J.; Sui, L.; Chen, X. Concomitant Statins and the Survival of Patients with Non-Small-Cell Lung Cancer Treated with Immune Checkpoint Inhibitors: A Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Buti, S.; Bersanelli, M.; Perrone, F.; Tiseo, M.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; et al. Effect of Concomitant Medications with Immune-Modulatory Properties on the Outcomes of Patients with Advanced Cancer Treated with Immune Checkpoint Inhibitors: Development and Validation of a Novel Prognostic Index. Eur. J. Cancer 2021, 142, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Dussart, C.; Decaux-Tramoni, B.; Quesada, S.; Thomas, Q.D.; Benzerouale, O.; Nicolas, E.; Fiteni, F. Combinaisons d’inhibiteurs de points de contrôle immunitaires en oncologie: état de l’art et perspectivesCombination strategies for checkpoint inhibition: Current practices and perspectives. Bull. Cancer, 2023; in press. [Google Scholar] [CrossRef]
| Author (Year) | Type of Cancer | ICI | GC Regimen and Indication | n Patients/Total (%) | Compared Arms | ORR [CI 95%] | PFS [CI 95%] | OS [CI 95%] |
|---|---|---|---|---|---|---|---|---|
| Margolin (2012) [54] | Melanoma with BM | Ipi | Systemic GC for symptomatic BM | 21/72 (29) | No statistical comparison | - | 1.3 m vs. 2.7 m * | 3.7 m vs. 7 m * |
| Chasset (2015) [55] | Melanoma | Ipi | ≥10 mg pred baseline multiple indications | 12/45 (27) | Overall | - | - | 4 m vs. 11 m HR: 5.82 [2.45, 13.8], p < 0.001 |
| Among BM | - | - | 4 m vs. 7 m, p = 0.043 | |||||
| Arbour (2018) [51] | NSCLC | Multiple | ≥10 mg pred baseline multiple indications | 90/640 (14) | Overall | 7% vs. 18%, p = 0.05 | HR: 1.31 [1.03, 1.67], p = 0.03 | HR: 1.66 [1.28, 2.16], p < 0.001 |
| Scott (2018) [52] | NSCLC | Nivo | (A) ≥10 mg pred in first 30 d | 25/210 (12) | A vs. non A | - | - | HR: 2.30 [1.27, 4.16], p = 0.006 |
| (B) ≥10 mg pred for irAEs | 31/210 (15) | B vs. non B | - | - | 16.1 m vs. 10.5 m, p = 0.50 | |||
| Hendriks (2019) [56] | NSCLC BM+ or BM− prospective | Multiple | (A) GC at baseline (overall) | 141/1025 (14) | A vs. non A | - | HR: 1.31 [1.07, 1.62], p = 0.01 | HR: 1.46 [1.16, 1.84], p = 0.001 |
| (B) GC at baseline among BM+ | 69/255 (27) | B vs. BM + non B | - | HR: 2.78 [1.90, 4.08], p < 0.001 | HR: 2.37 [1.54, 3.63], p < 0.001 | |||
| Ricciuti (2019) [53] | NSCLC | Multiple | (A) ≥10 mg pred baseline: SC | 56/640 (10) | A + B vs. C | 10.8% vs. 19.7%, p = 0.04 | 2.0 m vs. 3.4 m HR: 1.36 [1.08, 1.73], p = 0.01 | 4.9 m vs. 11.2 m HR: 1.68 [1.30, 2.17], p < 0.001 |
| (B) ≥10 mg pred baseline: non SC | 27/640 (4) | A vs. C | 6.1% vs. 19.7%, p = 0.01 | 1.4 m vs. 3.4 m HR: 1.87 [1.43, 2.45], p < 0.001 | 2.2 m vs. 11.2 m HR: 2.38 [1.78, 3.19], p = 0.001 | |||
| (C) 0 to <10 mg pred baseline | 557/640 (86) | B vs. C | 22.2% vs. 19.7% * | 4.6 m vs. 3.4 m HR: 0.77 [0.50, 1.19], p = 0.24 | 10.7 m vs. 11.2 m HR: 0.93 [0.59, 1.48], p = 0.77 | |||
| Pinato (2020) [57] | HCC | Multiple | (A) ≥10 mg pred baseline | 14/304 (5) | A vs. B + C | p = 0.62 | 6.7 m vs. 5.8 m, p = 0.37 | 10.4 m vs. 12.2 m, p = 0.48 |
| (B) ≥10 mg pred during ICI | 64/304 (20) | B vs. A + C | p = 0.62 | 8.1 m vs. 10.7 m, p = 0.46 | 16.1 m vs. 11.7 m, p = 0.25 | |||
| (C) no GC at all | 226/304 (75) | Among A + B: SC vs. non | SC: “more ICI refractory” p = 0.05 | 1.6 m vs. 8.8 m, p < 0.01 | 4.9 m vs. 15.4 m, p = 0.05 | |||
| Umehara (2021) [58] | NSCLC | Nivo | (A) GC at baseline multiple indications | 12/109 (11) | A vs. C | 8% vs. 14%, p = 0.03 | 0.9 m vs. 3.3 m, p < 0.01 | 2.2 m vs. 11.9 m, p < 0.01 |
| (B) GC during ICI: irAE or no | 19/109 (17) 14/109 (13) | B vs. C | 36% vs. 14%, p = 0.02 | 3.6 m vs. 3.3 m, p = 0.23 | 12.5 m vs. 11.9 m, p = 0.72 | |||
| (C) no GC at all | 64/109 (59) | Among B: irAE vs. no | 47% vs. 21%, p = 0.13 | 5.1 m vs. 2.2 m, p = 0.17 | 13.5 m vs. 12.5 m, p = 0.30 | |||
| Gaucher (2021) [59] | Multiple | Multiple | (A) concomitant GC: irAE | 21/372 (6) | A + B vs. C | 16.9% vs. 27.8%, p = 0.025 | - | HR: 1.25 [0.91, 1.71], p = 0.16 |
| (B) concomitant GC: other indication | 56/372 (15) | A vs. B + C | 28.6% vs. 27.8%, p = 0.30 | - | HR: 1.04 [0.56, 1.95], p = 0.90 | |||
| (C) no GC at all | 295/372 (79) | B vs. A + C | 12.5% vs. 27.8%, p = 0.008 | - | HR: 1.34 [1.05, 2.03], p = 0.046 |
| Author (Year) | Type of Cancer | ICI | GC Regimen | GC Indication | n Studies (n Patients) | PFS: HR, [95% CI] | OS: HR, [95% CI] |
|---|---|---|---|---|---|---|---|
| Petrelli (2020) [42] | Multiple | Multiple | Multiple | Overall | 16 (4045) | 1.34 [1.02, 1.76], p = 0.03 | 1.54 [1.24, 1.91], p = 0.0001 |
| SC | 3 (836) | - | 2.5 [1.41, 4.43], p < 0.01 | ||||
| BM | 3 (1164) | - | 1.51 [1.22, 1.87], p < 0.01 | ||||
| irAEs | 9 (926) | - | 1.08 [0.79, 1.49], p = 0.62 | ||||
| Zhang (2021) [60] | NSCLC | Multiple | Multiple | Overall | 14 (5461) | 1.69 [1.51, 2.04], p = 0.009 | 1.82 [1.51, 2.18], p = 0.003 |
| SC | NS | 1.55 [1.26, 1.92] * | 1.94 [1.57, 2.20] * | ||||
| BM | NS | 1.56 [1.23, 1.97] * | 1.62 [1.41, 1.86] * | ||||
| Jessurun (2021) [61] | Multiple with BM | Multiple | Multiple | Overall BM | 15 (1113) | 2.00 [1.37, 2.91], p = 0.007 | 1.84 [1.22, 2.77], p = 0.007 |
| NSCLC BM | 4 (505) | - | 2.43 [0.38, 15.77] * | ||||
| Melanoma BM | NS | - | 1.67 [1.49, 1.87] * |
| Author (Year) | Type of Cancer | Treatment | ATB Regimen | n Patients/Total (%) | Subgroup | ORR [CI 95%] | PFS [CI 95%] | OS [CI 95%] |
|---|---|---|---|---|---|---|---|---|
| Routy (2018) [26] | NSCLC RCC UC | ICI (multiple) | Within 2 m before or 1 m after ICI initiation | 69/246 (28) | Overall | - | 3.5 m vs. 4.1 m, p = 0.017 | 11.5 m vs. 20.6 m, p < 0.001 |
| 37/140 (26) | NSCLC | - | 3.5 m vs. 2.8 m, p = 0.57 | 8.3 m vs. 15.3 m, p = 0.001 | ||||
| 20/67 (30) | RCC | - | 4.3 m vs. 7.4 m, p = 0.012 | 23.4 m vs. 27.9 m, p = 0.15 | ||||
| 12/42 (29) | UC | - | 1.8 m vs. 4.3 m, p = 0.049 | 11.5 m vs. NR, p = 0.098 | ||||
| 68/239 (28) | Validation cohort | - | 2.6 m vs. 3.6 m, p = 0.24 | 9.8 m vs. 21.9 m, p = 0.002 | ||||
| Derosa (2018) [68] | RCC NSCLC | ICI (multiple) +/− TT | Within 30 d before ICI initiation | 16/121 (13) | RCC | 13% vs. 26%, p < 0.01 | 1.9 m vs. 7.4 m HR: 3.1 [1.4, 6.9], p < 0.01 | 17.3 m vs. 30.6 m HR: 3.5 [1.1, 10.8], p = 0.03 |
| 48/239 (20) | NSCLC | 13% vs. 23%, p = 0.26 | 1.9 m vs. 3.8 m HR: 1.5 [1.0, 2.2], p = 0.03 | 7.9 m vs. 24.6 m HR: 4.4 [2.6, 7.7], p < 0.01 | ||||
| Pinato (2019) [69] | Multiple | ICI (multiple) | (A) within 30 d before ICI initiation | 29/196 (15) | A: overall | 8% vs. 43%, p < 0.01 | - | 2 m vs. 26 m HR: 3.4 [1.9, 6.1], p < 0.01 |
| 6/107 (6) | A: NSCLC | - | - | 2.5 m vs. 26 m HR: 9.3 [4.3, 19], p < 0.01 | ||||
| 17/38 (45) | A: melanoma | - | - | 3.9 m vs. 14 m HR: 7.5 [1.7, 30.4], p < 0.001 | ||||
| (B) concomitant | 68/196 (35) | B | - | - | NR vs. 26 m HR: 0.9 [0.5, 1.4], p = 0.65 | |||
| Tinsley (2019) [70] | Multiple | ICI (multiple) | Between 2 w before and 6 w after ICI initiation: single vs. cumulative course | 92/291 (32) | Overall | 3.1 m vs. 6.3 m HR: 1.40 [1.03, 1.92], p = 0.033 | 10.4 m vs. 21.7 m HR: 1.47 [1.04, 2.11], p = 0.033 | |
| NS | Single course | - | 3.7 m vs. 6.3 m HR: 1.32 [0.80, 2.20], p = 0.28 | 17.7 m vs. 21.7 m HR: 1.26 [0.82, 1.93], p = 0.29 | ||||
| NS | Cumulative courses | - | 2.8 m vs. 6.3 m HR: 2.63 [1.25, 6.13], p = 0.026 | 6.3 m vs. 21.7 m HR: 1.90 [1.18, 2.08], p = 0.009 | ||||
| Cortellini (2021) [71] | NSCLC TPS > 50% | Pembro (A) vs. CT (B) | Within 30 d before initiation | (A) 131/950 (14) | A | 30.1% vs. 44.4% OR: 0.57 [0.37, 0.87], p = 0.01 | 4.8 m vs. 7.5 m HR: 1.29 [1.04, 1.59], p = 0.02 | 10.4 m vs. 17.2 m HR: 1.42 [1.13, 1.79], p = 0.002 |
| (B) 87/595 (15) | B | 33.3% vs. 37.6%, p = 0.50 | 5.1 m vs. 5.9 m HR: 1.10 [0.86, 1.40], p = 0.42 | 13.2 m vs. 14.9 m HR: 1.23 [0.95, 1.61], p = 0.11 | ||||
| Cortellini (2021) [72] | NSCLC | CT + ICI 1st line | (A) within 30 d before ICI initiation | 47/302 (16) | A: overall | 42.6% vs. 57.4% OR: 0.83 [0.42, 1.64], p = 0.60 | 5.6 m vs. 6.3 m HR: 1.12 [0.76, 1.63], p = 0.56 | 11.2 m vs. 16.6 m HR: 1.42 [0.91, 2.22], p = 0.12 |
| 17/302 (6) | A: ATB > 7 d | - | HR: 1.31 [0.73, 2.31] * | HR: 1.76 [0.83, 3.71] * | ||||
| 20/302 (7) | A: ATB IV | - | HR: 1.67 [0.88, 3.17] * | HR: 1.44 [0.69, 3.09] * | ||||
| 12/76 (16) | A: among TPS > 50% | - | 7.0 m vs. 9.8 m HR: 1.48 [0.62, 3.53], p = 0.37 | 16.3 m vs. 25.9 m HR: 1.61 [0.57, 4.49], p = 0.36 | ||||
| (B) concomitant | 117/302 (39) | B | - | HR: 1.20 [0.89, 1.63], p = 0.22 | HR: 1.29 [0.91, 1.84], p = 0.15 |
| Author (Year) | Type of Cancer | ICI | ATB Regimen | Subgroup | n Studies (n Patients) | ORR: OR, [95% CI] | PFS: HR, [95% CI] | OS: HR, [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Lurienne (2020) [74] | NSCLC | Multiple +/− CT or TT | Multiple | Overall | 23 (2208) | - | 1.47 [1.13, 1.90], p < 0.01 | 1.69 [1.25, 2.29], p < 0.01 |
| Within 90 d before ICI | 4 (708) | - | 1.56 [0.78, 3.13] * | 2.49 [0.95, 6.51] * | ||||
| Within 60 d before ICI | 3 (325) | - | 2.00 [1.34, 2.99] * | 2.94 [1.60, 5.40] * | ||||
| 60 d before to 60 d after ICI initiation | 12 (1624) | - | 1.72 [1.30, 2.27] * | 2.04 [1.49, 2.79] * | ||||
| Within 90 d before ICI and during ICI treatment | 5 (645) | - | 0.97 [0.44, 2.17] * | 1.24 [0.56, 2.76] * | ||||
| Xu (2020) [75] | Multiple | Multiple +/− CT or TT | Multiple | Overall | 20 (4331) | - | 1.53 [1.30, 1.79], p < 0.01 | 1.90 [1.55, 2.34], p < 0.01 |
| NSCLC | 12 (1880) | - | 1.39 [1.16, 1.67], p < 0.01 | 1.73 [1.26, 2.38], p < 0.01 | ||||
| NSCLC: ATB within 6 m before ICI | 3 (515) | - | - | 1.81 [0.91, 3.63], p = 0.09 | ||||
| NSCLC: ATB within 1 m before ICI or during ICI | 7 (1365) | - | - | 2.09 [1.31, 3.32], p = 0.002 | ||||
| Wu (2021) [76] | Multiple | Multiple +/− CT or TT | Multiple | Overall | 44 (12492) | 0.61 [0.42, 0.90], p = 0.01 | 1.18 [1.11, 1.25], p < 0.01 | 1.20 [1.15, 1.25], p < 0.01 |
| RCC | 4 (367) | 0.30 [0.14, 0.67], p < 0.01 | 1.29 [1.19, 1.40], p < 0.01 | 1.12 [1.01, 1.25], p = 0.028 | ||||
| NSCLC | 9 (1276) | 0.84 [0.50, 1.42], p = 0.51 | 1.13 [1.04, 1.23], p < 0.01 | 1.26 [1.15, 1.38], p < 0.01 | ||||
| Melanoma | 2 (182) | 0.37 [0.12, 1.10], p = 0.07 | 1.75 [1.34, 2.29], p < 0.01 | 1.36 [1.06, 1.75], p = 0.017 | ||||
| ATB before ICI | 8 (1060) | 0.47 [0.32, 0.71], p < 0.01 | 1.23 [1.14, 1.32], p < 0.01 | 1.39 [1.26, 1.54], p < 0.01 | ||||
| ATB before or after ICI within 1 m | 9 (1010) | 0.63 [0.32, 1.26], p = 0.19 | 1.16 [1.06, 1.26], p < 0.01 | 1.17 [1.10, 1.24], p < 0.01 | ||||
| Luo (2022) [77] | RCC | Multiple +/− TT | Multiple | Overall | 6 (1104) | 0.58 [0.41, 0.84] * | 1.77 [1.25, 2.50] * | 1.69 [1.34, 2.12] * |
| 60 d before to 60 d after ICI initiation | 4 (NS) | - | 1.86 [1.18, 2.95] * | 1.66 [1.30, 2.11] * | ||||
| Within 90 d before ICI | 2 (NS) | - | 1.75 [0.40, 7.55] * | 0.66 [0.13, 3.35] * |
| Author (Year) | Type of Cancer | Treatment | PPI Regimen | n Patients/Total (%) | Subgroup | ORR [CI 95%] | PFS [CI 95%] | OS [CI 95%] |
|---|---|---|---|---|---|---|---|---|
| Hopkins (2020) [88] | UC | Atezo or CT (IMvigor210, 211) | Within 30 d before (A) or after (B) ICI initiation | 286/896 (32) | Pooled atezo | OR: 0.51 [0.32, 0.82], p = 0.006 | HR: 1.38 [1.18, 1.62], p < 0.001 | HR: 1.52 [1.27, 1.83], p < 0.001 |
| 185/464 (40) | CT | OR: 1.04 [0.64, 1.71], p = 0.2 | HR: 1.11 [0.89, 1.37], p = 0.35 | HR: 1.16 [0.93, 1.47], p = 0.2 | ||||
| 272 | Atezo + PPI: A vs. B | - | HR: 0.71 [0.49, 1.03], p = 0.07 | HR: 0.65 [0.44, 0.97], p = 0.033 | ||||
| Chalabi (2020) [79] | NSCLC | Atezo or CT (OAK, POPLAR) | Within 30 d before or after ICI initiation | 234/757 (31) | Pooled atezo | - | 1.9 m vs. 2.8 m HR: 1.30 [1.10, 1.53], p = 0.001 | 9.6 m vs. 14.5 m HR: 1.45 [1.20, 1.75], p < 0.001 |
| 260/755 (34) | CT | 3.5 m vs. 3.9 m HR: 1.04 [0.89, 1.22] * | 9.1 m vs. 11.0 m HR: 1.17 [0.97, 1.40] * | |||||
| 74/757 (10) | Pooled atezo: PPI + ATB | - | 1.7 m vs. 2.8 m HR: 1.48 [1.16, 1.91] * | 6.6 m vs. 14.1 m HR: 1.89 [1.42, 2.52] * | ||||
| Stokes (2021) [89] | NSCLC (US veterans) | ICI (multiple) +/− CT | Within 90 d of ICI initiation | 2159/3634 (59) | Overall | - | - | 10 m vs. 10 m HR: 0.98 [0.90, 1.06], p = 0.59 |
| Baek (2022) [90] | NSCLC | Multiple (L2+) | Within 30 d before ICI initiation (new users or not) | 936/2963 (32) | Overall | - | - | 5.1 m vs. 8.0 m HR: 1.28 [1.13, 1.46], p < 0.001 |
| 168/2963 (6) | New PPI users | - | - | 3.8 m vs. 8.4 m HR: 1.64 [1.25, 2.17], p < 0.001 | ||||
| Peng (2022) [91] | Multiple | Nivo or pembro +/− CT | Within 30 d before or after ICI initiation | 89/233 (38) | Overall | HR: 1.05 [0.76, 1.45] * | HR: 1.22 [0.80, 1.86] * | |
| 46/117 (39) | NSCLC | - | HR: 1.33 [0.86, 2.04] * | HR: 1.18 [0.79, 2.01] * |
| Author (Year) | Type of Cancer | ICI | PPI Regimen | Subgroup | n Studies (n Patients) | PFS: HR, [95% CI] | OS: HR, [95% CI] |
|---|---|---|---|---|---|---|---|
| Li (2020) [93] | Multiple | Multiple | Prior or within | Overall | 7 (1482) | 0.90 [0.66, 1.23], p = 0.51 | 1.05 [0.79, 1.40], p = 0.73 |
| NSCLC | 4 (NS) | 1.17 [1.05, 1.31], p = 0.006 | 1.24 [1.00, 1.55], p = 0.05 | ||||
| Melanoma | 2 (NS) | 0.50 [0.28, 0.91], p = 0.02 | 0.67 [0.30, 1.52], p = 0.34 | ||||
| Liu (2022) [94] | Multiple | Multiple +/− TT | Prior or within | Overall | 17 (9978) | 1.19 [0.98, 1.44] * | 1.29 [1.10, 1.50] * |
| 30 d before and/or after ICI initiation | 5 (NS) | 1.23 [1.06, 1.43], p = 0.007 | 1.38 [1.18, 1.62], p < 0.001 | ||||
| Any time after ICI initiation | 7 (NS) | 0.72 [0.40, 1.28], p = 0.18 | 1.27 [1.01, 1.59], p = 0.038 | ||||
| NSCLC | 6 (NS) | 1.27 [1.10, 1.47], p = 0.001 | 1.19 [0.92, 1.54], p = 0.18 | ||||
| Melanoma | 2 (NS) | 0.48 [0.25, 0.90], p = 0.023 | 0.70 [0.31, 1.56], p = 0.38 | ||||
| Chen (2022) [95] | Multiple | Multiple | Prior or within | Overall | 33 (15,957) | 1.30 [1.17, 1.46], p < 0.001 | 1.31 [1.19, 1.44], p < 0.001 |
| At baseline | 3 (2194) | 1.29 [1.15, 1.44], p < 0.001 | 1.43 [1.21, 1.69], p < 0.001 | ||||
| Within 60 d before ICI initiation | 20 (7742) | 1.33 [1.20, 1.48], p < 0.001 | 1.35 [1.22, 1.51], p < 0.001 | ||||
| After ICI initiation | 12 (>4900) | 1.19 [0.65, 2.17], p = 0.58 | 1.18 [0.98, 1.41], p = 0.09 | ||||
| NSCLC | 13 (9200) | 1.33 [1.17, 1.51], p < 0.001 | 1.33 [1.15, 1.54], p < 0.001 | ||||
| RCC | 6 (433) | 1.11 [0.89, 1.38], p = 0.37 | 1.01 [0.77, 1.33], p = 0.92 | ||||
| Dar (2022) [96] | NSCLC | Multiple +/− TT | NS | Overall | 4 (2940) | 1.31 [1.17, 1.47], p < 0.01 | 1.46 [1.27, 1.67], p < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colard-Thomas, J.; Thomas, Q.D.; Viala, M. Comedications with Immune Checkpoint Inhibitors: Involvement of the Microbiota, Impact on Efficacy and Practical Implications. Cancers 2023, 15, 2276. https://doi.org/10.3390/cancers15082276
Colard-Thomas J, Thomas QD, Viala M. Comedications with Immune Checkpoint Inhibitors: Involvement of the Microbiota, Impact on Efficacy and Practical Implications. Cancers. 2023; 15(8):2276. https://doi.org/10.3390/cancers15082276
Chicago/Turabian StyleColard-Thomas, Julien, Quentin Dominique Thomas, and Marie Viala. 2023. "Comedications with Immune Checkpoint Inhibitors: Involvement of the Microbiota, Impact on Efficacy and Practical Implications" Cancers 15, no. 8: 2276. https://doi.org/10.3390/cancers15082276
APA StyleColard-Thomas, J., Thomas, Q. D., & Viala, M. (2023). Comedications with Immune Checkpoint Inhibitors: Involvement of the Microbiota, Impact on Efficacy and Practical Implications. Cancers, 15(8), 2276. https://doi.org/10.3390/cancers15082276





