MicroRNA, mRNA, and Proteomics Biomarkers and Therapeutic Targets for Improving Lung Cancer Treatment Outcomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. RNA Isolation and Quality Assessment
2.3. Microarray Analysis
2.4. Hierarchical Clustering Analysis and Heatmap
2.5. Nearest Centroid Classification
2.6. MiRNA Targeted Genes
2.7. Cancer Cell Line Encyclopedia
2.8. Drug Response
2.9. Proliferation Assays
2.10. Connectivity Map (CMap)
2.11. Statistical Analysis
3. Results
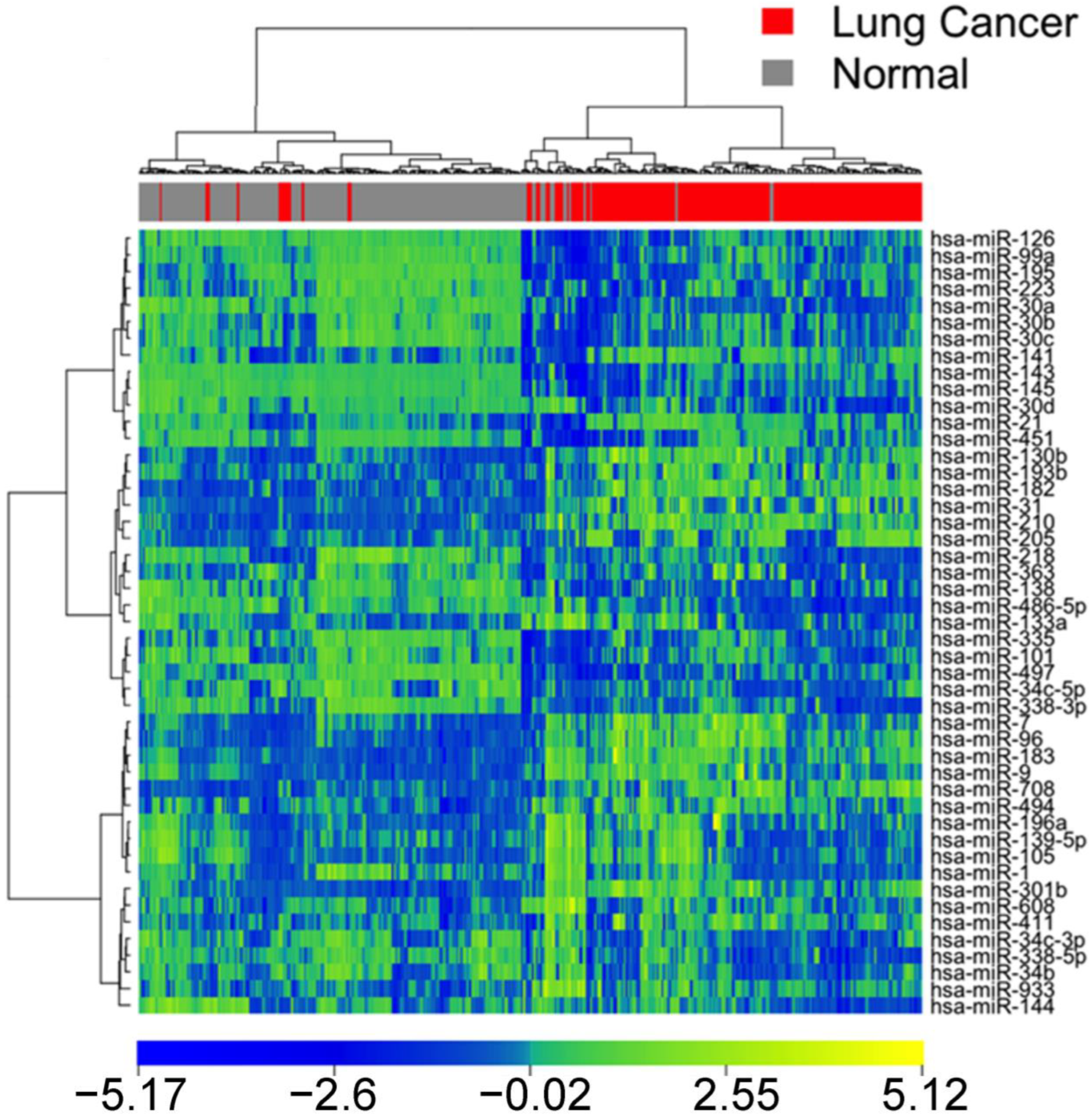
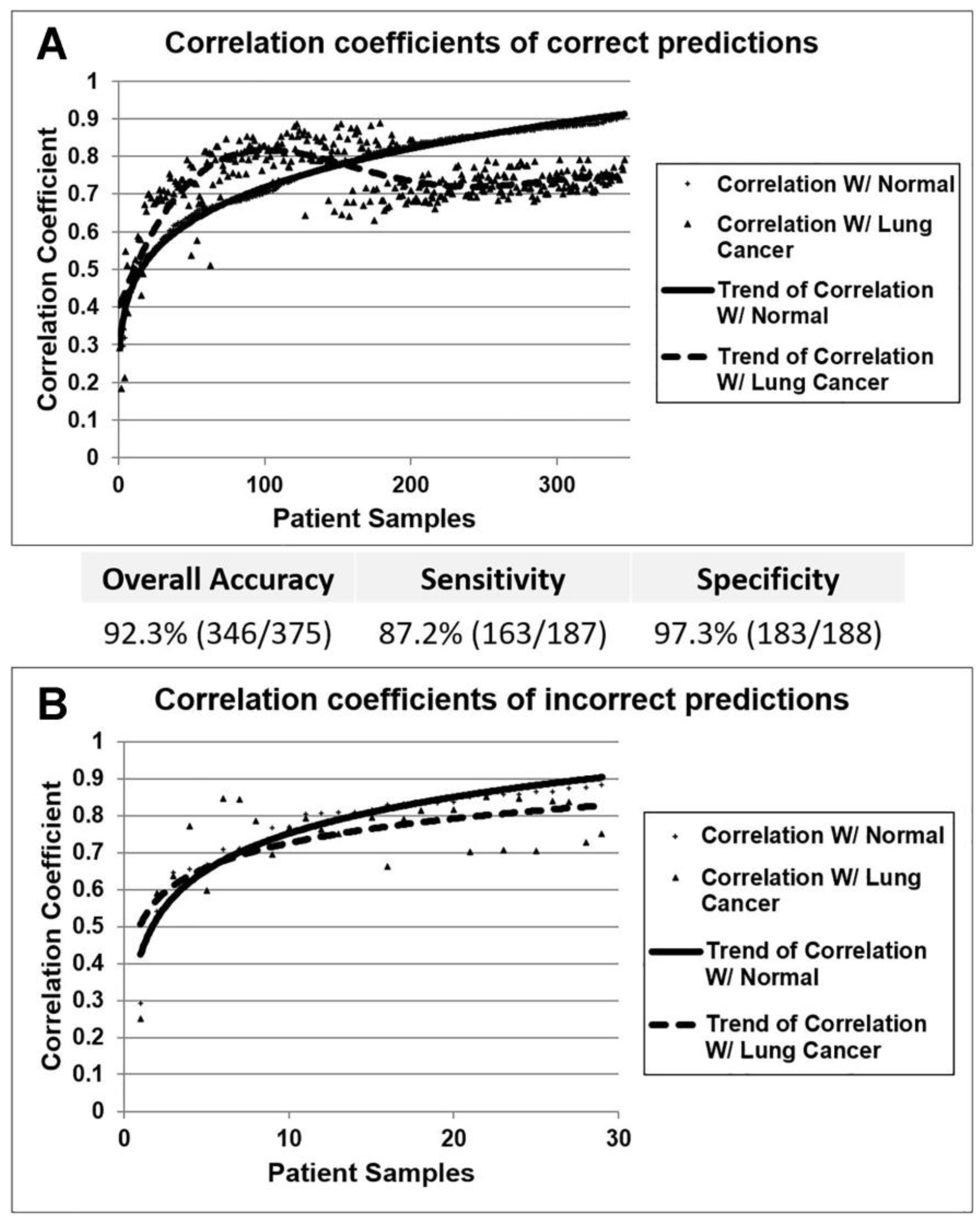
3.1. Identification of Diagnostic miRNA Markers
3.2. Comparison of miRNA Markers in Different Histology
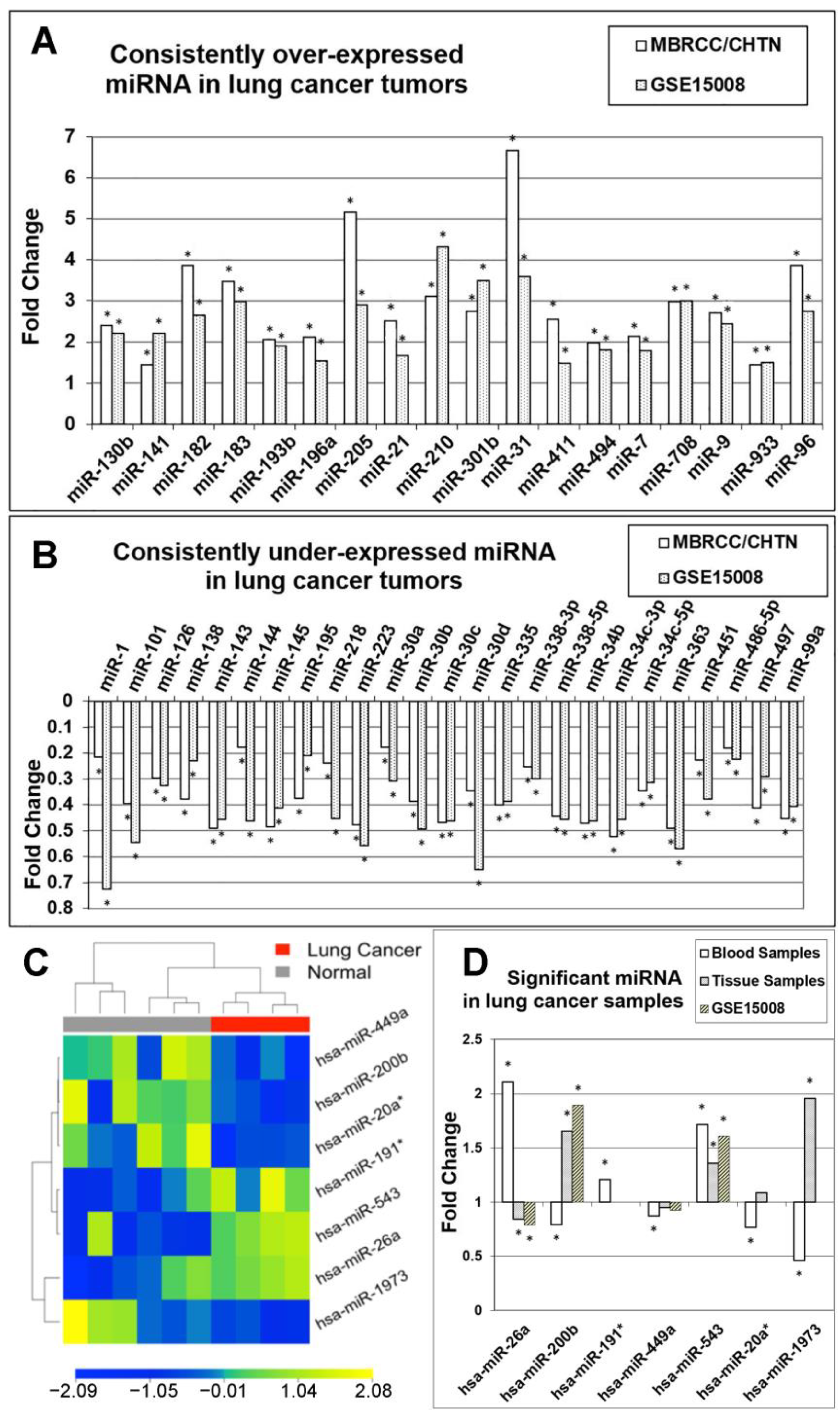
3.3. Confirmation of miRNA Expression Patterns in Multiple Cohorts
3.4. MicroRNA Markers in Blood Samples
3.5. Identification of Prognostic miRNAs
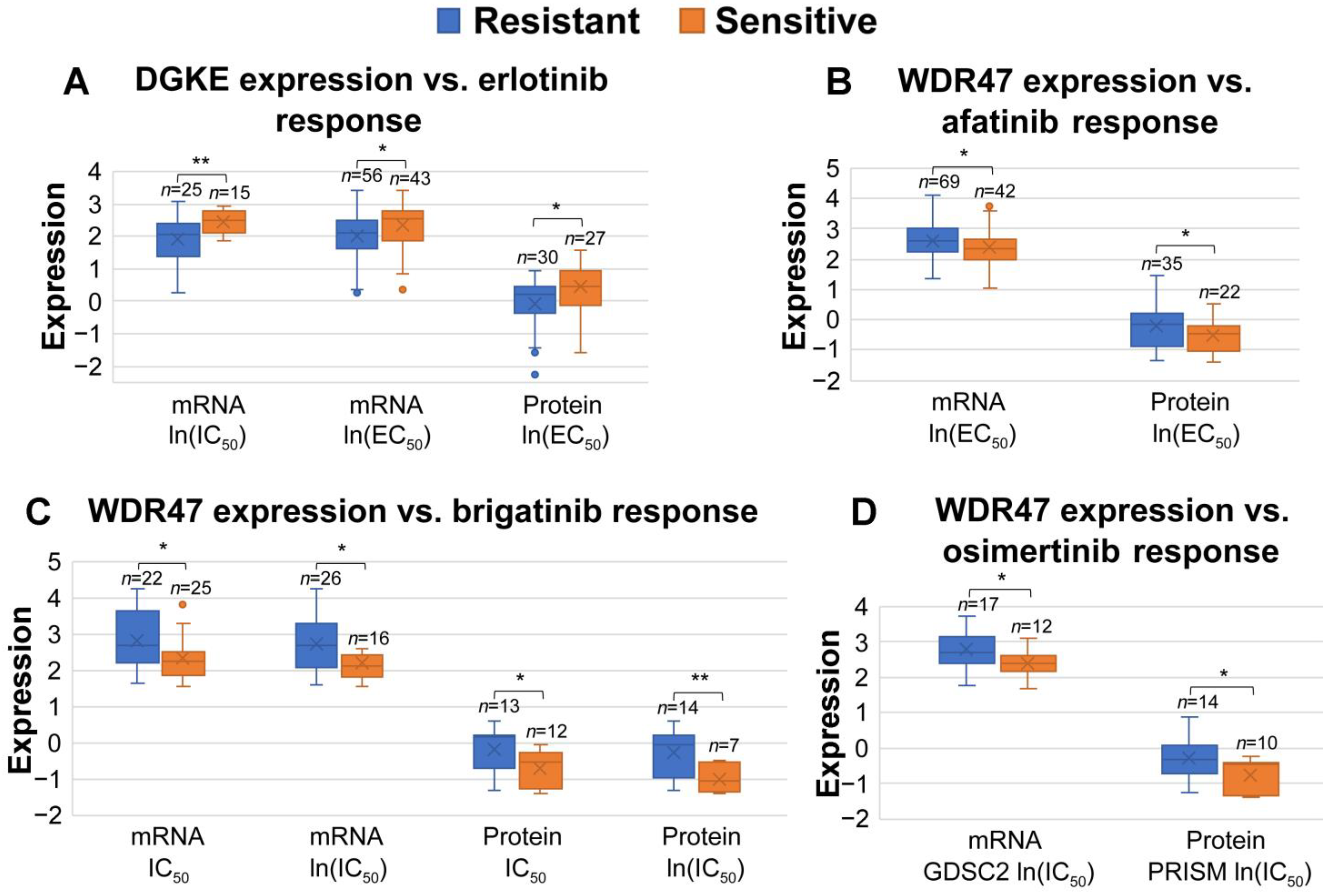
3.6. Association between miRNA-Targeted Genes and Responses to Systemic Therapies and Radiotherapy
3.7. Identification of Prognostic miRNA-Targeted Genes
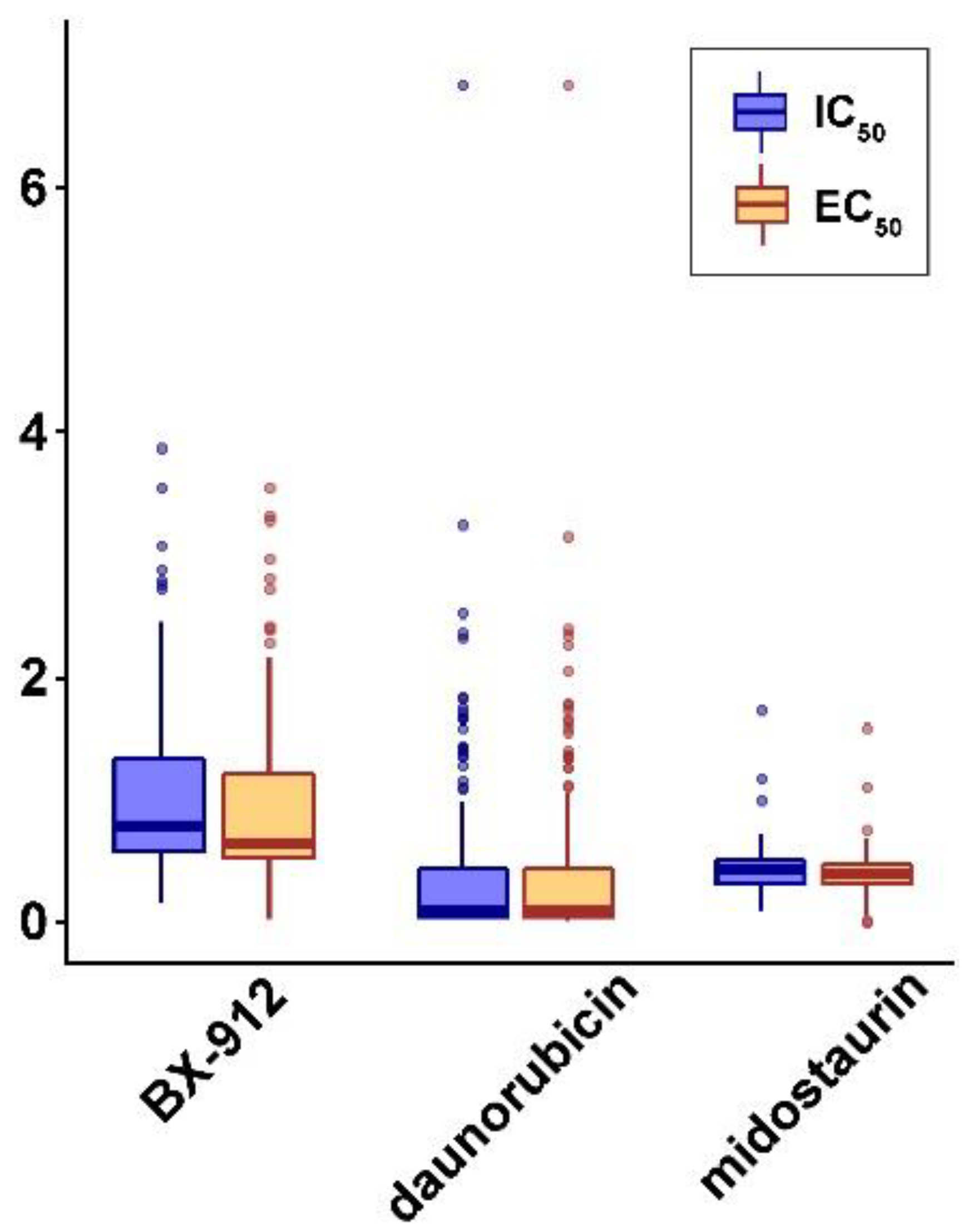
3.8. Discovery of Repositioning Drugs
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bishop, J.A.; Benjamin, H.; Cholakh, H.; Chajut, A.; Clark, D.P.; Westra, W.H. Accurate Classification of Non–Small Cell Lung Carcinoma Using a Novel MicroRNA-Based ApproachMicroRNA-Based Approach for Lung Cancer Classification. Clin. Cancer Res. 2010, 16, 610–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Chua, J.H.; Armugam, A.; Jeyaseelan, K. MicroRNAs: Biogenesis, function and applications. Curr. Opin. Mol. Ther. 2009, 11, 189–199. [Google Scholar] [PubMed]
- Farazi, T.A.; Spitzer, J.I.; Morozov, P.; Tuschl, T. miRNAs in human cancer. J. Pathol. 2011, 223, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Kasinski, A.L.; Slack, F.J. MicroRNAs en route to the clinic: Progress in validating and targeting microRNAs for cancer therapy. Nat. Rev. Cancer 2011, 11, 849–864. [Google Scholar] [CrossRef] [Green Version]
- Ferracin, M.; Veronese, A.; Negrini, M. Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Rev. Mol. Diagn. 2010, 10, 297–308. [Google Scholar] [CrossRef]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Boeri, M.; Verri, C.; Conte, D.; Roz, L.; Modena, P.; Facchinetti, F.; Calabrò, E.; Croce, C.M.; Pastorino, U.; Sozzi, G. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 3713–3718. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Hu, Z.; Wang, W.; Ba, Y.; Ma, L.; Zhang, C.; Wang, C.; Ren, Z.; Zhao, Y.; Wu, S.; et al. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Int. J. Cancer 2012, 130, 1620–1628. [Google Scholar] [CrossRef]
- Keller, A.; Leidinger, P.; Borries, A.; Wendschlag, A.; Wucherpfennig, F.; Scheffler, M.; Huwer, H.; Lenhof, H.-P.; Meese, E. miRNAs in lung cancer-studying complex fingerprints in patient’s blood cells by microarray experiments. BMC Cancer 2009, 9, 353. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Todd, N.W.; Xing, L.; Xie, Y.; Zhang, H.; Liu, Z.; Fang, H.; Zhang, J.; Katz, R.L.; Jiang, F. Early detection of lung adenocarcinoma in sputum by a panel of microRNA markers. Int. J. Cancer 2010, 127, 2870–2878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turchinovich, A.; Burwinkel, B. Distinct AGO1 and AGO2 associated miRNA profiles in human cells and blood plasma. RNA Biol. 2012, 9, 1066–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Ding, H.; Wang, Y.; Li, P.; Wang, K. Emerging Function and Clinical Values of Exosomal MicroRNAs in Cancer. Mol. Ther. Nucleic Acids 2019, 16, 791–804. [Google Scholar] [CrossRef] [Green Version]
- Mendell, J.T.; Olson, E.N. MicroRNAs in stress signaling and human disease. Cell 2012, 148, 1172–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.; Yadav, T.; Rani, V. Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit. Rev. Oncol. Hematol. 2016, 98, 12–23. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.Y.; Zhang, C.; Wang, X.; Zhai, L.; Ma, Y.; Mao, Y.; Qian, K.; Sun, C.; Liu, Z.; Jiang, S.; et al. Integrative Proteomic Characterization of Human Lung Adenocarcinoma. Cell 2020, 182, 245–261.e217. [Google Scholar] [CrossRef]
- Beekman, J.M.; Reischl, J.; Henderson, D.; Bauer, D.; Ternes, R.; Peña, C.; Lathia, C.; Heubach, J.F. Recovery of microarray-quality RNA from frozen EDTA blood samples. J. Pharmacol. Toxicol. Methods 2009, 59, 44–49. [Google Scholar] [CrossRef]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DepMap. DepMap 22Q2 Public 2022; Figshare: Los Angeles, CA, USA, 2022. [Google Scholar]
- Ghandi, M.; Huang, F.W.; Jane-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R., 3rd; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Nusinow, D.P.; Szpyt, J.; Ghandi, M.; Rose, C.M.; McDonald, E.R., 3rd; Kalocsay, M.; Jané-Valbuena, J.; Gelfand, E.; Schweppe, D.K.; Jedrychowski, M.; et al. Quantitative Proteomics of the Cancer Cell Line Encyclopedia. Cell 2020, 180, 387–402.e316. [Google Scholar] [CrossRef] [PubMed]
- Corsello, S.M.; Nagari, R.T.; Spangler, R.D.; Rossen, J.; Kocak, M.; Bryan, J.G.; Humeidi, R.; Peck, D.; Wu, X.; Tang, A.A.; et al. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat. Cancer 2020, 1, 235–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Soares, J.; Greninger, P.; Edelman, E.J.; Lightfoot, H.; Forbes, S.; Bindal, N.; Beare, D.; Smith, J.A.; Thompson, I.R.; et al. Genomics of Drug Sensitivity in Cancer (GDSC): A resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013, 41, D955–D961. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Falatovich, B.; Singh, S.; Ivanov, A.V.; Eubank, T.D.; Guo, N.L. A Multi-Omics Network of a Seven-Gene Prognostic Signature for Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 23, 219. [Google Scholar] [CrossRef]
- Ye, Q.; Singh, S.; Qian, P.R.; Guo, N.L. Immune-Omics Networks of CD27, PD1, and PDL1 in Non-Small Cell Lung Cancer. Cancers 2021, 13, 4296. [Google Scholar] [CrossRef]
- Meyers, R.M.; Bryan, J.G.; McFarland, J.M.; Weir, B.A.; Sizemore, A.E.; Xu, H.; Dharia, N.V.; Montgomery, P.G.; Cowley, G.S.; Pantel, S.; et al. Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat. Genet. 2017, 49, 1779–1784. [Google Scholar] [CrossRef] [Green Version]
- McFarland, J.M.; Ho, Z.V.; Kugener, G.; Dempster, J.M.; Montgomery, P.G.; Bryan, J.G.; Krill-Burger, J.M.; Green, T.M.; Vazquez, F.; Boehm, J.S.; et al. Improved estimation of cancer dependencies from large-scale RNAi screens using model-based normalization and data integration. Nat. Commun. 2018, 9, 4610. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e1417. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef] [Green Version]
- Tusher, V.G.; Tibshirani, R.; Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 2001, 98, 5116–5121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Hickey, J.; Summers, K.; Falatovich, B.; Gencheva, M.; Eubank, T.D.; Ivanov, A.V.; Guo, N.L. Multi-Omics Immune Interaction Networks in Lung Cancer Tumorigenesis, Proliferation, and Survival. Int. J. Mol. Sci. 2022, 23, 14978. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Guo, N.L. Single B Cell Gene Co-Expression Networks Implicated in Prognosis, Proliferation, and Therapeutic Responses in Non-Small Cell Lung Cancer Bulk Tumors. Cancers 2022, 14, 3123. [Google Scholar] [CrossRef]
- Mashukova, A.; Forteza, R.; Wald, F.A.; Salas, P.J. PDK1 in apical signaling endosomes participates in the rescue of the polarity complex atypical PKC by intermediate filaments in intestinal epithelia. Mol. Biol. Cell 2012, 23, 1664–1674. [Google Scholar] [CrossRef]
- Feldman, R.I.; Wu, J.M.; Polokoff, M.A.; Kochanny, M.J.; Dinter, H.; Zhu, D.; Biroc, S.L.; Alicke, B.; Bryant, J.; Yuan, S.; et al. Novel small molecule inhibitors of 3-phosphoinositide-dependent kinase-1. J. Biol. Chem. 2005, 280, 19867–19874. [Google Scholar] [CrossRef] [Green Version]
- Maegawa, S.; Chinen, Y.; Shimura, Y.; Tanba, K.; Takimoto, T.; Mizuno, Y.; Matsumura-Kimoto, Y.; Kuwahara-Ota, S.; Tsukamoto, T.; Kobayashi, T.; et al. Phosphoinositide-dependent protein kinase 1 is a potential novel therapeutic target in mantle cell lymphoma. Exp. Hematol. 2018, 59, 72–81.e72. [Google Scholar] [CrossRef]
- Saleem, T.; Kasi, A. Daunorubicin; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Mayer, L.D.; Tardi, P.; Louie, A.C. CPX-351: A nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int. J. Nanomed. 2019, 14, 3819–3830. [Google Scholar] [CrossRef] [Green Version]
- Gotlib, J.; Kluin-Nelemans, H.C.; George, T.I.; Akin, C.; Sotlar, K.; Hermine, O.; Awan, F.T.; Hexner, E.; Mauro, M.J.; Sternberg, D.W.; et al. Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. N. Engl. J. Med. 2016, 374, 2530–2541. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Cancer Stat Facts: Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 26 May 2021).
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.A.; Battafarano, R.J.; Meyers, B.F.; Zoole, J.B.; Cooper, J.D.; Patterson, G.A. Prevalence of benign disease in patients undergoing resection for suspected lung cancer. Ann. Thorac. Surg. 2006, 81, 1824–1828; discussion 1828–1829. [Google Scholar] [CrossRef]
- Lee, K.H.; Lim, K.Y.; Suh, Y.J.; Hur, J.; Han, D.H.; Kang, M.J.; Choo, J.Y.; Kim, C.; Kim, J.I.; Yoon, S.H.; et al. Diagnostic Accuracy of Percutaneous Transthoracic Needle Lung Biopsies: A Multicenter Study. Korean J. Radiol. 2019, 20, 1300–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quint, L.E.; Kretschmer, M.; Chang, A.; Nan, B. CT-guided thoracic core biopsies: Value of a negative result. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2006, 6, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [Green Version]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Kan, C.F.K.; Unis, G.D.; Li, L.Z.; Gunn, S.; Li, L.; Soyer, H.P.; Stark, M.S. Circulating Biomarkers for Early Stage Non-Small Cell Lung Carcinoma Detection: Supplementation to Low-Dose Computed Tomography. Front. Oncol. 2021, 11, 555331. [Google Scholar] [CrossRef] [PubMed]
- Boeri, M.; Milione, M.; Proto, C.; Signorelli, D.; Lo Russo, G.; Galeone, C.; Verri, C.; Mensah, M.; Centonze, G.; Martinetti, A.; et al. Circulating miRNAs and PD-L1 Tumor Expression Are Associated with Survival in Advanced NSCLC Patients Treated with Immunotherapy: A Prospective Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2166–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Zhang, J.; Fu, H.; Shen, L. miR-144 functions as a tumor suppressor in breast cancer through inhibiting ZEB1/2-mediated epithelial mesenchymal transition process. OncoTargets Ther. 2016, 9, 6247–6255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Cai, J.; Meng, F.; Sui, C.; Jiang, Y. MiR-144 suppresses proliferation, invasion, and migration of breast cancer cells through inhibiting CEP55. Cancer Biol. Ther. 2018, 19, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooshkaki, O.; Rezaei, Z.; Rahmati, M.; Vahedi, P.; Derakhshani, A.; Brunetti, O.; Baghbanzadeh, A.; Mansoori, B.; Silvestris, N.; Baradaran, B. MiR-144: A New Possible Therapeutic Target and Diagnostic/Prognostic Tool in Cancers. Int. J. Mol. Sci. 2020, 21, 2578. [Google Scholar] [CrossRef] [Green Version]
- Sheng, S.; Xie, L.; Wu, Y.; Ding, M.; Zhang, T.; Wang, X. MiR-144 inhibits growth and metastasis in colon cancer by down-regulating SMAD4. Biosci. Rep. 2019, 39, BSR20181895. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.B.; Chen, Y.W.; Yao, Q.S.; Chen, X.H.; He, M.; Chen, C.B.; Yang, Y.; Gong, X.X.; Huang, L. MicroRNA-144 Suppresses Prostate Cancer Growth and Metastasis by Targeting EZH2. Technol. Cancer Res. Treat. 2021, 20, 1533033821989817. [Google Scholar] [CrossRef]
- Wu, M.; Huang, C.; Huang, X.; Liang, R.; Feng, Y.; Luo, X. MicroRNA-144-3p suppresses tumor growth and angiogenesis by targeting SGK3 in hepatocellular carcinoma. Oncol. Rep. 2017, 38, 2173–2181. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Liu, X.; Li, J.; He, Y. MicroRNA-144 inhibits cell proliferation, migration and invasion in human hepatocellular carcinoma by targeting CCNB1. Cancer Cell Int. 2019, 19, 15. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, P.; Li, J.; Wang, Y.; Du, Y.; Chen, X.; Zang, W.; Wang, H.; Chu, H.; Zhao, G.; et al. MiR-144 inhibits proliferation and induces apoptosis and autophagy in lung cancer cells by targeting TIGAR. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 35, 997–1007. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, X.; Sun, Q.; Guo, H.; Wu, X.; Xie, F.; Xu, Q.; Yan, M.; Liu, J.; Han, Z.; et al. Transcriptional control of PAX4-regulated miR-144/451 modulates metastasis by suppressing ADAMs expression. Oncogene 2015, 34, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Manasa, V.G.; Thomas, S.; Kannan, S. MiR-144/451a cluster synergistically modulates growth and metastasis of Oral Carcinoma. Oral Dis. 2023, 29, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Qiu, Y.; Liu, X.; Huang, F. Biomimetic Nanosystems for the Synergistic Delivery of miR-144/451a for Oral Squamous Cell Carcinoma. Balk. Med. J. 2022, 39, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liang, X.; Li, X.; Zhang, Y.; Sun, Z.; Liu, Y.; Wang, J. MicroRNA-195: A review of its role in cancers. OncoTargets Ther. 2018, 11, 7109–7123. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Berg, N.; Lee, J.W.; Le, T.T.; Neudecker, V.; Jing, N.; Eltzschig, H. MicroRNA miR-223 as regulator of innate immunity. J. Leukoc. Biol. 2018, 104, 515–524. [Google Scholar] [CrossRef]
- Pulikkan, J.A.; Dengler, V.; Peramangalam, P.S.; Peer Zada, A.A.; Müller-Tidow, C.; Bohlander, S.K.; Tenen, D.G.; Behre, G. Cell-cycle regulator E2F1 and microRNA-223 comprise an autoregulatory negative feedback loop in acute myeloid leukemia. Blood 2010, 115, 1768–1778. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Liu, X.H.; Sun, Y.R. MiR-223-3p inhibits proliferation and metastasis of oral squamous cell carcinoma by targeting SHOX2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6927–6934. [Google Scholar] [CrossRef]
- Dou, L.; Han, K.; Xiao, M.; Lv, F. miR-223-5p Suppresses Tumor Growth and Metastasis in Non-Small Cell Lung Cancer by Targeting E2F8. Oncol. Res. 2019, 27, 261–268. [Google Scholar] [CrossRef]
- Fang, G.; Liu, J.; Wang, Q.; Huang, X.; Yang, R.; Pang, Y.; Yang, M. MicroRNA-223-3p Regulates Ovarian Cancer Cell Proliferation and Invasion by Targeting SOX11 Expression. Int. J. Mol. Sci. 2017, 18, 1208. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Chen, Y.; Zhang, B.; Liu, G. Increased microRNA-223 in Helicobacter pylori-associated gastric cancer contributed to cancer cell proliferation and migration. Biosci. Biotechnol. Biochem. 2014, 78, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Haneklaus, M.; Gerlic, M.; O’Neill, L.A.; Masters, S.L. miR-223: Infection, inflammation and cancer. J. Intern. Med. 2013, 274, 215–226. [Google Scholar] [CrossRef]
- Baraniskin, A.; Birkenkamp-Demtroder, K.; Maghnouj, A.; Zöllner, H.; Munding, J.; Klein-Scory, S.; Reinacher-Schick, A.; Schwarte-Waldhoff, I.; Schmiegel, W.; Hahn, S.A. MiR-30a-5p suppresses tumor growth in colon carcinoma by targeting DTL. Carcinogenesis 2012, 33, 732–739. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Yang, L.; Lin, X.; Chen, X.; Lin, X.; Wei, F.; Liang, X.; Luo, Y.; Wu, Y.; Gan, T.; et al. MiR-30a-5p suppresses cell growth and enhances apoptosis of hepatocellular carcinoma cells via targeting AEG-1. Int. J. Clin. Exp. Pathol. 2015, 8, 15632–15641. [Google Scholar] [PubMed]
- Tang, J.; Ahmad, A.; Sarkar, F.H. The Role of MicroRNAs in Breast Cancer Migration, Invasion and Metastasis. Int. J. Mol. Sci. 2012, 13, 13414–13437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhang, X.; Li, N.; Liu, Q.; Chen, D. miR-30b inhibits cancer cell growth, migration, and invasion by targeting homeobox A1 in esophageal cancer. Biochem. Biophys. Res. Commun. 2017, 485, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Fu, Z.; Zhang, S.; Chen, J. miR-30b-5p inhibits proliferation, invasion, and migration of papillary thyroid cancer by targeting GALNT7 via the EGFR/PI3K/AKT pathway. Cancer Cell Int. 2021, 21, 618. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Hou, S.; Cheng, Z.; Yuan, M. Non-small cell lung cancer: miR-30d suppresses tumor invasion and migration by directly targeting NFIB. Biotechnol. Lett. 2017, 39, 1827–1834. [Google Scholar] [CrossRef]
- Kanthaje, S.; Baikunje, N.; Kandal, I.; Ratnacaram, C.K. Repertoires of MicroRNA-30 family as gate-keepers in lung cancer. FBS 2021, 13, 141–156. [Google Scholar] [CrossRef]
- Su, W.; Hong, L.; Xu, X.; Huang, S.; Herpai, D.; Li, L.; Xu, Y.; Truong, L.; Hu, W.Y.; Wu, X.; et al. miR-30 disrupts senescence and promotes cancer by targeting both p16(INK4A) and DNA damage pathways. Oncogene 2018, 37, 5618–5632. [Google Scholar] [CrossRef]
- Liu, J.; Bian, T.; Feng, J.; Qian, L.; Zhang, J.; Jiang, D.; Zhang, Q.; Li, X.; Liu, Y.; Shi, J. miR-335 inhibited cell proliferation of lung cancer cells by target Tra2β. Cancer Sci. 2018, 109, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xiao, H.; Wu, D.; Zhang, D.; Zhang, Z. miR-335-5p Regulates Cell Cycle and Metastasis in Lung Adenocarcinoma by Targeting CCNB2. OncoTargets Ther. 2020, 13, 6255–6263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, T.; Huang, H.; Jiang, Y.; Yang, L.; Lin, Z.; He, H.; Liu, T.; Wu, B.; Chen, J.; et al. miR-363-3p inhibits tumor growth by targeting PCNA in lung adenocarcinoma. Oncotarget 2017, 8, 20133–20144. [Google Scholar] [CrossRef]
- Chang, J.; Gao, F.; Chu, H.; Lou, L.; Wang, H.; Chen, Y. miR-363-3p inhibits migration, invasion, and epithelial-mesenchymal transition by targeting NEDD9 and SOX4 in non-small-cell lung cancer. J. Cell. Physiol. 2020, 235, 1808–1820. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Li, L.H.; Tang, J.B.; Sheng, Y.L. Mir-451 inhibits proliferation and migration of non-small cell lung cancer cells via targeting LKB1/AMPK. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 274–280. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Cui, J.Y.; Yuan, J.; Wang, X. MiR-451a suppressed cell migration and invasion in non-small cell lung cancer through targeting ATF2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5554–5561. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Xu, Y.; Sun, C.; He, Z. MicroRNA-451 sensitizes lung cancer cells to cisplatin through regulation of Mcl-1. Mol. Cell. Biochem. 2016, 423, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, A.; Garcia-Mayea, Y.; Jubierre, L.; Mir, C.; Hummel, M.; Castellvi, J.; Hernández-Losa, J.; Paciucci, R.; Sansano, I.; Sun, Y.; et al. miR-99a reveals two novel oncogenic proteins E2F2 and EMR2 and represses stemness in lung cancer. Cell Death Dis. 2017, 8, e3141. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Ma, J.; Chen, L.; Piao, S.; Zhang, Y.; Zhang, S.; Ma, H.; Li, Y.; Qu, Y.; Wang, X.; et al. MiR-99a Enhances the Radiation Sensitivity of Non-Small Cell Lung Cancer by Targeting mTOR. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 471–481. [Google Scholar] [CrossRef]
- Bica-Pop, C.; Cojocneanu-Petric, R.; Magdo, L.; Raduly, L.; Gulei, D.; Berindan-Neagoe, I. Overview upon miR-21 in lung cancer: Focus on NSCLC. Cell. Mol. Life Sci. CMLS 2018, 75, 3539–3551. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Liu, X.; Hu, Y.; Ding, L.; Zhang, X.; Sun, Q.; Li, Y. Oncogenic microRNA-411 promotes lung carcinogenesis by directly targeting suppressor genes SPRY4 and TXNIP. Oncogene 2019, 38, 1892–1904. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.; Liu, Y.; Fang, X.; Liu, Y.; Fang, L.; Lin, L.; Liu, X.; Wang, N. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis 2015, 18, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Wang, X.; Ma, L.; Li, J.; Cao, G.; Zhang, S.; Lin, W. CircVAPA promotes small cell lung cancer progression by modulating the miR-377-3p and miR-494-3p/IGF1R/AKT axis. Mol. Cancer 2022, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, M.; Frémeaux-Bacchi, V.; Schaefer, F.; Choi, M.; Tang, W.H.; Le Quintrec, M.; Fakhouri, F.; Taque, S.; Nobili, F.; Martinez, F.; et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat. Genet. 2013, 45, 531–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Márquez, J.; Kohli, M.; Arteta, B.; Chang, S.; Li, W.B.; Goldblatt, M.; Vidal-Vanaclocha, F. Identification of hepatic microvascular adhesion-related genes of human colon cancer cells using random homozygous gene perturbation. Int. J. Cancer 2013, 133, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gutierrez, A.; Carbajal-Lopez, B.; Bui, T.M.; Mendoza-Rodriguez, M.; Campos-Parra, A.D.; Calderillo-Ruiz, G.; Cantú-De Leon, D.; Madrigal-Santillán, E.-O.; Sumagin, R.; Pérez-Plasencia, C.; et al. A microRNA panel that regulates proinflammatory cytokines as diagnostic and prognosis biomarkers in colon cancer. Biochem. Biophys. Rep. 2022, 30, 101252. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, J.; Zhu, L.; Xie, L.; Chen, Y.; Zhang, Y.; Zhang, W.; Yin, Y.; Peng, C.; Zhou, J.; et al. Wdr47, Camsaps, and Katanin cooperate to generate ciliary central microtubules. Nat. Commun. 2021, 12, 5796. [Google Scholar] [CrossRef]



| Clinical Information | N |
|---|---|
| Cancer Stage | 109 |
| 1 | 40 |
| 2 | 34 |
| 3 | 13 |
| Normal | 22 |
| Age | |
| <60 | 23 |
| 60–80 | 68 |
| >80 | 2 |
| Missing | 16 |
| Gender | |
| Male | 57 |
| Female | 51 |
| Missing | 1 |
| Tumor Grade | |
| 1 | 4 |
| 2 | 29 |
| 3 | 42 |
| Normal | 22 |
| Missing | 12 |
| Histology | |
| NSCLC | |
| Adenocarcinoma | 41 |
| Adenosquamous carcinoma | 1 |
| Large cell carcinoma | 2 |
| Non-small cell carcinoma | 13 |
| Sarcomatoid carcinoma | 1 |
| Squamous cell carcinoma | 1 |
| Small Cell Carcinoma | 1 |
| Carcinoid tumor | 2 |
| Normal Tissue | 22 |
| Potential Tumor Suppressive miRNAs | Potential Oncogenic miRNAs |
|---|---|
| hsa-miR-144, hsa-miR-195, hsa-miR-223, hsa-miR-30a, hsa-miR-30b, hsa-miR-30d, hsa-miR-335, hsa-miR-363, hsa-miR-451, hsa-miR-99a | hsa-miR-21, hsa-miR-31, hsa-miR-411, hsa-miR-494 |
| Drug | Pansensitive Genes | Panresistant Genes |
|---|---|---|
| afatinib | ADH7 | CCNT1, WDR47, TRIB1, hsa-miR-133a, hsa-miR-1280 |
| alectinib | CCNT1, hsa-miR-30b, hsa-miR-30d | |
| brigatinib | WDR47, hsa-miR-133a, hsa-miR-30a | |
| cabozantinib | hsa-miR-133a, hsa-miR-210 | |
| carboplatin | hsa-miR-210 | |
| cisplatin | CDK1, hsa-miR-133a, hsa-miR-30a, hsa-miR-30b | |
| crizotinib | hsa-miR-223, hsa-miR-218 | hsa-miR-30b, hsa-miR-30d |
| dabrafenib | hsa-miR-218 | GLI2, CLCN5, CDKN3 |
| dacomitinib | GLTP, FBXL4, hsa-miR-195, hsa-miR-30a | |
| docetaxel | RAB30, NCEH1, GLTP, COPG1, hsa-miR-133a, hsa-miR-210, hsa-miR-30a | |
| erlotinib | DGKE | GLTP, hsa-miR-1280 |
| gefitinib | hsa-miR-195 | |
| gemcitabine | CDK1, CDK16 | |
| osimertinib | WDR47, hsa-miR-30a | |
| paclitaxel | RAB30, hsa-miR-30a | |
| pemetrexed | CDK16, hsa-miR-34b | |
| trametinib | FOS, CDC42, PCBP1, TAOK2, NISCH, hsa-miR-195 | |
| vemurafenib | hsa-miR-195 | |
| vinorelbine | FOS, CDC42, RAB30, NCEH1, GLTP, hsa-miR-133a, hsa-miR-30a |
| Drug | Sensitive Genes | Resistant Genes |
|---|---|---|
| BX-912 | ARSD, ATG4C, CD44, CDK6, CNST, G3BP2, GSTA5, KDM6B, LGMN, MCM9, NCOR2, NEGR1, NPC2, NT5C, PTPRG, RAB32, RPTOR, RRBP1, SF3B5, SOGA3, STAU1, TGFB2, TMCC2 | BCAS1, CD14, CGNL1, PALM3, VTCN1 |
| daunorubicin | BCOR, KMT2B, NUDT8, OTUD4, SMS, SYT7 | |
| midostaurin | ACBD5, APLF, ASH1L, BSCL2, CENPB, FMO5, JAK1, NRCAM, OPA3, PCGF1, SHC1, WDR53, ZHX3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Raese, R.; Luo, D.; Cao, S.; Wan, Y.-W.; Qian, Y.; Guo, N.L. MicroRNA, mRNA, and Proteomics Biomarkers and Therapeutic Targets for Improving Lung Cancer Treatment Outcomes. Cancers 2023, 15, 2294. https://doi.org/10.3390/cancers15082294
Ye Q, Raese R, Luo D, Cao S, Wan Y-W, Qian Y, Guo NL. MicroRNA, mRNA, and Proteomics Biomarkers and Therapeutic Targets for Improving Lung Cancer Treatment Outcomes. Cancers. 2023; 15(8):2294. https://doi.org/10.3390/cancers15082294
Chicago/Turabian StyleYe, Qing, Rebecca Raese, Dajie Luo, Shu Cao, Ying-Wooi Wan, Yong Qian, and Nancy Lan Guo. 2023. "MicroRNA, mRNA, and Proteomics Biomarkers and Therapeutic Targets for Improving Lung Cancer Treatment Outcomes" Cancers 15, no. 8: 2294. https://doi.org/10.3390/cancers15082294
APA StyleYe, Q., Raese, R., Luo, D., Cao, S., Wan, Y.-W., Qian, Y., & Guo, N. L. (2023). MicroRNA, mRNA, and Proteomics Biomarkers and Therapeutic Targets for Improving Lung Cancer Treatment Outcomes. Cancers, 15(8), 2294. https://doi.org/10.3390/cancers15082294







