Extracellular Vesicles in Chronic Lymphocytic Leukemia: Tumor Microenvironment Messengers as a Basis for New Targeted Therapies?
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Chronic Lymphocytic Leukemia
1.2. The CLL Tumor Microenvironment
2. EV Generalities
2.1. Nomenclature and Biogenesis
- -
- Exosomes (EXOs) are 30–150 nm vesicles generated through endosome maturation, the formation of multivesicular bodies (MVBs), intraluminal vesicles, and the fusion of MVBs with the plasma membrane. The secretion of EXOs is regulated by the endosomal sorting complexes required for transport (ESCRT) machinery. Therefore, some common EXO markers include ESCRT proteins such as Tsg101 and Alix. Tetraspanins CD9, CD81, and CD63 are also amongst the most popular EXO markers.
- -
- Microvesicles (MVs) (previously referred to as ectosomes or microparticles) are 150–1000 nm vesicles resulting from the blebbing of the plasma membrane.
2.2. Purification
2.3. Uptake of EVs and Transfer of Their Cargo to Recipient Cells
3. CLL EVs and Their Role in the TME
3.1. Purification and Characterization of CLL EVs
3.2. Modulation of CLL Vesiculation by TME Signals
3.3. Influence of CLL EVs on the TME
3.3.1. Differentiation of Stromal Cells into CAFs
3.3.2. Induction of a Pro-Angiogenic Phenotype
3.3.3. Immunomodulation by CLL EVs
4. Clinical Implications of EV Biology in CLL
4.1. The EV Profile as a Biomarker in CLL
4.2. Interference with Immunotherapy
4.3. Novel EV-Driven Therapeutic Strategies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Hallek, M.; Al-Sawaf, O. Chronic Lymphocytic Leukemia: 2022 Update on Diagnostic and Therapeutic Procedures. Am. J. Hematol. 2021, 96, 1679–1705. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Sawitsky, A.; Cronkite, E.; Chanana, A.; Levy, R.; Pasternack, B. Clinical Staging of Chronic Lymphocytic Leukemia. Blood 1975, 46, 219–234. [Google Scholar] [CrossRef]
- Binet, J.L.; Auquier, A.; Dighiero, G.; Chastang, C.; Piguet, H.; Goasguen, J.; Vaugier, G.; Potron, G.; Colona, P.; Oberling, F.; et al. A New Prognostic Classification of Chronic Lymphocytic Leukemia Derived from a Multivariate Survival Analysis. Cancer 1981, 48, 198–206. [Google Scholar] [CrossRef]
- Haselager, M.V.; Kater, A.P.; Eldering, E. Proliferative Signals in Chronic Lymphocytic Leukemia; What Are We Missing? Front. Oncol. 2020, 10, 592205. [Google Scholar] [CrossRef]
- Redondo-Muñoz, J.; García-Pardo, A.; Teixidó, J. Molecular Players in Hematologic Tumor Cell Trafficking. Front. Immunol. 2019, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Tannoury, M.; Garnier, D.; Susin, S.A.; Bauvois, B. Current Status of Novel Agents for the Treatment of B Cell Malignancies: What’s Coming Next? Cancers 2022, 14, 6026. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Pagel, J.M. Current and Future Treatment Strategies in Chronic Lymphocytic Leukemia. J. Hematol. Oncol. 2021, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M. Chronic Lymphocytic Leukemia: 2020 Update on Diagnosis, Risk Stratification and Treatment. Am. J. Hematol. 2019, 94, 1266–1287. [Google Scholar] [CrossRef]
- Skanland, S.S.; Mato, A.R. Overcoming Resistance to Targeted Therapies in Chronic Lymphocytic Leukemia. Blood Adv. 2021, 5, 334–343. [Google Scholar] [CrossRef]
- Dubois, N.; Crompot, E.; Meuleman, N.; Bron, D.; Lagneaux, L.; Stamatopoulos, B. Importance of Crosstalk Between Chronic Lymphocytic Leukemia Cells and the Stromal Microenvironment: Direct Contact, Soluble Factors, and Extracellular Vesicles. Front. Oncol. 2020, 10, 1422. [Google Scholar] [CrossRef]
- van Attekum, M.H.A.; Eldering, E.; Kater, A.P. Chronic Lymphocytic Leukemia Cells Are Active Participants in Microenvironmental Cross-Talk. Haematologica 2017, 102, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- ten Hacken, E.; Burger, J.A. Microenvironment Interactions and B-Cell Receptor Signaling in Chronic Lymphocytic Leukemia: Implications for Disease Pathogenesis and Treatment. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.J.; Verschuer, L.A.; Harmon, B.V.; Prentice, R.L.; Pope, J.H.; Kerr, J.F.R. Spontaneous Programmed Death (Apoptosis) of B-Chronic Lymphocytic Leukaemia Cells Following Their Culture in Vitro. Br. J. Haematol. 2008, 71, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Cols, M.; Barra, C.M.; He, B.; Puga, I.; Xu, W.; Chiu, A.; Tam, W.; Knowles, D.M.; Dillon, S.R.; Leonard, J.P.; et al. Stromal Endothelial Cells Establish a Bidirectional Crosstalk with Chronic Lymphocytic Leukemia Cells through the TNF-Related Factors BAFF, APRIL, and CD40L. J. Immunol. 2012, 188, 6071–6083. [Google Scholar] [CrossRef] [PubMed]
- Calissano, C.; Damle, R.N.; Hayes, G.; Murphy, E.J.; Hellerstein, M.K.; Moreno, C.; Sison, C.; Kaufman, M.S.; Kolitz, J.E.; Allen, S.L.; et al. In Vivo Intraclonal and Interclonal Kinetic Heterogeneity in B-Cell Chronic Lymphocytic Leukemia. Blood 2009, 114, 4832–4842. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Vacca, A.; Pistoia, V.; Ribatti, D. Cancer Associated Fibroblasts in Hematological Malignancies. Oncotarget 2015, 6, 2589–2603. [Google Scholar] [CrossRef]
- Os, A.; Bürgler, S.; Ribes, A.P.; Funderud, A.; Wang, D.; Thompson, K.M.; Tjønnfjord, G.E.; Bogen, B.; Munthe, L.A. Chronic Lymphocytic Leukemia Cells Are Activated and Proliferate in Response to Specific T Helper Cells. Cell Rep. 2013, 4, 566–577. [Google Scholar] [CrossRef]
- Pascutti, M.F.; Jak, M.; Tromp, J.M.; Derks, I.A.M.; Remmerswaal, E.B.M.; Thijssen, R.; van Attekum, M.H.A.; van Bochove, G.G.; Luijks, D.M.; Pals, S.T.; et al. IL-21 and CD40L Signals from Autologous T Cells Can Induce Antigen-Independent Proliferation of CLL Cells. Blood 2013, 122, 3010–3019. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Vangapandu, H.V.; Ayres, M.L.; Bristow, C.A.; Wierda, W.G.; Keating, M.J.; Balakrishnan, K.; Stellrecht, C.M.; Gandhi, V. The Stromal Microenvironment Modulates Mitochondrial Oxidative Phosphorylation in Chronic Lymphocytic Leukemia Cells. Neoplasia 2017, 19, 762–771. [Google Scholar] [CrossRef]
- Zhang, W.; Trachootham, D.; Liu, J.; Chen, G.; Pelicano, H.; Garcia-Prieto, C.; Lu, W.; Burger, J.A.; Croce, C.M.; Plunkett, W.; et al. Stromal Control of Cystine Metabolism Promotes Cancer Cell Survival in Chronic Lymphocytic Leukaemia. Nat. Cell Biol. 2012, 14, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Jitschin, R.; Braun, M.; Qorraj, M.; Saul, D.; Le Blanc, K.; Zenz, T.; Mougiakakos, D. Stromal Cell–Mediated Glycolytic Switch in CLL Cells Involves Notch-c-Myc Signaling. Blood 2015, 125, 3432–3436. [Google Scholar] [CrossRef] [PubMed]
- Kluckova, K.; Clear, A.J.; D’Avola, A.; Rassenti, L.Z.; Kipps, T.J.; Gribben, J.G.; Riches, J.C. B-Cell Receptor Signaling Induced Metabolic Alterations in Chronic Lymphocytic Leukemia Can Be Partially Bypassed by TP53 Abnormalities. HemaSphere 2022, 6, e722. [Google Scholar] [CrossRef]
- Chen, Z.; Simon-Molas, H.; Cretenet, G.; Valle-Argos, B.; Smith, L.D.; Forconi, F.; Schomakers, B.V.; van Weeghel, M.; Bryant, D.J.; van Bruggen, J.A.C.; et al. Characterization of Metabolic Alterations of Chronic Lymphocytic Leukemia in the Lymph Node Microenvironment. Blood 2022, 140, 630–643. [Google Scholar] [CrossRef] [PubMed]
- van Bruggen, J.A.C.; Martens, A.W.J.; Fraietta, J.A.; Hofland, T.; Tonino, S.H.; Eldering, E.; Levin, M.-D.; Siska, P.J.; Endstra, S.; Rathmell, J.C.; et al. Chronic Lymphocytic Leukemia Cells Impair Mitochondrial Fitness in CD8+ T Cells and Impede CAR T-Cell Efficacy. Blood 2019, 134, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Siska, P.J.; van der Windt, G.J.W.; Kishton, R.J.; Cohen, S.; Eisner, W.; MacIver, N.J.; Kater, A.P.; Weinberg, J.B.; Rathmell, J.C. Suppression of Glut1 and Glucose Metabolism by Decreased Akt/MTORC1 Signaling Drives T Cell Impairment in B Cell Leukemia. J. Immunol. 2016, 197, 2532–2540. [Google Scholar] [CrossRef]
- Svanberg, R.; Janum, S.; Patten, P.E.M.; Ramsay, A.G.; Niemann, C.U. Targeting the Tumor Microenvironment in Chronic Lymphocytic Leukemia. Haematologica 2021, 106, 2312–2324. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Kalluri, R. The Biology and Function of Exosomes in Cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Nisticò, N.; Maisano, D.; Iaccino, E.; Vecchio, E.; Fiume, G.; Rotundo, S.; Quinto, I.; Mimmi, S. Role of Chronic Lymphocytic Leukemia (Cll)-Derived Exosomes in Tumor Progression and Survival. Pharmaceuticals 2020, 13, 244. [Google Scholar] [CrossRef]
- Gargiulo, E.; Morande, P.E.; Largeot, A.; Moussay, E.; Paggetti, J. Diagnostic and Therapeutic Potential of Extracellular Vesicles in B-Cell Malignancies. Front. Oncol. 2020, 10, 580874. [Google Scholar] [CrossRef]
- Aguilar-Hernandez, M.M.; Rincon Camacho, J.C.; Galicia Garcia, G. Extracellular Vesicles and Their Associated MiRNAs as Potential Prognostic Biomarkers in Chronic Lymphocytic Leukemia. Curr. Oncol. Rep. 2021, 23, 66. [Google Scholar] [CrossRef]
- Yang, C.; Yang, H.; Liu, J.; Zhu, L.; Yu, S.; Zhang, X.; Gao, L. Focus on Exosomes: Novel Pathogenic Components of Leukemia. Am. J. Cancer Res. 2019, 9, 1815–1829. [Google Scholar]
- Zhou, J.; Wang, S.; Sun, K.; Chng, W.J. The Emerging Roles of Exosomes in Leukemogeneis. Oncotarget 2016, 7, 50698–50707. [Google Scholar] [CrossRef]
- Wierz, M.; Pierson, S.; Gargiulo, E.; Guerin, C.; Moussay, E.; Paggetti, J. Purification of Leukemia-Derived Exosomes to Study Microenvironment Modulation. In Cancer Immunosurveillance: Methods and Protocols; Methods in Molecular Biology; Humana: Louisville, IL, USA, 2019. [Google Scholar]
- Elgamal, S.; Cocucci, E.; Sass, E.J.; Mo, X.M.; Blissett, A.R.; Calomeni, E.P.; Rogers, K.A.; Woyach, J.A.; Bhat, S.A.; Muthusamy, N.; et al. Optimizing Extracellular Vesicles’ Isolation from Chronic Lymphocytic Leukemia Patient Plasma and Cell Line Supernatant. JCI Insight 2021, 6, e137937. [Google Scholar] [CrossRef] [PubMed]
- Elgamal, S.; Colombo, F.; Cottini, F.; Byrd, J.C.; Cocucci, E. Imaging Intercellular Interaction and Extracellular Vesicle Exchange in a Co-Culture Model of Chronic Lymphocytic Leukemia and Stromal Cells by Lattice Light-Sheet Fluorescence Microscopy. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2020; pp. 79–107. ISBN 9780128206621. [Google Scholar]
- Farahani, M.; Rubbi, C.; Liu, L.; Slupsky, J.R.; Kalakonda, N. CLL Exosomes Modulate the Transcriptome and Behaviour of Recipient Stromal Cells and Are Selectively Enriched in MiR-202-3p. PLoS ONE 2015, 10, e0141429. [Google Scholar] [CrossRef] [PubMed]
- Boysen, J.; Nelson, M.; Magzoub, G.; Maiti, G.P.; Sinha, S.; Goswami, M.; Vesely, S.K.; Shanafelt, T.D.; Kay, N.E.; Ghosh, A.K. Dynamics of Microvesicle Generation in B-Cell Chronic Lymphocytic Leukemia: Implication in Disease Progression. Leukemia 2017, 31, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Reiners, K.S.; Shatnyeva, O.; Vasyutina, E.; Bösl, T.; Hansen, H.P.; Hallek, M.; Herling, M.; von Strandmann, E.P. Extracellular Vesicles Released from Chronic Lymphocytic Leukemia Cells Exhibit a Disease Relevant MRNA Signature and Transfer MRNA to Bystander Cells. Haematologica 2017, 102, e100–e103. [Google Scholar] [CrossRef]
- Yeh, Y.-Y.; Ozer, H.G.; Lehman, A.M.; Maddocks, K.; Yu, L.; Johnson, A.J.; Byrd, J.C. Characterization of CLL Exosomes Reveals a Distinct MicroRNA Signature and Enhanced Secretion by Activation of BCR Signaling. Blood 2015, 125, 3297–3305. [Google Scholar] [CrossRef]
- De Luca, L.; D’Arena, G.; Simeon, V.; Trino, S.; Laurenzana, I.; Caivano, A.; La Rocca, F.; Villani, O.; Mansueto, G.; Deaglio, S.; et al. Characterization and Prognostic Relevance of Circulating Microvesicles in Chronic Lymphocytic Leukemia. Leuk. Lymphoma 2017, 58, 1424–1432. [Google Scholar] [CrossRef]
- Belov, L.; Matic, K.J.; Hallal, S.; Best, O.G.; Mulligan, S.P.; Christopherson, R.I. Extensive Surface Protein Profiles of Extracellular Vesicles from Cancer Cells May Provide Diagnostic Signatures from Blood Samples. J. Extracell. Vesicles 2016, 5, 25355. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Secreto, C.R.; Knox, T.R.; Ding, W.; Mukhopadhyay, D.; Kay, N.E. Circulating Microvesicles in B-Cell Chronic Lymphocytic Leukemia Can Stimulate Marrow Stromal Cells: Implications for Disease Progression. Blood 2010, 115, 1755–1764. [Google Scholar] [CrossRef]
- Smallwood, D.T.; Apollonio, B.; Willimott, S.; Lezina, L.; Alharthi, A.; Ambrose, A.R.; De Rossi, G.; Ramsay, A.G.; Wagner, S.D. Extracellular Vesicles Released by CD40/IL-4–Stimulated CLL Cells Confer Altered Functional Properties to CD4+ T Cells. Blood 2016, 128, 542–552. [Google Scholar] [CrossRef]
- Paggetti, J.; Haderk, F.; Seiffert, M.; Janji, B.; Distler, U.; Ammerlaan, W.; Kim, Y.J.; Adam, J.; Lichter, P.; Solary, E.; et al. Exosomes Released by Chronic Lymphocytic Leukemia Cells Induce the Transition of Stromal Cells into Cancer-Associated Fibroblasts. Blood 2015, 126, 1106–1117. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Geng, Y. Exosomes Derived from Chronic Lymphocytic Leukaemia Cells Transfer MiR-146a to Induce the Transition of Mesenchymal Stromal Cells into Cancer-Associated Fibroblasts. J. Biochem. 2020, 168, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Crompot, E.; Van Damme, M.; Pieters, K.; Vermeersch, M.; Perez-Morga, D.; Mineur, P.; Maerevoet, M.; Meuleman, N.; Bron, D.; Lagneaux, L.; et al. Extracellular Vesicles of Bone Marrow Stromal Cells Rescue Chronic Lymphocytic Leukemia B Cells from Apoptosis, Enhance Their Migration and Induce Gene Expression Modifications. Haematologica 2017, 102, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Feng, Z.; Zhang, J.; Li, G. Exosomal CLIC1 Released by CLL Promotes HUVECs Angiogenesis by Regulating ITGβ1-MAPK/ERK Axis. Kaohsiung J. Med. Sci. 2021, 37, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Haderk, F.; Schulz, R.; Iskar, M.; Cid, L.L.; Worst, T.; Willmund, K.V.; Schulz, A.; Warnken, U.; Seiler, J.; Benner, A.; et al. Tumor-Derived Exosomes Modulate PD-L1 Expression in Monocytes. Sci. Immunol. 2017, 2, eaah5509. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, J.; Liu, J.; Fan, L.; Lin, X.; Yu, H.; Sun, G. Exosomal NAMPT from Chronic Lymphocytic Leukemia Cells Orchestrate Monocyte Survival and Phenotype under Endoplasmic Reticulum Stress. Hematol. Oncol. 2022, 41, 61–70. [Google Scholar] [CrossRef]
- Bruns, H.; Böttcher, M.; Qorraj, M.; Fabri, M.; Jitschin, S.; Dindorf, J.; Busch, L.; Jitschin, R.; Mackensen, A.; Mougiakakos, D. CLL-Cell-Mediated MDSC Induction by Exosomal MiR-155 Transfer Is Disrupted by Vitamin D. Leukemia 2017, 31, 985–988. [Google Scholar] [CrossRef]
- Böttcher, M.; Böttcher-Loschinski, R.; Kahlfuss, S.; Aigner, M.; Gießl, A.; Mackensen, A.; Schlötzer-Schrehardt, U.; Tüting, T.; Bruns, H.; Mougiakakos, D. CLL-Derived Extracellular Vesicles Impair T-Cell Activation and Foster T-Cell Exhaustion via Multiple Immunological Checkpoints. Cells 2022, 11, 2176. [Google Scholar] [CrossRef]
- Gargiulo, E.; Viry, E.; Morande, P.E.; Largeot, A.; Gonder, S.; Xian, F.; Ioannou, N.; Benzarti, M.; Kleine Borgmann, F.B.; Mittelbronn, M.; et al. Extracellular Vesicle Secretion by Leukemia Cells In Vivo Promotes CLL Progression by Hampering Antitumor T-Cell Responses. Blood Cancer Discov. 2023, 4, 54–77. [Google Scholar] [CrossRef]
- Caivano, A.; La Rocca, F.; Simeon, V.; Girasole, M.; Dinarelli, S.; Laurenzana, I.; De Stradis, A.; De Luca, L.; Trino, S.; Traficante, A.; et al. MicroRNA-155 in Serum-Derived Extracellular Vesicles as a Potential Biomarker for Hematologic Malignancies—A Short Report. Cell. Oncol. 2017, 40, 97–103. [Google Scholar] [CrossRef]
- Caivano, A.; Laurenzana, I.; De Luca, L.; La Rocca, F.; Simeon, V.; Trino, S.; D’Auria, F.; Traficante, A.; Maietti, M.; Izzo, T.; et al. High Serum Levels of Extracellular Vesicles Expressing Malignancy-Related Markers Are Released in Patients with Various Types of Hematological Neoplastic Disorders. Tumor Biol. 2015, 36, 9739–9752. [Google Scholar] [CrossRef]
- Ishdorj, G.; Nugent, Z.; Squires, M.; Kost, S.; Banerji, V.; Davidson, L.; Katyal, C.S.; Marshall, A.; Gibson, S.B.; Johnston, J.B. Rapid Improvement in Symptoms and Physical Function Following Ibrutinib Initiation in Chronic Lymphocytic Leukemia and the Associated Changes in Plasma Cytokines. Leuk. Res. 2021, 109, 106628. [Google Scholar] [CrossRef] [PubMed]
- Saunderson, S.C.; Schuberth, P.C.; Dunn, A.C.; Miller, L.; Hock, B.D.; MacKay, P.A.; Koch, N.; Jack, R.W.; McLellan, A.D. Induction of Exosome Release in Primary B Cells Stimulated via CD40 and the IL-4 Receptor. J. Immunol. 2008, 180, 8146–8152. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, D.; Han, Y.; Huang, T.; He, X.; Wang, J.; Ou, C. Emerging Role of Cancer-Associated Fibroblasts-Derived Exosomes in Tumorigenesis. Front. Immunol. 2021, 12, 795372. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Teixeira, A.F.; Zhu, H.-J.; ten Dijke, P. Cancer Associated-Fibroblast-Derived Exosomes in Cancer Progression. Mol. Cancer 2021, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Masoudi-Khoram, N.; Soheilifar, M.H.; Ghorbanifar, S.; Nobari, S.; Hakimi, M.; Hassani, M. Exosomes Derived from Cancer-Associated Fibroblasts Mediate Response to Cancer Therapy. Crit. Rev. Oncol. Hematol. 2023, in press. [CrossRef] [PubMed]
- de Oliveira, T.D.; vom Stein, A.; Rebollido-Rios, R.; Lobastova, L.; Lettau, M.; Janssen, O.; Wagle, P.; Nguyen, P.-H.; Hallek, M.; Hansen, H.P. Stromal Cells Support the Survival of Human Primary Chronic Lymphocytic Leukemia (CLL) Cells through Lyn-Driven Extracellular Vesicles. Front. Med. 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Lu, R.N.; Li, J. Angiogenic Factors in Chronic Lymphocytic Leukemia. Leuk. Res. 2012, 36, 1211–1217. [Google Scholar] [CrossRef]
- Gargiulo, E.; Viry, E.; Moussay, E.; Paggetti, J. Small Extracellular Vesicles: Multi-Faceted Tools for Leukemia Immune Evasion in Vivo. Oncoimmunology 2022, 11, 2127507. [Google Scholar] [CrossRef]
- Reiners, K.S.; Topolar, D.; Henke, A.; Simhadri, V.R.; Kessler, J.; Sauer, M.; Bessler, M.; Hansen, H.P.; Tawadros, S.; Herling, M.; et al. Soluble Ligands for NK Cell Receptors Promote Evasion of Chronic Lymphocytic Leukemia Cells from NK Cell Anti-Tumor Activity. Blood 2013, 121, 3658–3665. [Google Scholar] [CrossRef]
- Caivano, A.; La Rocca, F.; Laurenzana, I.; Trino, S.; De Luca, L.; Lamorte, D.; Del Vecchio, L.; Musto, P. Extracellular Vesicles in Hematological Malignancies: From Biology to Therapy. Int. J. Mol. Sci. 2017, 18, 1183. [Google Scholar] [CrossRef]
- Trino, S.; Lamorte, D.; Caivano, A.; De Luca, L.; Sgambato, A.; Laurenzana, I. Clinical Relevance of Extracellular Vesicles in Hematological Neoplasms: From Liquid Biopsy to Cell Biopsy. Leukemia 2021, 35, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Khalife, J.; Sanchez, J.F.; Pichiorri, F. Extracellular Vesicles in Hematological Malignancies: From Biomarkers to Therapeutic Tools. Diagnostics 2020, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Hill, A.F. Therapeutically Harnessing Extracellular Vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef] [PubMed]
- Katsaraki, K.; Karousi, P.; Artemaki, P.I.; Scorilas, A.; Pappa, V.; Kontos, C.K.; Papageorgiou, S.G. MicroRNAs: Tiny Regulators of Gene Expression with Pivotal Roles in Normal B-Cell Development and B-Cell Chronic Lymphocytic Leukemia. Cancers 2021, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Fathullahzadeh, S.; Khanmohammadi, R.; Darijani, M.; Momeni, F.; Masoudifar, A.; Goodarzi, M.; Mardanshah, O.; Stenvang, J.; Jaafari, M.R.; et al. State of the Art in MicroRNA as Diagnostic and Therapeutic Biomarkers in Chronic Lymphocytic Leukemia. J. Cell. Physiol. 2018, 233, 888–900. [Google Scholar] [CrossRef]
- Ferrajoli, A.; Shanafelt, T.D.; Ivan, C.; Shimizu, M.; Rabe, K.G.; Nouraee, N.; Ikuo, M.; Ghosh, A.K.; Lerner, S.; Rassenti, L.Z.; et al. Prognostic Value of MiR-155 in Individuals with Monoclonal B-Cell Lymphocytosis and Patients with B Chronic Lymphocytic Leukemia. Blood 2013, 122, 1891–1899. [Google Scholar] [CrossRef]
- Cui, B.; Chen, L.; Zhang, S.; Mraz, M.; Fecteau, J.-F.; Yu, J.; Ghia, E.M.; Zhang, L.; Bao, L.; Rassenti, L.Z.; et al. MicroRNA-155 Influences B-Cell Receptor Signaling and Associates with Aggressive Disease in Chronic Lymphocytic Leukemia. Blood 2014, 124, 546–554. [Google Scholar] [CrossRef]
- Stamatopoulos, B.; Van Damme, M.; Crompot, E.; Dessars, B.; El Housni, H.; Mineur, P.; Meuleman, N.; Bron, D.; Lagneaux, L. Opposite Prognostic Significance of Cellular and Serum Circulating MicroRNA-150 in Patients with Chronic Lymphocytic Leukemia. Mol. Med. 2015, 21, 123–133. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, H.; Wang, C.; Liu, W.; Liu, M.; Zhu, Y.; Xu, W.; Jin, H.; Li, J. Mitochondrial Genome-Derived CircRNA Mc-COX2 Functions as an Oncogene in Chronic Lymphocytic Leukemia. Mol. Ther.-Nucleic Acids 2020, 20, 801–811. [Google Scholar] [CrossRef]
- Prieto, D.; Sotelo, N.; Seija, N.; Sernbo, S.; Abreu, C.; Durán, R.; Gil, M.; Sicco, E.; Irigoin, V.; Oliver, C.; et al. S100-A9 Protein in Exosomes from Chronic Lymphocytic Leukemia Cells Promotes NF-ΚB Activity during Disease Progression. Blood 2017, 130, 777–788. [Google Scholar] [CrossRef]
- Lortholary, P.; Boiron, M.; Ripault, P.; Levy, J.P.; Manus, A.; Bernard, J. Chronic Lymphoid Leukemia Secondarily Associated with a Malignant Reticulopathy: Richter’s Syndrome. Nouv. Rev. Fr. Hematol. 1964, 4, 621–644. [Google Scholar] [PubMed]
- Fabbri, G.; Khiabanian, H.; Holmes, A.B.; Wang, J.; Messina, M.; Mullighan, C.G.; Pasqualucci, L.; Rabadan, R.; Dalla-Favera, R. Genetic Lesions Associated with Chronic Lymphocytic Leukemia Transformation to Richter Syndrome. J. Exp. Med. 2013, 210, 2273–2288. [Google Scholar] [CrossRef] [PubMed]
- Jurj, A.; Pop, L.; Petrushev, B.; Pasca, S.; Dima, D.; Frinc, I.; Deak, D.; Desmirean, M.; Trifa, A.; Fetica, B.; et al. Exosome-Carried MicroRNA-Based Signature as a Cellular Trigger for the Evolution of Chronic Lymphocytic Leukemia into Richter Syndrome. Crit. Rev. Clin. Lab. Sci. 2018, 55, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.J.; Lucien, F.; Sakemura, R.; Boysen, J.C.; Kim, Y.; Horvei, P.; Manriquez Roman, C.; Hansen, M.J.; Tapper, E.E.; Siegler, E.L.; et al. Leukemic Extracellular Vesicles Induce Chimeric Antigen Receptor T Cell Dysfunction in Chronic Lymphocytic Leukemia. Mol. Ther. 2021, 29, 1529–1540. [Google Scholar] [CrossRef]
- Aung, T.; Chapuy, B.; Vogel, D.; Wenzel, D.; Oppermann, M.; Lahmann, M.; Weinhage, T.; Menck, K.; Hupfeld, T.; Koch, R.; et al. Exosomal Evasion of Humoral Immunotherapy in Aggressive B-Cell Lymphoma Modulated by ATP-Binding Cassette Transporter A3. Proc. Natl. Acad. Sci. USA 2011, 108, 15336–15341. [Google Scholar] [CrossRef]
- Oksvold, M.P.; Kullmann, A.; Forfang, L.; Kierulf, B.; Li, M.; Brech, A.; Vlassov, A.V.; Smeland, E.B.; Neurauter, A.; Pedersen, K.W. Expression of B-Cell Surface Antigens in Subpopulations of Exosomes Released from B-Cell Lymphoma Cells. Clin. Ther. 2014, 36, 847–862.e1. [Google Scholar] [CrossRef]
- Ruiss, R.; Jochum, S.; Mocikat, R.; Hammerschmidt, W.; Zeidler, R. EBV-Gp350 Confers B-Cell Tropism to Tailored Exosomes and Is a Neo-Antigen in Normal and Malignant B Cells—A New Option for the Treatment of B-CLL. PLoS ONE 2011, 6, e25294. [Google Scholar] [CrossRef]
- Gärtner, K.; Luckner, M.; Wanner, G.; Zeidler, R. Engineering Extracellular Vesicles as Novel Treatment Options: Exploiting Herpesviral Immunity in CLL. J. Extracell. Vesicles 2019, 8, 1573051. [Google Scholar] [CrossRef]
- Xiu, H.; Nan, X.; Guo, D.; Wang, J.; Li, J.; Peng, Y.; Xiong, G.; Wang, S.; Wang, C.; Zhang, G.; et al. Gp350-Anchored Extracellular Vesicles: Promising Vehicles for Delivering Therapeutic Drugs of B Cell Malignancies. Asian J. Pharm. Sci. 2022, 17, 462–474. [Google Scholar] [CrossRef]
- Zhang, F.; Li, R.; Yang, Y.; Shi, C.; Shen, Y.; Lu, C.; Chen, Y.; Zhou, W.; Lin, A.; Yu, L.; et al. Specific Decrease in B-Cell-Derived Extracellular Vesicles Enhances Post-Chemotherapeutic CD8 + T Cell Responses. Immunity 2019, 50, 738–750.e7. [Google Scholar] [CrossRef]
- Fu, W.; Lei, C.; Liu, S.; Cui, Y.; Wang, C.; Qian, K.; Li, T.; Shen, Y.; Fan, X.; Lin, F.; et al. CAR Exosomes Derived from Effector CAR-T Cells Have Potent Antitumour Effects and Low Toxicity. Nat. Commun. 2019, 10, 4355. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Vaiselbuh, S.R. CD19 Chimeric Antigen Receptor-Exosome Targets CD19 Positive B-Lineage Acute Lymphocytic Leukemia and Induces Cytotoxicity. Cancers 2021, 13, 1401. [Google Scholar] [CrossRef] [PubMed]
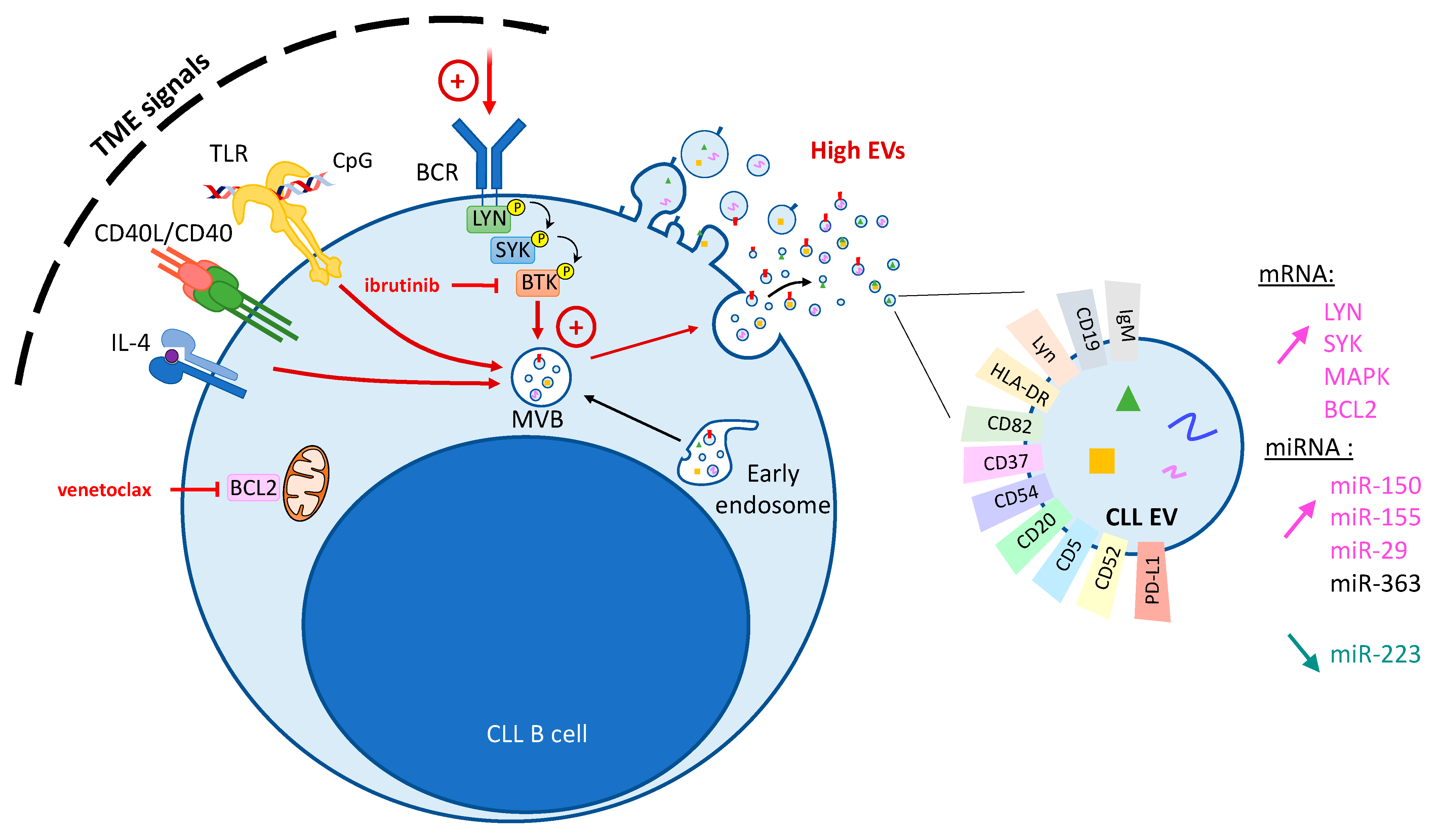
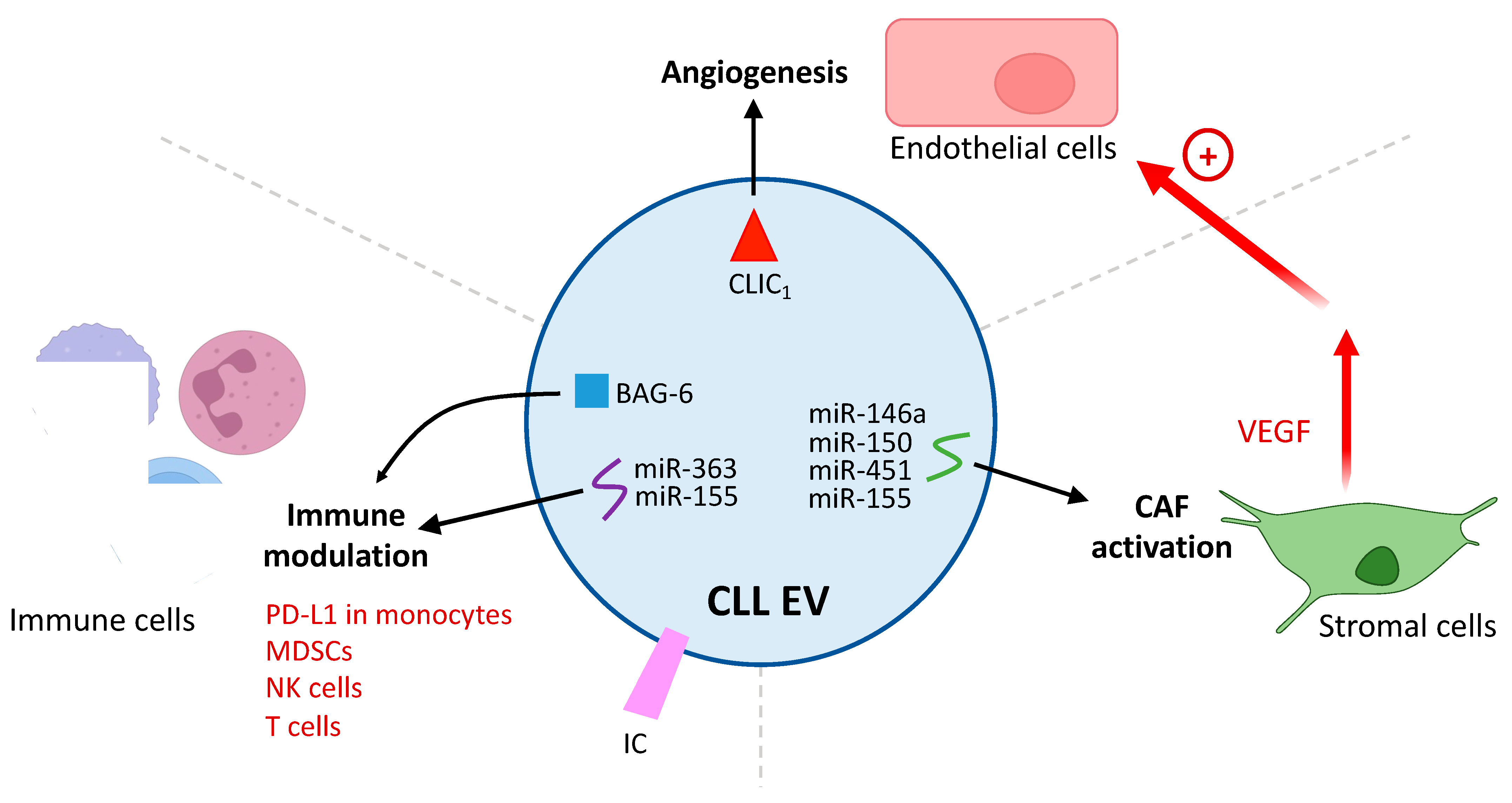
| Donor Cells | EVs | EV Markers | Cargo | Purification Protocol | Target Cells | Results | Ref |
|---|---|---|---|---|---|---|---|
| Untreated CLL patients (n = 34) and healthy donors, MEC-1 | EXO | TSG101, MHC I/II, IgM, Lyn kinase, CD81, CD37, ITGA4 | miR-202-3p | 10 min at 500× g, 10 min at 4000× g, 30 min at 18,000× g, filtration (Immuno-magnetic isolation), UC 90 min at 100,000× g (×2) | HS-5 | CLL EXOs ↑ expression of genes such as c-fos and ATM; ↑ proliferation of recipient HS-5 cells; CLL EXOs enriched in certain miRNAs | [44] |
| Primary CLL B-cells and normal B cells, plasma (n = 33 CLL/n = 9 treatment-naïve CLL patients/n = 5 CLL patients under ibrutinib) | MV | CD52 | - | 20 min at 2500× g (×3); 1 h at 16,000× g at 4 °C | - | ↑ CD52+ MVs with BCR stimulation in CLL B-cells; ↑ plasma CD52+ MVs correlated to tumor progression; ↓ plasma CD52+ MVs after ibrutinib therapy | [45] |
| Primary CLL and healthy B cells | EV | CD63, CD9, CD54, CD82 | TCL1A-mRNA | 10 min at 300× g, 15 min at 6800× g (×2), UC 90 min at 100,000× g (×2) | HDFn and THP-1 | CLL-CpG-EVs contain disease-relevant mRNA; ↑ CLL-EVs compared to healthy B cells | [46] |
| CD19+ B cells from CLL patients and healthy donors | EXO | CD63, CD9, CD37 | miR-155, miR-150 | 10 min at 300× g, 10 min at 2000× g, 30 min at 10,000× g at 4 °C, UC 70 min at 100,000× g at 4 °C (×2) | - | ↑ EXOs in CLL patients’ plasma; ↑ EXOs with BCR activation by α-IgM in CLL B cells; ibrutinib impedes α-IgM-stimulation EXO release; ↑ EXO miR-150 and miR-155 with BCR activation | [47] |
| Serum (n = 131 CLL/n = 28 healthy controls) | MV | CD19, CD37 | 20 min at 2000× g at 4 °C, 30 min at 10,000× g at 4 °C, UC 70 min at 100,000× g at 4 °C (×2) | - | ↑ MVs in CLL patients’ plasma; CD19+ CD37+ MVs correlate to tumor progression; total MVs predict for overall survival and time to treatment | [48] | |
| Plasma (n = 60 CLL, n = 5 healthy controls) | MV | CD19 | 20 min at 2500× g (×3), 1 h at 16,000× g at 4 °C | HS-5, primary BMSCs | ↑ MVs in CLL patients’ plasma; ↑ VEGF, B-catenin pathway, cyclin D1, and c-myc in CLL-BMSCs | [50] | |
| CD19+ CD5+ B cells CLL patient and healthy donors; plasma CLL patients | EV comprise EXO | - | miR-363, miR-155, miR-374b | 5 min at 250× g at 4 °C, 10 min at 2000× g at 4 °C, 30 min at 10,000× g at 4 °C, UC 110 min at 100,000× g | CD4+ T cellsfrom CLL patients | CD40/IL-4–stimulated CLL cells released specific EV miRNAs; ↑ migration; proliferation of CD4+ T cells; immunological synapse signaling | [51] |
| Primary CLL (n = 21), MEC-1 | EXO | ALIX, TSG101, HLA-DR | 10 min at 400× g (×2), 20 min at 2000× g, filtration 0.45 μm, UC at 110,000× g at 4 °C, flotation on Optiprep cushion (Axis-Shield, 17%) for 75 min at 100,000× g at 4 °C, filtration 0.45 μm | Human BM-MSCs, HMEC-1, HS5 | CLL-EXO transfer protein and miRNA into stromal cells that induce a CAF-like phenotype; uptake by endothelial cells ↑ angiogenesis | [52] | |
| MEC-1 | EXO | CD63, CD9 | miR-146a | 10 min at 400× g (×2), 20 min at 2000× g, filtration 0.45 μm, UC 70 min at 110,000× g (×2), 75 min at 100,000× g at 4 °C, filtration 0.45 μm | Human BM-MSCs | CLL cells deliver miR-146a to BM-MSCs that induce CAFs phenotype by down-regulation of USP16 mRNA expression | [53] |
| Human BM-MSCs | EV | CD63 | 10 min at 300× g (×2), concentrated on 3 K centrifugal device, UC 1 h at 150,000× g at 4 °C (×2) | Primary CLL | BM-MSCs ↓ B CLL spontaneous apoptosis and ↑ chemoresistance to fludarabine, ibrutinib, idelalisib and venetoclax; ↑ CLL B cells migration | [54] | |
| Plasma CLL patients, MEC-1 | EXO | CD63, CD81, TSG101 | CLIC1 | 20 min at 400× g, 40 min at 2000× g, filtration 0,45 μm, UC 70 min at 110,000× g at 4 °C, floatation on Optiprep cushion (Axis-Shield, 17%) 75 min at 100,000× g at 4 °C, filtration 0.45 μm | HUVECs | MEC-1 EXO invasion; metastasis and angiogenesis of HUVECs by transferring CLIC1 | [55] |
| Plasma CLL patients and healthy donors, MEC-1 | EXO | RAB5a, HSP70, HLA-DR, CD81 | noncoding Y RNA hY4 | 300× g and 10,000× g, UC at 100,000× g, UC on 40% sucrose cushion | Human monocytes or murine BM-MDSCs | ↑ release of cytokines, such as CCL2, CCL4, and interleukin-6,and expression of PD-L1 | [56] |
| Untreated CLL patients (n = 26, aggressive /indolent)and healthy donors, MEC1 and HG3 | EXO | CD63, CD81 | NAMPT | 15 min at 3000× g, ExoQuick -TC reagent overnight at 4 °C, 30 min at 1500× g | Primary monocytes (CD14+ CD16+) | CLL-EXO transfer NAMPT to monocytes; ↑ NAD+ (nicotinamide adenine dinucleotide) which activatedSIRT1-C/EBPβ signaling pathway in monocytes | [57] |
| CLL patients (n = 56) and healthy donors, EHEB and MEC1, serum CLL patients and healthy donors | EXO | CD63, CD81 | 10 min at 500× g, 20 min at 16,500× g, filtration 0.2 μm, UC 70 min at 110,000× g at 4 °C (×2) and filtration 0.2 μm | CD14+HLA-Drlow monocytes (MDSCs) | miR-155 in CLL-EXO induces MDSCs; is disrupted by vitamin D | [58] | |
| PB and plasma samples, CLL patient and healthy donors, MEC-1, HG-3, EHEB, and PGA1 | EV | CD9, CD63, CD81, CD19, CD20, CD40 | ICs | filtration 0.2 μm, UC 70 min at 110,000× g at 4 °C | T-cells | CLL-EV contain ICs that may hamper T-cell viability, proliferation, activation, and metabolism | [59] |
| Eμ-TCL1 CLL murine model (WT B cells from C57BL/6 mouse) | sEV (EXO) | Alix, TSG101, CD63, CD9, CD81 | miR-150, -155, -21, -146a, -378a, and -27a, IC ligands | 5 min at 400× g, 20 min at 400× g, 40 min at 2000× g, 60 min at 10,000× g, filtration 0.2 μm, UC 70 min at 110,000× g at 4 °C, flotation on 17% Optiprep cushion, 75 min at 100,000× g at 4 °C, UC 70 min at 110,000× g at 4 °C, filtration 0.45 μm and 0.22 μm | CD8+ T cells | small EVs secreted by CLL cells in mouse model inhibit CD8+ T-cell immune response against tumor cells | [60] |
| Serum (n = 9 CLL/n = 18 healthy controls) | EV | - | - | ↑ miR-155 in CLL EVs | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubois, K.; Tannoury, M.; Bauvois, B.; Susin, S.A.; Garnier, D. Extracellular Vesicles in Chronic Lymphocytic Leukemia: Tumor Microenvironment Messengers as a Basis for New Targeted Therapies? Cancers 2023, 15, 2307. https://doi.org/10.3390/cancers15082307
Dubois K, Tannoury M, Bauvois B, Susin SA, Garnier D. Extracellular Vesicles in Chronic Lymphocytic Leukemia: Tumor Microenvironment Messengers as a Basis for New Targeted Therapies? Cancers. 2023; 15(8):2307. https://doi.org/10.3390/cancers15082307
Chicago/Turabian StyleDubois, Kenza, Mariana Tannoury, Brigitte Bauvois, Santos A. Susin, and Delphine Garnier. 2023. "Extracellular Vesicles in Chronic Lymphocytic Leukemia: Tumor Microenvironment Messengers as a Basis for New Targeted Therapies?" Cancers 15, no. 8: 2307. https://doi.org/10.3390/cancers15082307
APA StyleDubois, K., Tannoury, M., Bauvois, B., Susin, S. A., & Garnier, D. (2023). Extracellular Vesicles in Chronic Lymphocytic Leukemia: Tumor Microenvironment Messengers as a Basis for New Targeted Therapies? Cancers, 15(8), 2307. https://doi.org/10.3390/cancers15082307







