Review of Cardiovascular Risk of Androgen Deprivation Therapy and the Influence of Race in Men with Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
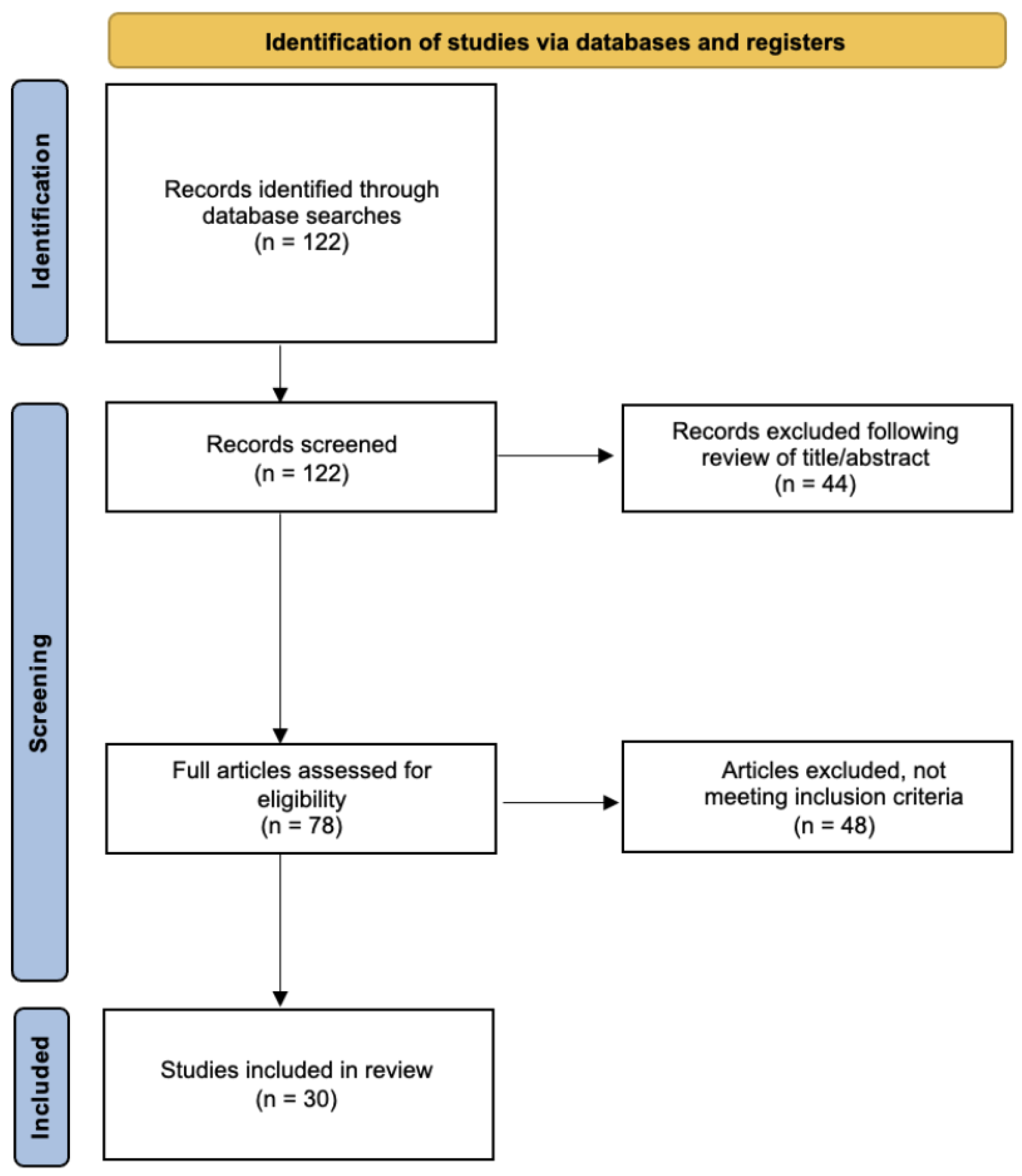
2. Materials and Methods
3. Androgen Deprivation Therapy: Overview and Mechanisms
3.1. Rationale of Therapy
3.2. Medical ADT
3.3. Surgical ADT
4. Cardiovascular Risk of Hormone Therapy
4.1. Mechanisms of CV Risk and Metabolic Considerations
4.2. Preclinical Models
4.3. Evidence of CV Risk of ADT
4.4. Types of Medical ADT and CVD Risk
5. Racial Disparities
5.1. Race and CV Health
5.2. Race and PCa
5.3. Challenges Ahead
6. Risk Management
6.1. ABCDE Algorithm
6.1.1. A: Awareness, Aspirin, and Arrhythmia
6.1.2. B: Blood Pressure and Biomarkers
6.1.3. C: Cigarettes, Cholesterol, and CT/Cardiac Imaging
6.1.4. D: Diabetes, Diet, Diversity, and Drug Choice
6.1.5. E: Exercise, EKG, and Exposure

6.2. Authors’ Suggestions for ADT Choice in High-Risk Patients
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Author | Study Title | MI | Nonfatal CVD | Stroke | CV Mortality |
|---|---|---|---|---|---|
| Carneiro et al. [71] | Cardiovascular events associated with androgen deprivation therapy in patients with prostate cancer: a systematic review and meta-analysis | OR, 1.23; 95% CI, 0.92–1.64 | OR, 1.55; 95% CI, 1.09–2.20 | OR, 1.02; 95% CI, 0.71–1.46 | OR, 0.97; 95% CI, 0.81–1.18 |
| Carneiro et al. [71] | Cardiovascular events associated with androgen deprivation therapy in patients with prostate cancer: a systematic review and meta-analysis | OR, 2.05; 95% CI, 1.93–2.17 | OR, 1.06; 95% CI, 0.70–1.61 | OR, 1.07; 95% CI, 0.66–1.72 | OR, 1.92; 95% CI, 0.79–4.68 |
| Meng et al. [72] | Stroke related to androgen deprivation therapy for prostate cancer: a meta-analysis and systematic review | HR, 1.12; 95% CI, 0.95–1.32; p = 0.16 | |||
| Zhao et al. [73] | Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: a meta-analysis of population-based observational studies | HR, 1.10; 95% CI, 0.97–1.26; p = 0.14 | HR, 1.10; 95% CI, 1.00–1.21; p = 0.06 | HR, 1.17; 95% CI, 1.04–1.32; p = 0.01 | |
| Nguyen et al. [74] | Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials | RR, 0.93; 95% CI, 0.79–1.10; p = 0.41 | |||
| Bourke et al. [75] | Endocrine therapy in prostate cancer: time for reappraisal of risks, benefits and cost-effectiveness? | RR, 1.06; 95% CI, 0.80–1.40 |
| Author | Study Title | MI | Nonfatal CVD | Stroke | CV Mortality |
|---|---|---|---|---|---|
| Bosco et al. [76] | Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis | RR, 1.57; 95% CI, 1.26–1.94 | RR, 1.38; 95% CI, 1.29–1.48 | RR, 1.51; 95% CI, 1.24–1.84 | |
| Zhao et al. [73] | Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: a meta-analysis of population-based observational studies | HR, 1.20; 95% CI, 1.05–1.38; p = 0.008 | HR, 1.19; 95% CI, 1.04–1.36; p < 0.001 | HR, 1.36; 95% CI 1.10–1.68; p = 0.004 | |
| Meng et al. [72] | Stroke related to androgen deprivation therapy for prostate cancer: a meta-analysis and systematic review | HR, 1.20; 95% CI, 1.12–1.28; p < 0.001 | |||
| Keating et al. [77] | Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer |
HR, 1.28; 95% CI, 1.08–1.52 |
HR, 1.19; 95% CI, 1.10–1.28 |
HR, 1.22; 95% CI, 1.10–1.36 |
HR, 1.35; 95% CI, 1.18–1.54 |
| Author | Study Title | Risk of Adverse CV Events: GnRH Antagonist as Compared to Agonists |
|---|---|---|
| Shore et al. [33] | Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer | HR, 0.46; 95% CI, 0.24–0.88 |
| Abufaraj et al. [78] | Differential Impact of Gonadotropin-releasing Hormone Antagonist Versus Agonist on Clinical Safety and Oncologic Outcomes on Patients with Metastatic Prostate Cancer: A Meta-analysis of Randomized Controlled Trials | RR, 0.52; 95% CI, 0.34–0.80 |
| Perrone et al. [79] | Cardiovascular Risk Profile in Prostate Cancer Patients Treated with GnRH Agonists versus Antagonists: An Italian Real-World Analysis | HR, 0.76; 95% CI, 0.60–0.95; p = 0.018 |
| Merseburger et al. [80] | Cardiovascular risk patients under androgen deprivation therapy: Lower risk with GnRH antagonists compared to LHRH agonists? | HR, 0.597; 95% CI, 0.380–0.938; p = 0.0253 |
| Chen et al. [81] | Gonadotropin-releasing hormone antagonist associated with lower cardiovascular risk compared with gonadotropin-releasing hormone agonist in prostate cancer: A nationwide cohort and in vitro study | HR, 0.21; 95% CI, 0.06–0.70 |
| Albertsen et al. [29] | Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist | HR, 0.44; 95% CI, 0.26–0.74; p = 0.002 |
| Davey et al. [82] | Cardiovascular risk profiles of GnRH agonists and antagonists: real-world analysis from UK general practice | RR, 0.39; 95% CI, 0.191–0.799; p = 0.01 |
| Author | Study Title | Any Cardiac Event | HTN |
|---|---|---|---|
| Moreira et al. [34] | Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: a meta-analysis of randomized controlled trials | RR, 1.06; 95% CI, 0.67–1.65 | |
| Iacovelli et al. [37] | The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer | RR, 1.41; 95% CI, 0.75–2.63; p = 0.28 | RR 2.74; 95% CI, 2.07–3.63; p ≤ 0.001 |
| Iacovelli et al. [35] | The incidence and relative risk of cardiovascular toxicity in patients treated with new hormonal agents for castration-resistant prostate cancer | RR, 1.32; 95% CI, 1.08–1.60; p = 0.006 | RR, 1.84; 95% CI, 1.37–2.46; p < 0.001 |
| Moreira et al. [34] | Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: a meta-analysis of randomized controlled trials | RR, 1.28; 95% CI, 1.06–1.55 | |
| Iacovelli et al. [37] | The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer | RR, 1.41; 95% CI, 1.21–1.64; p ≤ 0.001 | RR, 1.79; 95% CI, 1.45–2.21; p ≤ 0.001 |
| Author | Study Title | 1–2 Preexisting CVD | 3+ CVD |
|---|---|---|---|
| Lu-Yao et al. [36] | Mortality and Hospitalization Risk Following Oral Androgen Signaling Inhibitors Among Men with Advanced Prostate Cancer by Pre-existing Cardiovascular Comorbidities. | RR, 1.16; 95% CI, 1.00–1.36 | RR, 1.56; 95% CI, 1.29–1.88 |
References
- Prostate-Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 12 June 2022).
- Maggi, M.; Cowan, J.E.; Fasulo, V.; Washington, S.L.; Lonergan, P.E.; Sciarra, A.; Nguyen, H.G.; Carroll, P.R. The Long-Term Risks of Metastases in Men on Active Surveillance for Early Stage Prostate Cancer. J. Urol. 2020, 204, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Boccon-Gibod, L.; Shore, N.D.; Andreou, C.; Persson, B.-E.; Cantor, P.; Jensen, J.-K.; Olesen, T.K.; Schröder, F.H. The efficacy and safety of degarelix: A 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008, 102, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Fradet, V.; Shayegan, B.; Duceppe, E.; Siemens, R.; Niazi, T.; Klotz, L.; Brown, I.; Chin, J.; Lavallee, L.; et al. Cardiovascular Risk in Men with Prostate Cancer: Insights from the RADICAL PC Study. J. Urol. 2020, 203, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Challa, A.A.; Calaway, A.C.; Cullen, J.; Garcia, J.; Desai, N.; Weintraub, N.L.; Deswal, A.; Kutty, S.; Vallakati, A.; Addison, D.; et al. Cardiovascular toxicities of androgen deprivation therapy. Curr. Treat. Options Oncol. 2021, 22, 47. [Google Scholar] [CrossRef]
- Hu, J.-R.; Duncan, M.S.; Morgans, A.K.; Brown, J.D.; Meijers, W.C.; Freiberg, M.S.; Salem, J.-E.; Beckman, J.A.; Moslehi, J.J. Cardiovascular Effects of Androgen Deprivation Therapy in Prostate Cancer: Contemporary Meta-Analyses. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e55–e64. [Google Scholar] [CrossRef]
- Shahinian, V.B.; Kuo, Y.-F.; Freeman, J.L.; Goodwin, J.S. Risk of fracture after androgen deprivation for prostate cancer. N. Engl. J. Med. 2005, 352, 154–164. [Google Scholar] [CrossRef] [Green Version]
- DiBlasio, C.J.; Malcolm, J.B.; Derweesh, I.H.; Womack, J.H.; Kincade, M.C.; Mancini, J.G.; Ogles, M.L.; Lamar, K.D.; Patterson, A.L.; Wake, R.W. Patterns of sexual and erectile dysfunction and response to treatment in patients receiving androgen deprivation therapy for prostate cancer. BJU Int. 2008, 102, 39–43. [Google Scholar] [CrossRef]
- Spetz, A.-C.; Zetterlund, E.-L.; Varenhorst, E.; Hammar, M. Incidence and management of hot flashes in prostate cancer. J. Support. Oncol. 2003, 1, 263–266, 269. [Google Scholar]
- Higano, C.S. Update on cardiovascular and metabolic risk profiles of hormonal agents used in managing advanced prostate cancer. Urol. Oncol. 2020, 38, 912–917. [Google Scholar] [CrossRef]
- Karzai, F.H.; Madan, R.A.; Dahut, W.L. Metabolic syndrome in prostate cancer: Impact on risk and outcomes. Future Oncol. 2016, 12, 1947–1955. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.H.M.; Xu, N.; Lim, J.; Feng, K.-K.; Chan, W.K.W.; Chan, M.T.Y.; Leung, S.C.; Chen, D.-N.; Lin, Y.-Z.; Chiu, P.K.F.; et al. Adverse metabolic consequences of androgen deprivation therapy (ADT) on Asian patients with prostate cancer: Primary results from the real-life experience of ADT in Asia (READT) study. Prostate 2023. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Lee, H.; Nathan, D.M. Insulin sensitivity during combined androgen blockade for prostate cancer. J. Clin. Endocrinol. Metab. 2006, 91, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.S.; Hoermann, R.; Dupuis, P.; Joon, D.L.; Zajac, J.D.; Grossmann, M. Relationships between insulin resistance and frailty with body composition and testosterone in men undergoing androgen deprivation therapy for prostate cancer. Eur. J. Endocrinol. 2016, 175, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, J.; Choi, W.; Lee, M.; Lin, J. Cardiovascular toxicity of androgen deprivation therapy. Curr. Cardiol. Rep. 2021, 23, 109. [Google Scholar] [CrossRef]
- Gandaglia, G.; Sun, M.; Popa, I.; Schiffmann, J.; Abdollah, F.; Trinh, Q.-D.; Saad, F.; Graefen, M.; Briganti, A.; Montorsi, F.; et al. The impact of androgen-deprivation therapy (ADT) on the risk of cardiovascular (CV) events in patients with non-metastatic prostate cancer: A population-based study. BJU Int. 2014, 114, E82–E89. [Google Scholar] [CrossRef] [Green Version]
- Pischon, T.; Boeing, H.; Weikert, S.; Allen, N.; Key, T.; Johnsen, N.F.; Tjønneland, A.; Severinsen, M.T.; Overvad, K.; Rohrmann, S.; et al. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 3252–3261. [Google Scholar] [CrossRef] [Green Version]
- Möller, E.; Wilson, K.M.; Batista, J.L.; Mucci, L.A.; Bälter, K.; Giovannucci, E. Body size across the life course and prostate cancer in the Health Professionals Follow-up Study. Int. J. Cancer 2016, 138, 853–865. [Google Scholar] [CrossRef] [Green Version]
- Dickerman, B.A.; Torfadottir, J.E.; Valdimarsdottir, U.A.; Wilson, K.M.; Steingrimsdottir, L.; Aspelund, T.; Batista, J.L.; Fall, K.; Giovannucci, E.; Sigurdardottir, L.G.; et al. Midlife metabolic factors and prostate cancer risk in later life. Int. J. Cancer 2018, 142, 1166–1173. [Google Scholar] [CrossRef] [Green Version]
- Allott, E.H.; Howard, L.E.; Cooperberg, M.R.; Kane, C.J.; Aronson, W.J.; Terris, M.K.; Amling, C.L.; Freedland, S.J. Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 2349–2356. [Google Scholar] [CrossRef] [Green Version]
- Xi, L.; Kraskauskas, D.; Muniyan, S.; Batra, S.K.; Kukreja, R.C. Androgen deprivation therapy with leuprolide increases abdominal adiposity without causing cardiac dysfunction in middle-aged male mice: Effect of sildenafil. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 324, R589–R600. [Google Scholar] [CrossRef]
- Knutsson, A.; Hsiung, S.; Celik, S.; Rattik, S.; Mattisson, I.Y.; Wigren, M.; Scher, H.I.; Nilsson, J.; Hultgårdh-Nilsson, A. Treatment with a GnRH receptor agonist, but not the GnRH receptor antagonist degarelix, induces atherosclerotic plaque instability in ApoE(-/-) mice. Sci. Rep. 2016, 6, 26220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, C.B.; Mortensen, M.B.; Koechling, W.; Sørensen, C.B.; Bentzon, J.F. Differences in hypercholesterolemia and atherogenesis induced by common androgen deprivation therapies in male mice. J. Am. Heart Assoc. 2016, 5, e002800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keating, N.L.; O’Malley, A.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2006, 24, 4448–4456. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, S.; Garmo, H.; Holmberg, L.; Adolfsson, J.; Stattin, P.; Van Hemelrijck, M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J. Clin. Oncol. 2015, 33, 1243–1251. [Google Scholar] [CrossRef] [Green Version]
- Jespersen, C.G.; Nørgaard, M.; Borre, M. Androgen-deprivation therapy in treatment of prostate cancer and risk of myocardial infarction and stroke: A nationwide Danish population-based cohort study. Eur. Urol. 2014, 65, 704–709. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Freedland, S.J.; Smith, M.R. Does comorbidity influence the risk of myocardial infarction or diabetes during androgen-deprivation therapy for prostate cancer? Eur. Urol. 2013, 64, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Efstathiou, J.A.; Bae, K.; Shipley, W.U.; Hanks, G.E.; Pilepich, M.V.; Sandler, H.M.; Smith, M.R. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J. Clin. Oncol. 2009, 27, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Albertsen, P.C.; Klotz, L.; Tombal, B.; Grady, J.; Olesen, T.K.; Nilsson, J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur. Urol. 2014, 65, 565–573. [Google Scholar] [CrossRef]
- Dragomir, A.; Touma, N.; Hu, J.; Perreault, S.; Aprikian, A.G. Androgen deprivation therapy and risk of cardiovascular disease in patients with prostate cancer based on existence of cardiovascular risk. J. Natl. Compr. Canc. Netw. 2023, 21, 163–171. [Google Scholar] [CrossRef]
- Margel, D.; Peer, A.; Ber, Y.; Shavit-Grievink, L.; Tabachnik, T.; Sela, S.; Witberg, G.; Baniel, J.; Kedar, D.; Duivenvoorden, W.C.M.; et al. Cardiovascular Morbidity in a Randomized Trial Comparing GnRH Agonist and GnRH Antagonist among Patients with Advanced Prostate Cancer and Preexisting Cardiovascular Disease. J. Urol. 2019, 202, 1199–1208. [Google Scholar] [CrossRef]
- Sciarra, A.; Busetto, G.M.; Salciccia, S.; Del Giudice, F.; Maggi, M.; Crocetto, F.; Ferro, M.; De Berardinis, E.; Scarpa, R.M.; Porpiglia, F.; et al. Does Exist a Differential Impact of Degarelix Versus LHRH Agonists on Cardiovascular Safety? Evidences From Randomized and Real-World Studies. Front. Endocrinol. 2021, 12, 695170. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D.; Saad, F.; Cookson, M.S.; George, D.J.; Saltzstein, D.R.; Tutrone, R.; Akaza, H.; Bossi, A.; van Veenhuyzen, D.F.; Selby, B.; et al. HERO Study Investigators Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N. Engl. J. Med. 2020, 382, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.B.; Debiasi, M.; Francini, E.; Nuzzo, P.V.; Velasco, G.D.; Maluf, F.C.; Fay, A.P.; Bellmunt, J.; Choueiri, T.K.; Schutz, F.A. Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: A meta-analysis of randomized controlled trials. Oncotarget 2017, 8, 84572–84578. [Google Scholar] [CrossRef] [Green Version]
- Iacovelli, R.; Verri, E.; Cossu Rocca, M.; Aurilio, G.; Cullurà, D.; De Cobelli, O.; Nolè, F. The incidence and relative risk of cardiovascular toxicity in patients treated with new hormonal agents for castration-resistant prostate cancer. Eur. J. Cancer 2015, 51, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Lu-Yao, G.; Nikita, N.; Keith, S.W.; Nightingale, G.; Gandhi, K.; Hegarty, S.E.; Rebbeck, T.R.; Chapman, A.; Kantoff, P.W.; Cullen, J.; et al. Mortality and Hospitalization Risk Following Oral Androgen Signaling Inhibitors Among Men with Advanced Prostate Cancer by Pre-existing Cardiovascular Comorbidities. Eur. Urol. 2020, 77, 158–166. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Bria, E.; Romano, M.; Fantinel, E.; Bimbatti, D.; Muraglia, A.; Porcaro, A.B.; Siracusano, S.; Brunelli, M.; et al. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin. Genitourin. Cancer 2018, 16, e645–e653. [Google Scholar] [CrossRef]
- Rosenberg, M.T. Cardiovascular risk with androgen deprivation therapy. Int. J. Clin. Pract. 2020, 74, e13449. [Google Scholar] [CrossRef]
- Carnethon, M.R.; Pu, J.; Howard, G.; Albert, M.A.; Anderson, C.A.M.; Bertoni, A.G.; Mujahid, M.S.; Palaniappan, L.; Taylor, H.A.; Willis, M.; et al. American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council Cardiovascular health in african americans: A scientific statement from the american heart association. Circulation 2017, 136, e393–e423. [Google Scholar] [CrossRef]
- Leigh, J.A.; Alvarez, M.; Rodriguez, C.J. Ethnic minorities and coronary heart disease: An update and future directions. Curr. Atheroscler. Rep. 2016, 18, 9. [Google Scholar] [CrossRef] [Green Version]
- National Center for Health Statistics Health, United States, 2019. Available online: https://www.cdc.gov/nchs/hus/index.htm (accessed on 11 July 2021).
- Folsom, A.R.; Yatsuya, H.; Nettleton, J.A.; Lutsey, P.L.; Cushman, M.; Rosamond, W.D. ARIC Study Investigators Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J. Am. Coll. Cardiol. 2011, 57, 1690–1696. [Google Scholar] [CrossRef] [Green Version]
- Mensah, G.A. Cardiovascular diseases in african americans: Fostering community partnerships to stem the tide. Am. J. Kidney Dis. 2018, 72, S37–S42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healthy People 2030, U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Available online: https://health.gov/healthypeople/objectives-and-data/social-determinants-health (accessed on 19 July 2021).
- Edelstein, L.C.; Simon, L.M.; Montoya, R.T.; Holinstat, M.; Chen, E.S.; Bergeron, A.; Kong, X.; Nagalla, S.; Mohandas, N.; Cohen, D.E.; et al. Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat. Med. 2013, 19, 1609–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelstein, L.C.; Simon, L.M.; Lindsay, C.R.; Kong, X.; Teruel-Montoya, R.; Tourdot, B.E.; Chen, E.S.; Ma, L.; Coughlin, S.; Nieman, M.; et al. Common variants in the human platelet PAR4 thrombin receptor alter platelet function and differ by race. Blood 2014, 124, 3450–3458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, V.D.; Gerhard, G.S.; McNamara, D.M.; Tomar, D.; Madesh, M.; Kaniper, S.; Ramsey, F.V.; Fisher, S.G.; Ingersoll, R.G.; Kasch-Semenza, L.; et al. Association of variants in BAG3 with cardiomyopathy outcomes in african american individuals. JAMA Cardiol. 2018, 3, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saigal, C.S.; Gore, J.L.; Krupski, T.L.; Hanley, J.; Schonlau, M.; Litwin, M.S. Urologic Diseases in America Project Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer 2007, 110, 1493–1500. [Google Scholar] [CrossRef]
- Rewers, M.; Shetterly, S.M.; Hoag, S.; Baxter, J.; Marshall, J.; Hamman, R.F. Is the risk of coronary heart disease lower in Hispanics than in non-Hispanic whites? The San Luis Valley Diabetes Study. Ethn. Dis. 1993, 3, 44–54. [Google Scholar]
- Teoh, J.Y.C.; Ng, C.-F. Cardiovascular risk after androgen deprivation therapy for prostate cancer: An Asian perspective. Int. Urol. Nephrol. 2016, 48, 1429–1435. [Google Scholar] [CrossRef]
- Möhlig, M.; Arafat, A.M.; Osterhoff, M.A.; Isken, F.; Weickert, M.O.; Spranger, J.; Pfeiffer, A.F.H.; Schöfl, C. Androgen receptor CAG repeat length polymorphism modifies the impact of testosterone on insulin sensitivity in men. Eur. J. Endocrinol. 2011, 164, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Smigal, C.; Thun, M.J. Cancer statistics, 2006. CA Cancer J. Clin. 2006, 56, 106–130. [Google Scholar] [CrossRef] [Green Version]
- Labriola, M.; George, D.J. Differences in toxicity and outcomes in clinical trial participants from minority populations. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 1–5. [Google Scholar] [CrossRef]
- Siegel, D.A.; O’Neil, M.E.; Richards, T.B.; Dowling, N.F.; Weir, H.K. Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity-United States, 2001–2017. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.L.; Eggener, S.E.; Murphy, A.B. African-American Prostate Cancer Disparities. Curr. Urol. Rep. 2017, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Powell, I.J.; Dyson, G.; Land, S.; Ruterbusch, J.; Bock, C.H.; Lenk, S.; Herawi, M.; Everson, R.; Giroux, C.N.; Schwartz, A.G.; et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, S.; Grass, G.D.; Torres-Roca, J.; Johnstone, P.A.S.; Pow-Sang, J.; Dhillon, J.; Park, J.; Rounbehler, R.J.; Davicioni, E.; Hakansson, A.; et al. Genomic testing in localized prostate cancer can identify subsets of african americans with aggressive disease. J. Natl. Cancer Inst. 2022, 114, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Barocas, D.A.; Grubb, R.; Black, A.; Penson, D.F.; Fowke, J.H.; Andriole, G.; Crawford, E.D. Association between race and follow-up diagnostic care after a positive prostate cancer screening test in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer 2013, 119, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.K.; Carpenter, W.R.; Spain, P.; Clark, J.A.; Hamilton, R.J.; Galanko, J.A.; Jackman, A.; Talcott, J.A.; Godley, P.A. Race, healthcare access and physician trust among prostate cancer patients. Cancer Causes Control 2010, 21, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhard, R.S.; Patil, D.; Liu, Y.; Ogan, K.; Alemozaffar, M.; Jani, A.B.; Kucuk, O.N.; Master, V.A.; Gillespie, T.W.; Filson, C.P. Treatment of men with high-risk prostate cancer based on race, insurance coverage, and access to advanced technology. Urol. Oncol. 2017, 35, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, N.; Santos, M.; Jones, L.W.; Beckman, J.A.; Penson, D.F.; Morgans, A.K.; Moslehi, J. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation 2016, 133, 537–541. [Google Scholar] [CrossRef]
- Campbell, C.M.; Zhang, K.W.; Collier, A.; Linch, M.; Calaway, A.C.; Ponsky, L.; Guha, A.; Ghosh, A.K. Cardiovascular complications of prostate cancer therapy. Curr. Treat. Options Cardiovasc. Med. 2020, 22, 69. [Google Scholar] [CrossRef]
- Jacobs, E.J.; Newton, C.C.; Stevens, V.L.; Campbell, P.T.; Freedland, S.J.; Gapstur, S.M. Daily aspirin use and prostate cancer-specific mortality in a large cohort of men with nonmetastatic prostate cancer. J. Clin. Oncol. 2014, 32, 3716–3722. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Xia, S.; Li, T.; Liu, R. Could aspirin be a lifesaver for prostate cancer patients in prostate cancer-specific mortality?: An update systematic review and meta-analysis. BMC Cancer 2019, 19, 1186. [Google Scholar] [CrossRef] [Green Version]
- Calderone, D.; Greco, A.; Ingala, S.; Agnello, F.; Franchina, A.G.; Scalia, L.; Buccheri, S.; Capodanno, D. Efficacy and safety of aspirin for cardiovascular risk prevention in younger and older age: An updated systematic review and meta-analysis. Thromb. Haemost. 2021, 122, 445–455. [Google Scholar] [CrossRef]
- Margel, D.; Ber, Y.; Peer, A.; Shavit-Grievink, L.; Pinthus, J.H.; Witberg, G.; Baniel, J.; Kedar, D.; Rosenbaum, E. Cardiac biomarkers in patients with prostate cancer and cardiovascular disease receiving gonadotrophin releasing hormone agonist vs antagonist. Prostate Cancer Prostatic Dis. 2021, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Murta-Nascimento, C.; Romero, A.I.; Sala, M.; Lorente, J.A.; Bellmunt, J.; Rodero, N.J.; Lloreta, J.; Hospital, À.; Burón, A.; Castells, X.; et al. The effect of smoking on prostate cancer survival: A cohort analysis in Barcelona. Eur. J. Cancer Prev. 2015, 24, 335–339. [Google Scholar] [CrossRef]
- Saad, F.; Shore, N.D. Relugolix: A novel androgen deprivation therapy for management of patients with advanced prostate cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835921998586. [Google Scholar] [CrossRef] [PubMed]
- George, D.J.; Dearnaley, D.P. Relugolix, an oral gonadotropin-releasing hormone antagonist for the treatment of prostate cancer. Future Oncol. 2021, 17, 4431–4446. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Stanton, W.; Mandair, D. Darolutamide: An Evidenced-Based Review of Its Efficacy and Safety in the Treatment of Prostate Cancer. Cancer Manag. Res. 2020, 12, 5667–5676. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.; Sasse, A.D.; Wagner, A.A.; Peixoto, G.; Kataguiri, A.; Neto, A.S.; Bianco, B.A.V.; Chang, P.; Pompeo, A.C.L.; Tobias-Machado, M. Cardiovascular events associated with androgen deprivation therapy in patients with prostate cancer: A systematic review and meta-analysis. World J. Urol. 2015, 33, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhu, S.; Zhao, J.; Vados, L.; Wang, L.; Zhao, Y.; Zhao, D.; Niu, Y. Stroke related to androgen deprivation therapy for prostate cancer: A meta-analysis and systematic review. BMC Cancer 2016, 16, 180. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhu, S.; Sun, L.; Meng, F.; Zhao, L.; Zhao, Y.; Tian, H.; Li, P.; Niu, Y. Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: A meta-analysis of population-based observational studies. PLoS ONE 2014, 9, e107516. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Je, Y.; Schutz, F.A.B.; Hoffman, K.E.; Hu, J.C.; Parekh, A.; Beckman, J.A.; Choueiri, T.K. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: A meta-analysis of randomized trials. JAMA 2011, 306, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.; Kirkbride, P.; Hooper, R.; Rosario, A.J.; Chico, T.J.A.; Rosario, D.J. Endocrine therapy in prostate cancer: Time for reappraisal of risks, benefits and cost-effectiveness? Br. J. Cancer 2013, 108, 9–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco, C.; Bosnyak, Z.; Malmberg, A.; Adolfsson, J.; Keating, N.L.; Van Hemelrijck, M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: A meta-analysis. Eur. Urol. 2015, 68, 386–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keating, N.L.; O’Malley, A.J.; Freedland, S.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: Observational study of veterans with prostate cancer. J. Natl. Cancer Inst. 2010, 102, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abufaraj, M.; Iwata, T.; Kimura, S.; Haddad, A.; Al-Ani, H.; Abusubaih, L.; Moschini, M.; Briganti, A.; Karakiewicz, P.I.; Shariat, S.F. Differential Impact of Gonadotropin-releasing Hormone Antagonist Versus Agonist on Clinical Safety and Oncologic Outcomes on Patients with Metastatic Prostate Cancer: A Meta-analysis of Randomized Controlled Trials. Eur. Urol. 2021, 79, 44–53. [Google Scholar] [CrossRef]
- Perrone, V.; Degli Esposti, L.; Giacomini, E.; Veronesi, C.; Blini, V.; Oderda, M. Cardiovascular Risk Profile in Prostate Cancer Patients Treated with GnRH Agonists versus Antagonists: An Italian Real-World Analysis. Ther. Clin. Risk Manag. 2020, 16, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Merseburger, A.S.; Sedding, D.; Hüter, K. Cardiovascular risk patients under androgen deprivation therapy: Lower risk with GnRH antagonists compared to LHRH agonists? Urol. A 2016, 55, 218–225. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Su, P.-J.; See, L.-C.; Liu, J.-R.; Chuang, C.-K.; Pang, S.-T.; Tseng, C.-N.; Chen, S.-W.; Hsieh, I.-C.; Chu, P.-H.; et al. Gonadotropin-releasing hormone antagonist associated with lower cardiovascular risk compared with gonadotropin-releasing hormone agonist in prostate cancer: A nationwide cohort and in vitro study. Prostate 2021, 81, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Kirby, M.G. Cardiovascular risk profiles of GnRH agonists and antagonists: Real-world analysis from UK general practice. World J. Urol. 2021, 39, 307–315. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fradin, J.; Kim, F.J.; Lu-Yao, G.L.; Storozynsky, E.; Kelly, W.K. Review of Cardiovascular Risk of Androgen Deprivation Therapy and the Influence of Race in Men with Prostate Cancer. Cancers 2023, 15, 2316. https://doi.org/10.3390/cancers15082316
Fradin J, Kim FJ, Lu-Yao GL, Storozynsky E, Kelly WK. Review of Cardiovascular Risk of Androgen Deprivation Therapy and the Influence of Race in Men with Prostate Cancer. Cancers. 2023; 15(8):2316. https://doi.org/10.3390/cancers15082316
Chicago/Turabian StyleFradin, James, Felix J. Kim, Grace L. Lu-Yao, Eugene Storozynsky, and William K. Kelly. 2023. "Review of Cardiovascular Risk of Androgen Deprivation Therapy and the Influence of Race in Men with Prostate Cancer" Cancers 15, no. 8: 2316. https://doi.org/10.3390/cancers15082316
APA StyleFradin, J., Kim, F. J., Lu-Yao, G. L., Storozynsky, E., & Kelly, W. K. (2023). Review of Cardiovascular Risk of Androgen Deprivation Therapy and the Influence of Race in Men with Prostate Cancer. Cancers, 15(8), 2316. https://doi.org/10.3390/cancers15082316






