Obesity Measured via Body Mass Index May Be Associated with Increased Incidence but Not Worse Outcomes of Immune-Mediated Diarrhea and Colitis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Clinical Data Selection
2.2. IMDC Characteristics and Outcomes Assessed
2.3. Obesity Measurement
2.4. Statistical Analysis
3. Results
3.1. Patient Baseline Characteristics
3.2. Characteristics of IMDC Stratified by BMI
3.3. Outcomes of IMDC Stratified by BMI
3.4. Correlation between BMI and Visceral Body Fat Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Anandacoomarasamy, A.; Caterson, I.; Sambrook, P.; Fransen, M.; March, L. The impact of obesity on the musculoskeletal system. Int. J. Obes. 2008, 32, 211–222. [Google Scholar] [CrossRef]
- Anstey, K.J.; Cherbuin, N.; Budge, M.; Young, J. Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obes. Rev. 2011, 12, e426–e437. [Google Scholar] [CrossRef]
- Czernichow, S.; Kengne, A.P.; Stamatakis, E.; Hamer, M.; Batty, G.D. Body mass index, waist circumference and waist-hip ratio: Which is the better discriminator of cardiovascular disease mortality risk?: Evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes. Rev. 2011, 12, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef]
- Hurt, R.T.; Frazier, T.H.; McClave, S.A.; Kaplan, L.M. Obesity epidemic: Overview, pathophysiology, and the intensive care unit conundrum. JPEN J. Parenter. Enteral. Nutr. 2011, 35, 4S–13S. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Peterson, L.L. Obesity and Cancer: Evidence, Impact, and Future Directions. Clin. Chem. 2018, 64, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Sepesi, B.; Gold, K.A.; Correa, A.M.; Heymach, J.V.; Vaporciyan, A.A.; Roszik, J.; Dmitrovsky, E.; Liu, X. The Influence of Body Mass Index on Overall Survival Following Surgical Resection of Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 1280–1287. [Google Scholar] [CrossRef]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H.; Alexeeff, S.; Xiao, J.; Weltzien, E.; Feliciano, E.C.; Castillo, A.L.; Quesenberry, C.P.; Kwan, M.L.; et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol. Biomark. Prev. 2017, 26, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, S.; Song, J.H.; Choi, S.; Cho, M.; Kwon, I.G.; Son, T.; Kim, H.I.; Cheong, J.H.; Hyung, W.J.; et al. Prognostic significance of body mass index and prognostic nutritional index in stage II/III gastric cancer. Eur. J. Surg. Oncol. 2020, 46, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chan, H.L.; Chen, P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr. Med. Chem. 2019, 26, 3009–3025. [Google Scholar] [CrossRef] [PubMed]
- Baxi, S.; Yang, A.; Gennarelli, R.L.; Khan, N.; Wang, Z.; Boyce, L.; Korenstein, D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ 2018, 360, k793. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Ali, F.S.; Naqash, A.R.; Owen, D.H.; Patel, S.; Otterson, G.A.; Kendra, K.; Ricciuti, B.; Chiari, R.; De Giglio, A.; et al. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. J. Clin. Oncol. 2019, 37, 2738–2745. [Google Scholar] [CrossRef]
- Force, U.S.P.S.T. Screening for obesity in adults: Recommendations and rationale. Ann. Intern. Med. 2003, 139, 930–932. [Google Scholar] [CrossRef]
- Murphy, W.J.; Longo, D.L. The Surprisingly Positive Association Between Obesity and Cancer Immunotherapy Efficacy. JAMA 2019, 321, 1247–1248. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother Cancer 2019, 7, 57. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Santini, D.; Buti, S.; Tiseo, M.; Cannita, K.; Perrone, F.; Giusti, R.; De Tursi, M.; Zoratto, F.; et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events. Eur. J. Cancer 2020, 128, 17–26. [Google Scholar] [CrossRef]
- Xu, H.; Cao, D.; He, A.; Ge, W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: A pooled analysis of 4090 cancer patients. Int. Immunopharmacol. 2019, 74, 105745. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.C.; Chen, G.M.; Wang, Y.; Yuan, S.Q.; Zhou, J.; Duan, J.L.; Liu, W.W.; Chen, S.; Cai, M.Y.; Li, Y.F. Association between Body Mass Index and Survival Outcomes in Patients Treated with Immune Checkpoint Inhibitors: Meta-analyses of Individual Patient Data. J. Immunother. 2021, 44, 371–375. [Google Scholar] [CrossRef]
- WHO. Physical Status: The Use and Interpretation of Anthropometry, Report of a WHO Expert Committee; WHO Technical Report Series; WHO: Geneva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar]
- Okorodudu, D.O.; Jumean, M.F.; Montori, V.M.; Romero-Corral, A.; Somers, V.K.; Erwin, P.J.; Lopez-Jimenez, F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int. J. Obes. 2010, 34, 791–799. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Despres, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Hiuge-Shimizu, A.; Kishida, K.; Funahashi, T.; Ishizaka, Y.; Oka, R.; Okada, M.; Suzuki, S.; Takaya, N.; Nakagawa, T.; Fukui, T.; et al. Reduction of visceral fat correlates with the decrease in the number of obesity-related cardiovascular risk factors in Japanese with Abdominal Obesity (VACATION-J Study). J. Atheroscler. Thromb. 2012, 19, 1006–1018. [Google Scholar] [CrossRef]
- Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ. J. 2002, 66, 987–992. [Google Scholar] [CrossRef]
- Esposito, A.; Marra, A.; Bagnardi, V.; Frassoni, S.; Morganti, S.; Viale, G.; Zagami, P.; Varano, G.M.; Buccimazza, G.; Orsi, F.; et al. Body mass index, adiposity and tumour infiltrating lymphocytes as prognostic biomarkers in patients treated with immunotherapy: A multi-parametric analysis. Eur. J. Cancer 2021, 145, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.J.; Kline, M.R.; Liu, Y.; Shabto, J.M.; Williams, M.A.; Khan, A.I.; Lewis, C.; Collins, H.; Akce, M.; Kissick, H.T.; et al. Adiposity may predict survival in patients with advanced stage cancer treated with immunotherapy in phase 1 clinical trials. Cancer 2020, 126, 575–582. [Google Scholar] [CrossRef]
- Young, A.C.; Quach, H.T.; Song, H.; Davis, E.J.; Moslehi, J.J.; Ye, F.; Williams, G.R.; Johnson, D.B. Impact of body composition on outcomes from anti-PD1 +/− anti-CTLA-4 treatment in melanoma. J. Immunother. Cancer 2020, 8, e000821. [Google Scholar] [CrossRef]
- Minami, S.; Ihara, S.; Tanaka, T.; Komuta, K. Sarcopenia and Visceral Adiposity Did Not Affect Efficacy of Immune-Checkpoint Inhibitor Monotherapy for Pretreated Patients With Advanced Non-Small Cell Lung Cancer. World J. Oncol. 2020, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.J.; Olsen, T.A.; Goyal, S.; Liu, Y.; Evans, S.T.; Magod, B.; Brown, J.T.; Yantorni, L.; Russler, G.A.; Caulfield, S.; et al. Body Composition Variables as Radiographic Biomarkers of Clinical Outcomes in Metastatic Renal Cell Carcinoma Patients Receiving Immune Checkpoint Inhibitors. Front. Oncol. 2021, 11, 707050. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhu, Y.; Wang, H.; Zhang, H.; Shi, G.; Liu, X.; Ye, D. Prognostic value of components of body composition in patients treated with targeted therapy for advanced renal cell carcinoma: A retrospective case series. PLoS ONE 2015, 10, e0118022. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Thomas, E.L.; Saeed, N.; Hajnal, J.V.; Brynes, A.; Goldstone, A.P.; Frost, G.; Bell, J.D. Magnetic resonance imaging of total body fat. J. Appl. Physiol. 1998, 85, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Grummer-Strawn, L.M.; Pietrobelli, A.; Goulding, A.; Goran, M.I.; Dietz, W.H. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am. J. Clin. Nutr. 2002, 75, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Cahill, F.; Gulliver, W.; Yi, Y.; Xie, Y.; Bridger, T.; Pace, D.; Zhang, H. Concordance of BAI and BMI with DXA in the Newfoundland population. Obesity 2013, 21, 499–503. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, L.; Dong, S.; Zha, X.; Ran, L.; Li, Y.; Chen, S.; Gao, J.; Li, S.; Lu, Y.; et al. CT-derived abdominal adiposity: Distributions and better predictive ability than BMI in a nationwide study of 59,429 adults in China. Metabolism 2021, 115, 154456. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Dorosty, A.R.; Emmett, P.M. Identification of the obese child: Adequacy of the body mass index for clinical practice and epidemiology. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1623–1627. [Google Scholar] [CrossRef]
- Barlow, S.E.; Dietz, W.H. Obesity evaluation and treatment: Expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics 1998, 102, E29. [Google Scholar] [CrossRef] [PubMed]
- Power, C.; Lake, J.K.; Cole, T.J. Measurement and long-term health risks of child and adolescent fatness. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Reeder, B.A.; Elliott, S.; Joffres, M.R.; Pahwa, P.; Raine, K.D.; Kirkland, S.A.; Paradis, G. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Can. J. Public. Health 2012, 103, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.; Picone, G.; Sloan, F.; Yashkin, A. Relation between BMI and diabetes mellitus and its complications among US older adults. South Med. J. 2015, 108, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.L.; Korinek, J.; Allison, T.G.; Batsis, J.A.; Sert-Kuniyoshi, F.H.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef]
- Franzosi, M.G. Should we continue to use BMI as a cardiovascular risk factor? Lancet 2006, 368, 624–625. [Google Scholar] [CrossRef]
- Shah, N.R.; Braverman, E.R. Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. PLoS ONE 2012, 7, e33308. [Google Scholar] [CrossRef]
- Reilly, J.J.; El-Hamdouchi, A.; Diouf, A.; Monyeki, A.; Somda, S.A. Determining the worldwide prevalence of obesity. Lancet 2018, 391, 1773–1774. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ambrosi, J.; Silva, C.; Galofre, J.C.; Escalada, J.; Santos, S.; Millan, D.; Vila, N.; Ibanez, P.; Gil, M.J.; Valenti, V.; et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int. J. Obes. 2012, 36, 286–294. [Google Scholar] [CrossRef]
- Crudele, L.; Piccinin, E.; Moschetta, A. Visceral Adiposity and Cancer: Role in Pathogenesis and Prognosis. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Harvie, M.; Hooper, L.; Howell, A.H. Central obesity and breast cancer risk: A systematic review. Obes. Rev. 2003, 4, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Komninou, D.; Stephenson, G.D. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes. Rev. 2004, 5, 153–165. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Thun, M.J. Obesity and cancer. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.; Chandran, U.; Bandera, E.V. Obesity in cancer survival. Annu. Rev. Nutr. 2012, 32, 311–342. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chlebowski, R.T. Obesity and Cancer: Insights for Clinicians. J. Clin. Oncol. 2016, 34, 4197–4202. [Google Scholar] [CrossRef]
- Slawinski, C.G.V.; Barriuso, J.; Guo, H.; Renehan, A.G. Obesity and Cancer Treatment Outcomes: Interpreting the Complex Evidence. Clin. Oncol. 2020, 32, 591–608. [Google Scholar] [CrossRef]
- Woodall, M.J.; Neumann, S.; Campbell, K.; Pattison, S.T.; Young, S.L. The Effects of Obesity on Anti-Cancer Immunity and Cancer Immunotherapy. Cancers 2020, 12, 1230. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Abu-Sbeih, H.; Ma, W.; Peng, Y.; Qiao, W.; Wang, J.; Shah, A.Y.; Glitza Oliva, I.C.; Piha-Paul, S.A.; Thompson, J.A.; et al. Association of Chronic Immune-Mediated Diarrhea and Colitis With Favorable Cancer Response. J. Natl. Compr. Canc. Netw. 2020, 19, 700–708. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hebuterne, X.; Klek, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- Van Der Sloot, K.W.; Joshi, A.D.; Bellavance, D.R.; Gilpin, K.K.; Stewart, K.O.; Lochhead, P.; Garber, J.J.; Giallourakis, C.; Yajnik, V.; Ananthakrishnan, A.N.; et al. Visceral Adiposity, Genetic Susceptibility, and Risk of Complications among Individuals with Crohn’s Disease. Inflamm. Bowel. Dis. 2017, 23, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hass, D.J.; Brensinger, C.M.; Lewis, J.D.; Lichtenstein, G.R. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006, 4, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.; Karagiannides, I.; Bakirtzi, K.; Pothoulakis, C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm. Bowel. Dis. 2012, 18, 1550–1557. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, X.R.; Remer, E.M.; Lian, L.; Stocchi, L.; Li, Y.; McCullough, A.; Remzi, F.H.; Shen, B. Association between high visceral fat area and postoperative complications in patients with Crohn’s disease following primary surgery. Colorectal. Dis. 2016, 18, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, W.; Gong, J.; Zhang, W.; Gu, L.; Guo, Z.; Cao, L.; Shen, B.; Li, N.; Li, J. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn’s disease. Colorectal. Dis. 2015, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Cao, L.; Li, Y.; Cai, X.; Ge, Y.; Zhu, W. Visceral Fat Is Associated With Mucosal Healing of Infliximab Treatment in Crohn’s Disease. Dis. Colon. Rectum. 2018, 61, 706–712. [Google Scholar] [CrossRef]
- Doyle, S.L.; Donohoe, C.L.; Lysaght, J.; Reynolds, J.V. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc. Nutr. Soc. 2012, 71, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Lysaght, J.; Cathcart, M.C.; Donohoe, C.L.; Cummins, R.; McGarrigle, S.A.; Kay, E.; Reynolds, J.V.; Pidgeon, G.P. MMP9 expression in oesophageal adenocarcinoma is upregulated with visceral obesity and is associated with poor tumour differentiation. Mol. Carcinog. 2013, 52, 144–154. [Google Scholar] [CrossRef]
- Ohki, T.; Tateishi, R.; Shiina, S.; Goto, E.; Sato, T.; Nakagawa, H.; Masuzaki, R.; Goto, T.; Hamamura, K.; Kanai, F.; et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut 2009, 58, 839–844. [Google Scholar] [CrossRef]
- Bruun, J.M.; Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): Implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 2005, 90, 2282–2289. [Google Scholar] [CrossRef]
- Curat, C.A.; Wegner, V.; Sengenes, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumie, A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006, 49, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Marin, P.; Andersson, B.; Ottosson, M.; Olbe, L.; Chowdhury, B.; Kvist, H.; Holm, G.; Sjostrom, L.; Bjorntorp, P. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism 1992, 41, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Vikram, N.K. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 2003, 19, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, R.; Almeida, M.; Colombatti, A.; Calin, G.A. Long non-coding RNAs and cancer: A new frontier of translational research? Oncogene 2012, 31, 4577–4587. [Google Scholar] [CrossRef]
- Smolle, M.A.; Prinz, F.; Calin, G.A.; Pichler, M. Current concepts of non-coding RNA regulation of immune checkpoints in cancer. Mol. Asp. Med. 2019, 70, 117–126. [Google Scholar] [CrossRef]
- Yau, M.Y.-C.; Xu, L.; Huang, C.-L.; Wong, C.-M. Long Non-Coding RNAs in Obesity-Induced Cancer. Non-Coding RNA 2018, 4, 19. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Taheri, M. The expression profile and role of non-coding RNAs in obesity. Eur. J. Pharmacol. 2021, 892, 173809. [Google Scholar] [CrossRef]
- Doyle, S.L.; Bennett, A.M.; Donohoe, C.L.; Mongan, A.M.; Howard, J.M.; Lithander, F.E.; Pidgeon, G.P.; Reynolds, J.V.; Lysaght, J. Establishing computed tomography-defined visceral fat area thresholds for use in obesity-related cancer research. Nutr. Res. 2013, 33, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, M.; Tsuchihashi, K.; Saitoh, S.; Odawara, Y.; Hirano, T.; Nakata, T.; Miura, T.; Ura, N.; Hareyama, M.; Shimamoto, K. Visceral obesity in Japanese patients with metabolic syndrome: Reappraisal of diagnostic criteria by CT scan. Hypertens Res. 2007, 30, 315–323. [Google Scholar] [CrossRef]
- Trejo-Avila, M.; Bozada-Gutiérrez, K.; Valenzuela-Salazar, C.; Herrera-Esquivel, J.; Moreno-Portillo, M. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: A systematic review and meta-analysis. Int. J. Colorectal. Dis. 2021, 36, 1077–1096. [Google Scholar] [CrossRef]
- Ishihara, H.; Kondo, T.; Omae, K.; Takagi, T.; Iizuka, J.; Kobayashi, H.; Hashimoto, Y.; Tanabe, K. Sarcopenia predicts survival outcomes among patients with urothelial carcinoma of the upper urinary tract undergoing radical nephroureterectomy: A retrospective multi-institution study. Int. J. Clin. Oncol. 2017, 22, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, S.; Nau, P.N.; Mezhir, J.J. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J. Surg. Oncol. 2015, 112, 503–509. [Google Scholar] [CrossRef]
- Everard, A.; Geurts, L.; Van Roye, M.; Delzenne, N.M.; Cani, P.D. Tetrahydro iso-alpha acids from hops improve glucose homeostasis and reduce body weight gain and metabolic endotoxemia in high-fat diet-fed mice. PLoS ONE 2012, 7, e33858. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and Anticancer Immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Impact of the gut microbiome on mucosal inflammation. Trends Immunol 2013, 34, 423–430. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef]
- Qin, Y.; Roberts, J.D.; Grimm, S.A.; Lih, F.B.; Deterding, L.J.; Li, R.; Chrysovergis, K.; Wade, P.A. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome. Biol. 2018, 19, 7. [Google Scholar] [CrossRef]
- Chen, X.; Devaraj, S. Gut Microbiome in Obesity, Metabolic Syndrome, and Diabetes. Curr. Diab. Rep. 2018, 18, 129. [Google Scholar] [CrossRef]
- Sehgal, K.; Khanna, S. Gut microbiome and checkpoint inhibitor colitis. Intest. Res. 2021, 19, 360–364. [Google Scholar] [CrossRef]
- Dai, C.; Liu, W.X. Refractory Immune Checkpoint Inhibitor-induced Colitis Improved by Fecal Microbiota Transplantation: A Case Report. Inflamm. Bowel. Dis. 2022, 28, e43–e44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; DuPont, H.L.; Jiang, Z.D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.C.; et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.S.; Halsey, T.; Jiang, Z.-D.; DuPont, H.; Jenq, R.; Wang, Y. S156 Microbiome Alteration via Fecal Microbiota Transplantation (FMT) Is Effective for Immune Checkpoint Inhibitor (ICI) Induced-Colitis (IMC) Refractory to Immunosuppressive Therapy. Am. J. Gastroenterol. 2021, 116, S68–S69. [Google Scholar] [CrossRef]
- Pokrovskaya, E.V.; Sklyanik, I.A.; Shestakova, E.A.; Shestakova, M.V. Prospects for the use of fecal microbiota transplantation in obese patients with Type 2 Diabetes Mellitus for weight loss and improvement of insulin sensitivity. Diabetes Mellit. 2021, 23, 541–547. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clement, K.; Nieuwdorp, M. Fecal Microbiota Transplantation: A Future Therapeutic Option for Obesity/Diabetes? Curr. Diab. Rep. 2019, 19, 51. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
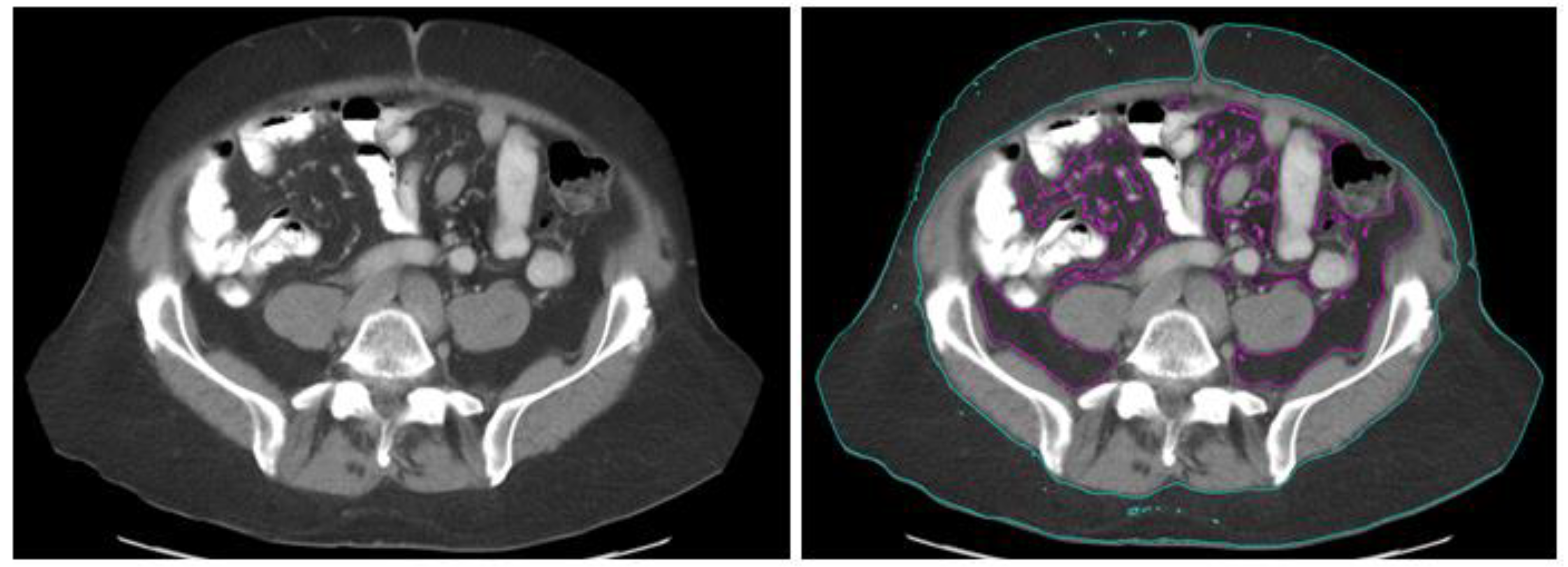
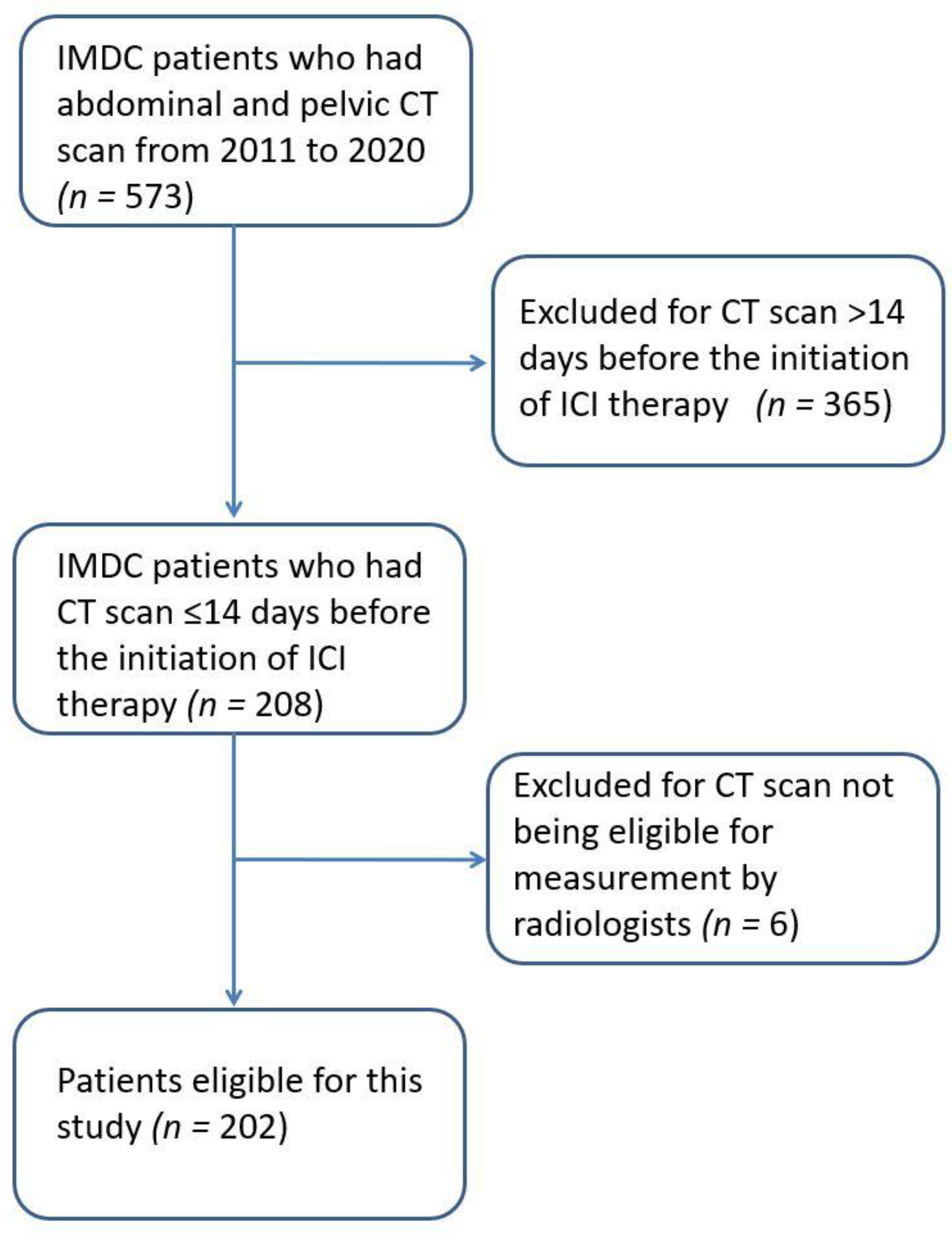
| Characteristic | Value (N = 202) |
|---|---|
| Age, median (IQR), years | 61 (49–70) |
| Race/ethnicity, n (%) | |
| White | 181 (89.6%) |
| Others | 21 (10.4%) |
| Male sex, n (%) | 136 (67.3%) |
| Cancer type, n (%) | |
| Melanoma | 89 (44.1%) |
| Genitourinary cancer | 60 (29.7%) |
| Lung cancer | 16 (7.9%) |
| Others | 37 (18.3%) |
| Cancer stage, n (%) | |
| III | 24 (11.9%) |
| IV | 176 (87.1%) |
| Other | 2 (1.0%) |
| Metabolic syndrome, n (%) | |
| Hypertension | 120 (59.4%) |
| Hyperlipidemia | 90 (44.6%) |
| Diabetes | 65 (32.2%) |
| Charlson comorbidity index, median (IQR) | 8 (6–9) |
| ICI therapy types, n (%) | |
| CTLA-4 monotherapy | 57 (28.2%) |
| PD-1/PD-L1 monotherapy | 75 (37.1%) |
| Combination of CTLA-4 and PD-1/PD-L1 | 70 (34.7%) |
| Follow-up duration from ICI initiation to last follow-up/death, median (IQR), months | 28.8 (14.2–48.8) |
| All-cause mortality, n (%) | 89 (44.1%) |
| IMDC Measures | BMI | p Value | ||
|---|---|---|---|---|
| <25 N = 58 | ≥25 but <30 N = 68 | ≥30 N = 76 | ||
| Incidence of GI irAEs | 7.96% | 9.12% | 11.41% | 0.085 † |
| Characteristics of IMDC | ||||
| Duration between first dose of ICI to IMDC, median (IQR), months | 1.9 (0.9–5.3) | 1.8 (1.0–3.5) | 2.4 (1.4–4.2) | 0.33 |
| Peak grade of colitis, n (%) | 0.03 | |||
| ≤2 | 34 (59%) | 43 (63%) | 60 (79%) | |
| 3–4 | 24 (41%) | 25 (37%) | 16 (21%) | |
| Peak grade of diarrhea, n (%) | 0.38 | |||
| ≤2 | 22 (38%) | 31 (46%) | 38 (50%) | |
| 3–4 | 36 (62%) | 37 (54%) | 38 (50%) | |
| Duration of IMDC symptoms, median (IQR), days | 19.0 (7.0–41.0) | 21.0 (10.0–45.0) | 18.0 (6.0–37.0) | 0.32 |
| Duration of steroid therapy, median (IQR), days | 37.0 (19.8–52.5) | 40.0 (17.0–60.0) | 30.0 (20.5–70.0) | 0.91 |
| Use of intravenous steroids, n (%) | 25 (43%) | 37 (54%) | 35 (46%) | 0.45 |
| Use of non-steroidal immunosuppressants, n (%) | 26 (45%) | 28 (41%) | 26 (34%) | 0.48 |
| Outcomes of IMDC | ||||
| Hospitalization of IMDC, n (%) | 36 (62%) | 41 (60%) | 46 (61%) | 0.98 |
| Duration of hospitalization, median (IQR), days | 7.0 (5.0–12.0) | 6.0 (4.0–11.0) | 5.0 (3.0–8.0) | 0.40 |
| Clinical remission after IMDC treatment, n (%) | 40 (69%) | 47 (69%) | 56 (74%) | 0.43 |
| Recurrent IMDC, n (%) | 14 (24%) | 14 (21%) | 12 (16%) | 0.62 |
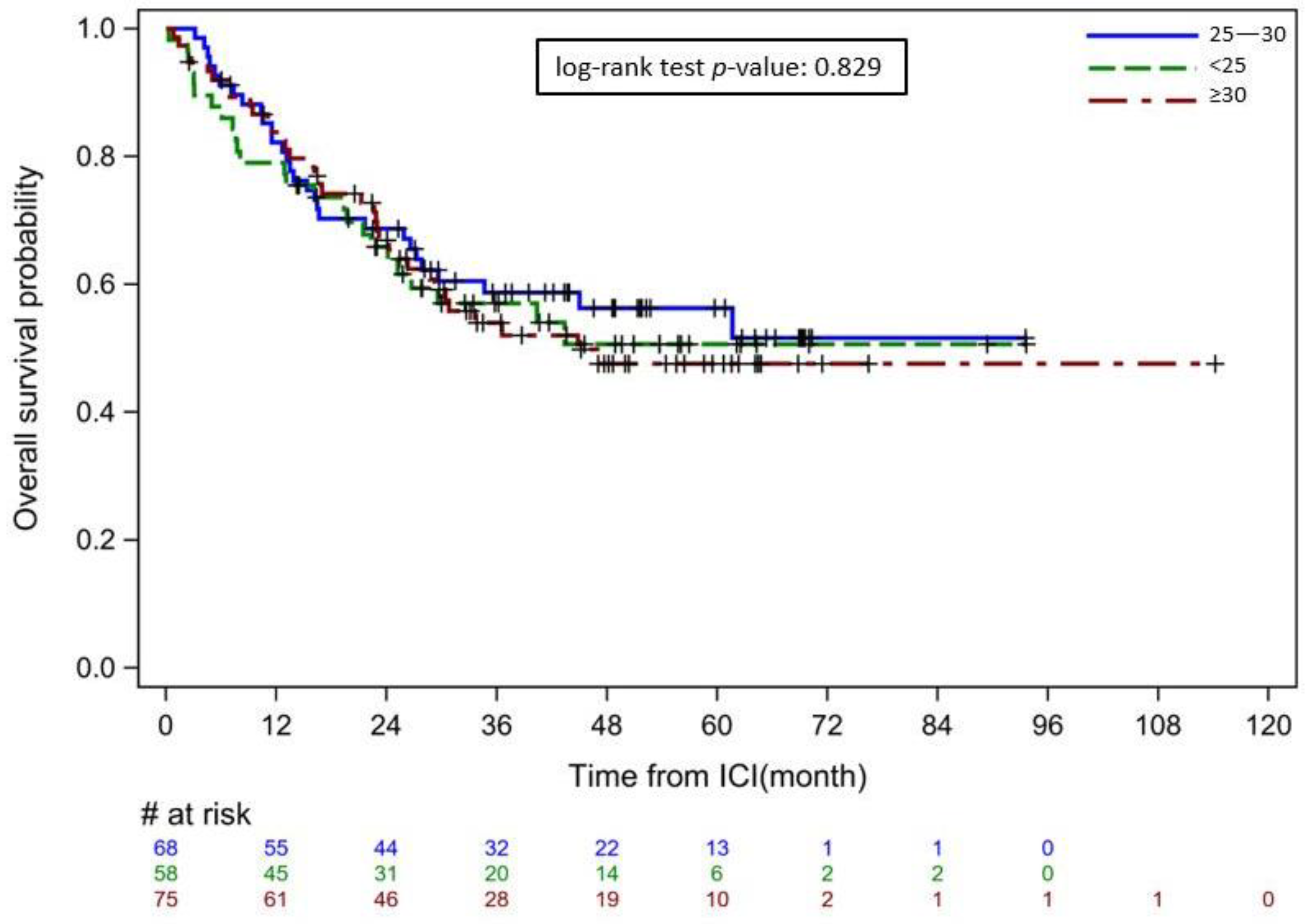
| Overall survival, median (IQR), months | 25.5 (13.4–45.0) | 33.1 (15.0–51.7) | 29.0 (15.9–47.8) | 0.32 |
| Parameter | Pearson Correlation Coefficient | p Value |
|---|---|---|
| SFA | 0.635 | <0.0001 |
| VFA | 0.579 | <0.0001 |
| TFA | 0.673 | <0.0001 |
| V/S ratio | 0.115 | 0.105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kono, M.; Shatila, M.; Xu, G.; Lu, Y.; Mathew, A.; Mohajir, W.; Varatharajalu, K.; Qiao, W.; Thomas, A.S.; Wang, Y. Obesity Measured via Body Mass Index May Be Associated with Increased Incidence but Not Worse Outcomes of Immune-Mediated Diarrhea and Colitis. Cancers 2023, 15, 2329. https://doi.org/10.3390/cancers15082329
Kono M, Shatila M, Xu G, Lu Y, Mathew A, Mohajir W, Varatharajalu K, Qiao W, Thomas AS, Wang Y. Obesity Measured via Body Mass Index May Be Associated with Increased Incidence but Not Worse Outcomes of Immune-Mediated Diarrhea and Colitis. Cancers. 2023; 15(8):2329. https://doi.org/10.3390/cancers15082329
Chicago/Turabian StyleKono, Miho, Malek Shatila, Guofan Xu, Yang Lu, Antony Mathew, Wasay Mohajir, Krishnavathana Varatharajalu, Wei Qiao, Anusha S. Thomas, and Yinghong Wang. 2023. "Obesity Measured via Body Mass Index May Be Associated with Increased Incidence but Not Worse Outcomes of Immune-Mediated Diarrhea and Colitis" Cancers 15, no. 8: 2329. https://doi.org/10.3390/cancers15082329
APA StyleKono, M., Shatila, M., Xu, G., Lu, Y., Mathew, A., Mohajir, W., Varatharajalu, K., Qiao, W., Thomas, A. S., & Wang, Y. (2023). Obesity Measured via Body Mass Index May Be Associated with Increased Incidence but Not Worse Outcomes of Immune-Mediated Diarrhea and Colitis. Cancers, 15(8), 2329. https://doi.org/10.3390/cancers15082329








