Repurposing Sulfasalazine as a Radiosensitizer in Hypoxic Human Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. TCGA Colorectal Cancer Cohort Analysis
2.2. Cell Lines
2.3. Treatment
2.4. Western Blot
2.5. MTT Assays
2.6. Kinetic Growth Assay
2.7. Radioresponse Determining Assay
2.8. Glutathione Assay
2.9. Thioredoxin Reductase Assay
2.10. Superoxide Dismutase Assay
2.11. NAD(P)H Levels
2.12. ROS Levels
2.13. DNA Damage Analysis
2.14. Ferroptosis Levels
2.15. Mitochondrial Membrane Potential
2.16. Seahorse Metabolic Profiling
2.17. Three-Dimensional (3D) Cell Cultures
2.18. In Vivo Tumor Xenograft Model
2.19. Data Analysis and Statistics
3. Results
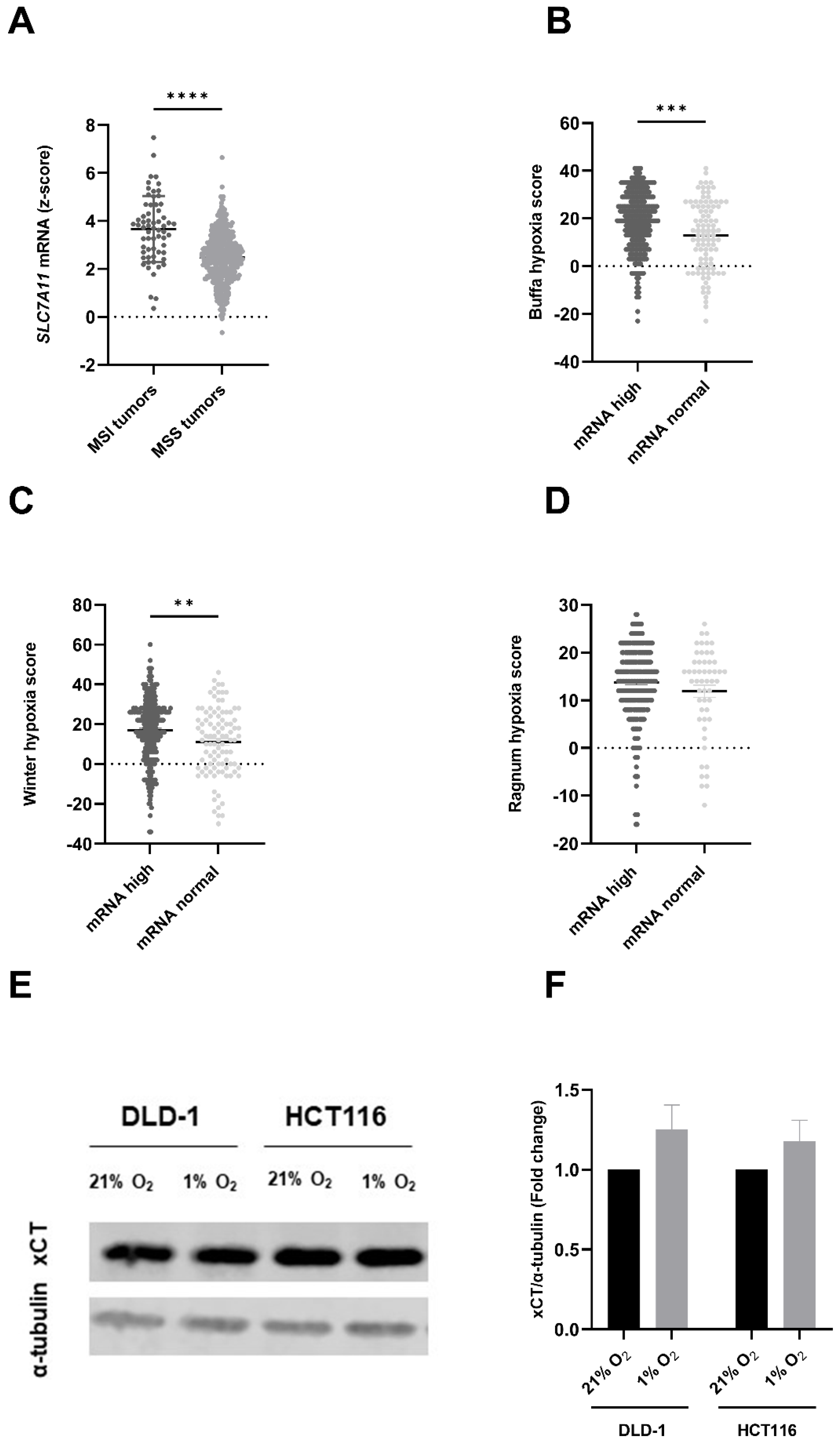
3.1. SLC7A11/xCT Expression Levels Are Linked to Mutational Status of CRC Patients and Correlated with Hypoxic Conditions
3.2. SSZ Radiosensitized Hypoxic CRC Cells While Exerting No Effect on Normoxic CRC Cells
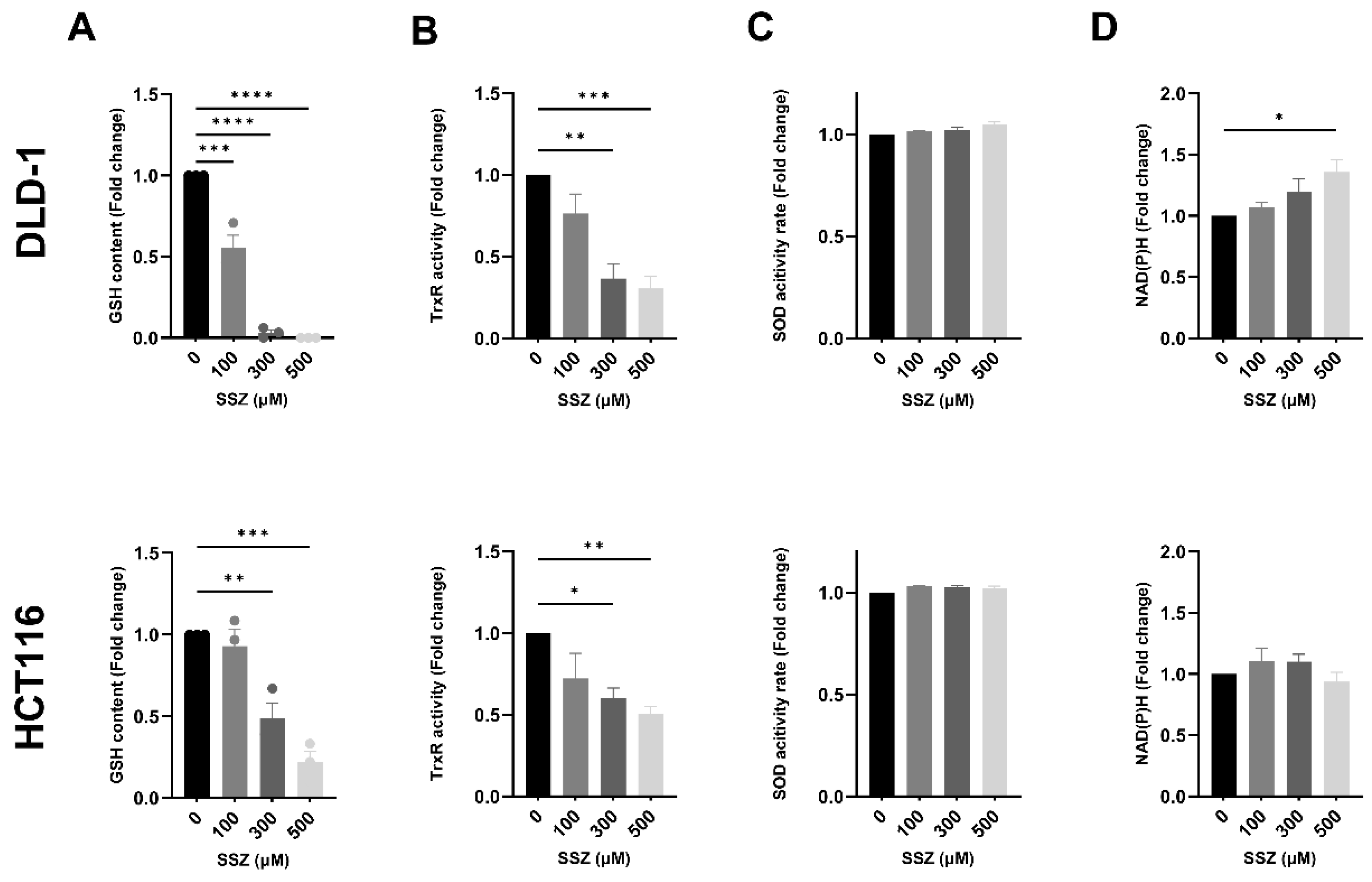
3.3. SSZ Inhibits Antioxidants GSH and TrxR, Inducing Disturbed Redox Homeostasis, Leading to Increased DNA Double-Strand Breaks under Hypoxic Conditions
3.4. SSZ Causes Lipid Peroxidation Resulting in Ferroptosis in the DLD-1 Cell Line under Hypoxic Conditions
3.5. SSZ Radiosensitizes 3D-Colorectal Cancer Models
3.6. SSZ Radiosensitizes DLD-1 Tumor Xenografts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, M.; Chen, G.; Wang, P.; Lu, W.; Zhu, C.; Song, M.; Yang, J.; Wen, S.; Xu, R.; Hu, Y.; et al. Xc−Inhibitor Sulfasalazine Sensitizes Colorectal Cancer to Cisplatin by a GSH-Dependent Mechanism. Cancer Lett. 2015, 368, 88–96. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of Oxidative Stress as an Anticancer Strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Kirtonia, A.; Sethi, G.; Garg, M. The Multifaceted Role of Reactive Oxygen Species in Tumorigenesis. Cell. Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar] [CrossRef]
- Liu, J.; Xia, X.; Huang, P. XCT: A Critical Molecule That Links Cancer Metabolism to Redox Signaling. Mol. Ther. 2020, 28, 2358–2366. [Google Scholar] [CrossRef]
- Lin, W.; Wang, C.; Liu, G.; Bi, C.; Wang, X.; Zhou, Q.; Jin, H. SLC7A11/XCT in Cancer: Biological Functions and Therapeutic Implications. Am. J. Cancer Res. 2020, 10, 3106–3126. [Google Scholar] [PubMed]
- Wu, Y.; Yu, C.; Luo, M.; Cen, C.; Qiu, J.; Zhang, S.; Hu, K. Ferroptosis in Cancer Treatment: Another Way to Rome. Front. Oncol. 2020, 10, 571127. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The Role of Ferroptosis in Ionizing Radiation-Induced Cell Death and Tumor Suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef]
- Lim, J.K.M.; Delaidelli, A.; Minaker, S.W.; Zhang, H.-F.; Colovic, M.; Yang, H.; Negri, G.L.; von Karstedt, S.; Lockwood, W.W.; Schaffer, P.; et al. Cystine/Glutamate Antiporter XCT (SLC7A11) Facilitates Oncogenic RAS Transformation by Preserving Intracellular Redox Balance. Proc. Natl. Acad. Sci. USA 2019, 116, 9433–9442. [Google Scholar] [CrossRef]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C.A. The Concentration of Oxygen Dissolved in Tissues at the Time of Irradiation as a Factor in Radiotherapy. BJR 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Ye, L.F.; Chaudhary, K.R.; Zandkarimi, F.; Harken, A.D.; Kinslow, C.J.; Upadhyayula, P.S.; Dovas, A.; Higgins, D.M.; Tan, H.; Zhang, Y.; et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS Chem. Biol. 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Lang, X.; Green, M.D.; Wang, W.; Yu, J.; Choi, J.E.; Jiang, L.; Liao, P.; Zhou, J.; Zhang, Q.; Dow, A.; et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019, 9, 1673–1685. [Google Scholar] [CrossRef]
- Takeuchi, S.; Wada, K.; Nagatani, K.; Otani, N.; Osada, H.; Nawashiro, H. Sulfasalazine and Temozolomide with Radiation Therapy for Newly Diagnosed Glioblastoma. Neurol. India 2014, 62, 42. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.C.; Buffa, F.M.; Silva, P.; Miller, C.; Valentine, H.R.; Turley, H.; Shah, K.A.; Cox, G.J.; Corbridge, R.J.; Homer, J.J.; et al. Relation of a Hypoxia Metagene Derived from Head and Neck Cancer to Prognosis of Multiple Cancers. Cancer Res. 2007, 67, 3441–3449. [Google Scholar] [CrossRef] [PubMed]
- Buffa, F.M.; Harris, A.L.; West, C.M.; Miller, C.J. Large Meta-Analysis of Multiple Cancers Reveals a Common, Compact and Highly Prognostic Hypoxia Metagene. Br. J. Cancer 2010, 102, 428–435. [Google Scholar] [CrossRef]
- Ragnum, H.B.; Vlatkovic, L.; Lie, A.K.; Axcrona, K.; Julin, C.H.; Frikstad, K.M.; Hole, K.H.; Seierstad, T.; Lyng, H. The Tumour Hypoxia Marker Pimonidazole Reflects a Transcriptional Programme Associated with Aggressive Prostate Cancer. Br. J. Cancer 2015, 112, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Noeparast, A.; Teugels, E.; Giron, P.; Verschelden, G.; De Brakeleer, S.; Decoster, L.; De Grève, J. Non-V600 BRAF Mutations Recurrently Found in Lung Cancer Predict Sensitivity to the Combination of Trametinib and Dabrafenib. Oncotarget 2017, 8, 60094–60108. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; De Ridder, M.; Verovski, V.N.; Sonveaux, P.; Jordan, B.F.; Law, K.; Monsaert, C.; Van den Berge, D.L.; Verellen, D.; Feron, O.; et al. Activated Macrophages as a Novel Determinant of Tumor Cell Radioresponse: The Role of Nitric Oxide–Mediated Inhibition of Cellular Respiration and Oxygen Sparing. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, H.; Corbet, C.; de Mey, S.; Law, K.; Gevaert, T.; Feron, O.; De Ridder, M. Piperlongumine Increases Sensitivity of Colorectal Cancer Cells to Radiation: Involvement of ROS Production via Dual Inhibition of Glutathione and Thioredoxin Systems. Cancer Lett. 2019, 450, 42–52. [Google Scholar] [CrossRef]
- de Mey, S.; Dufait, I.; Jiang, H.; Corbet, C.; Wang, H.; Van De Gucht, M.; Kerkhove, L.; Law, K.L.; Vandenplas, H.; Gevaert, T.; et al. Dichloroacetate Radiosensitizes Hypoxic Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9367. [Google Scholar] [CrossRef]
- Van de Gucht, M.; Dufait, I.; Kerkhove, L.; Corbet, C.; de Mey, S.; Jiang, H.; Law, K.L.; Gevaert, T.; Feron, O.; De Ridder, M. Inhibition of Phosphoglycerate Dehydrogenase Radiosensitizes Human Colorectal Cancer Cells under Hypoxic Conditions. Cancers 2022, 14, 5060. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 7th ed.; Wolters Kluwer: Alphen aan Den Rijn, The Nederlands, 2010. [Google Scholar]
- Benhar, M.; Shytaj, I.L.; Stamler, J.S.; Savarino, A. Dual Targeting of the Thioredoxin and Glutathione Systems in Cancer and HIV. J. Clin. Investig. 2016, 126, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Arce-Rodríguez, A.; Pankratz, D.; Preusse, M.; Nikel, P.I.; Häussler, S. Dual Effect: High NADH Levels Contribute to Efflux-Mediated Antibiotic Resistance but Drive Lethality Mediated by Reactive Oxygen Species. mBio 2022, 13, e0243421. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- de Mey, S.; Jiang, H.; Corbet, C.; Wang, H.; Dufait, I.; Law, K.; Bastien, E.; Verovski, V.; Gevaert, T.; Feron, O.; et al. Antidiabetic Biguanides Radiosensitize Hypoxic Colorectal Cancer Cells Through a Decrease in Oxygen Consumption. Front. Pharmacol. 2018, 9, 1073. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal Cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Sugano, K.; Maeda, K.; Ohtani, H.; Nagahara, H.; Shibutani, M.; Hirakawa, K. Expression of XCT as a Predictor of Disease Recurrence in Patients with Colorectal Cancer. Anticancer Res. 2015, 35, 677–682. [Google Scholar]
- Sleire, L.; Skeie, B.S.; Netland, I.A.; Førde, H.E.; Dodoo, E.; Selheim, F.; Leiss, L.; Heggdal, J.I.; Pedersen, P.-H.; Wang, J.; et al. Drug Repurposing: Sulfasalazine Sensitizes Gliomas to Gamma Knife Radiosurgery by Blocking Cystine Uptake through System Xc−, Leading to Glutathione Depletion. Oncogene 2015, 34, 5951–5959. [Google Scholar] [CrossRef]
- Nagane, M.; Kanai, E.; Shibata, Y.; Shimizu, T.; Yoshioka, C.; Maruo, T.; Yamashita, T. Sulfasalazine, an Inhibitor of the Cystine-Glutamate Antiporter, Reduces DNA Damage Repair and Enhances Radiosensitivity in Murine B16F10 Melanoma. PLoS ONE 2018, 13, e0195151. [Google Scholar] [CrossRef]
- De Ridder, M.; Verellen, D.; Verovski, V.; Storme, G. Hypoxic Tumor Cell Radiosensitization through Nitric Oxide. Nitric Oxide 2008, 19, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Doi, T.; Nagano, O.; Imamura, C.K.; Ozeki, T.; Ishii, Y.; Tsuchihashi, K.; Takahashi, S.; Nakajima, T.E.; Hironaka, S.; et al. Dose-Escalation Study for the Targeting of CD44v+ Cancer Stem Cells by Sulfasalazine in Patients with Advanced Gastric Cancer (EPOC1205). Gastric Cancer 2017, 20, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, K.; Nosaki, K.; Imamura, C.K.; Ogata, H.; Fujita, A.; Sakata, S.; Hirai, F.; Toyokawa, G.; Iwama, E.; Harada, T.; et al. Phase I Study of Salazosulfapyridine in Combination with Cisplatin and Pemetrexed for Advanced Non-small-cell Lung Cancer. Cancer Sci. 2017, 108, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Ogunrinu, T.A.; Sontheimer, H. Hypoxia Increases the Dependence of Glioma Cells on Glutathione. J. Biol. Chem. 2010, 285, 37716–37724. [Google Scholar] [CrossRef]
- Sims, B.; Clarke, M.; Francillion, L.; Kindred, E.; Hopkins, E.S.; Sontheimer, H. Hypoxic Preconditioning Involves System Xc− Regulation in Mouse Neural Stem Cells. Stem Cell Res. 2012, 8, 285–291. [Google Scholar] [CrossRef]
- Chen, S.; Bu, D.; Zhu, J.; Yue, T.; Guo, S.; Wang, X.; Pan, Y.; Liu, Y.; Wang, P. Endogenous Hydrogen Sulfide Regulates XCT Stability through Persulfidation of OTUB1 at Cysteine 91 in Colon Cancer Cells. Neoplasia 2021, 23, 461–472. [Google Scholar] [CrossRef]
- Conrad, M.; Sato, H. The Oxidative Stress-Inducible Cystine/Glutamate Antiporter, System x c −: Cystine Supplier and Beyond. Amino Acids 2012, 42, 231–246. [Google Scholar] [CrossRef]
- Rodman, S.N.; Spence, J.M.; Ronnfeldt, T.J.; Zhu, Y.; Solst, S.R.; O’Neill, R.A.; Allen, B.G.; Guan, X.; Spitz, D.R.; Fath, M.A. Enhancement of Radiation Response in Breast Cancer Stem Cells by Inhibition of Thioredoxin- and Glutathione-Dependent Metabolism. Radiat. Res. 2016, 186, 385. [Google Scholar] [CrossRef]
- Wang, H.; Bouzakoura, S.; de Mey, S.; Jiang, H.; Law, K.; Dufait, I.; Corbet, C.; Verovski, V.; Gevaert, T.; Feron, O.; et al. Auranofin Radiosensitizes Tumor Cells through Targeting Thioredoxin Reductase and Resulting Overproduction of Reactive Oxygen Species. Oncotarget 2017, 8, 35728–35742. [Google Scholar] [CrossRef]
- Su, J.; Zhao, Q.; Zheng, Z.; Wang, H.; Bian, C.; Meng, L.; Xin, Y.; Jiang, X. Prospective Application of Ferroptosis in Hypoxic Cells for Tumor Radiotherapy. Antioxidants 2022, 11, 921. [Google Scholar] [CrossRef]
- Singhal, R.; Mitta, S.R.; Das, N.K.; Kerk, S.A.; Sajjakulnukit, P.; Solanki, S.; Andren, A.; Kumar, R.; Olive, K.P.; Banerjee, R.; et al. HIF-2α Activation Potentiates Oxidative Cell Death in Colorectal Cancers by Increasing Cellular Iron. J. Clin. Investig. 2021, 131, e143691. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Zhuang, L.; Olszewski, K.; Gan, B. NADPH Debt Drives Redox Bankruptcy: SLC7A11/XCT-Mediated Cystine Uptake as a Double-Edged Sword in Cellular Redox Regulation. Genes Dis. 2021, 8, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Kuang, F.; Liu, J.; Tang, D.; Kang, R. Oxidative Damage and Antioxidant Defense in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 586578. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor P53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-Omics of 34 Colorectal Cancer Cell Lines—A Resource for Biomedical Studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T Cells Regulate Tumour Ferroptosis during Cancer Immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.R.; Brash, A.R.; Conolly, M.E.; Lennard-Jones, J.E. Studies of Prostaglandins and Sulphasalazine in Ulcerative Colitis. Prostaglandins Med. 1981, 6, 165–182. [Google Scholar] [CrossRef]
- Weppelmann, B.; Mönkemeier, D. The Influence of Prostaglandin Antagonists on Radiation Therapy of Carcinoma of the Cervix. Gynecol. Oncol. 1984, 17, 196–199. [Google Scholar] [CrossRef]
- Allgayer, H.; Kruis, W.; Eisenburg, J.; Paumgartner, G. Comparative Pharmacokinetics of Sulphasalazine and Sulphapyridine after Rectal and Oral Administration to Patients with Ulcerative Colitis. Eur. J. Clin. Pharmacol. 1984, 26, 275–277. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerkhove, L.; Geirnaert, F.; Rifi, A.L.; Law, K.L.; Gutiérrez, A.; Oudaert, I.; Corbet, C.; Gevaert, T.; Dufait, I.; De Ridder, M. Repurposing Sulfasalazine as a Radiosensitizer in Hypoxic Human Colorectal Cancer. Cancers 2023, 15, 2363. https://doi.org/10.3390/cancers15082363
Kerkhove L, Geirnaert F, Rifi AL, Law KL, Gutiérrez A, Oudaert I, Corbet C, Gevaert T, Dufait I, De Ridder M. Repurposing Sulfasalazine as a Radiosensitizer in Hypoxic Human Colorectal Cancer. Cancers. 2023; 15(8):2363. https://doi.org/10.3390/cancers15082363
Chicago/Turabian StyleKerkhove, Lisa, Febe Geirnaert, Amir Laraki Rifi, Ka Lun Law, Adrián Gutiérrez, Inge Oudaert, Cyril Corbet, Thierry Gevaert, Inès Dufait, and Mark De Ridder. 2023. "Repurposing Sulfasalazine as a Radiosensitizer in Hypoxic Human Colorectal Cancer" Cancers 15, no. 8: 2363. https://doi.org/10.3390/cancers15082363
APA StyleKerkhove, L., Geirnaert, F., Rifi, A. L., Law, K. L., Gutiérrez, A., Oudaert, I., Corbet, C., Gevaert, T., Dufait, I., & De Ridder, M. (2023). Repurposing Sulfasalazine as a Radiosensitizer in Hypoxic Human Colorectal Cancer. Cancers, 15(8), 2363. https://doi.org/10.3390/cancers15082363






