Inflammation-Based Prognostic Scores in Pancreatic Cancer Patients—A Single-Center Analysis of 1294 Patients within the Last Decade
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Determination of Cut-Off Values
3.3. Predictive Factors of Survival
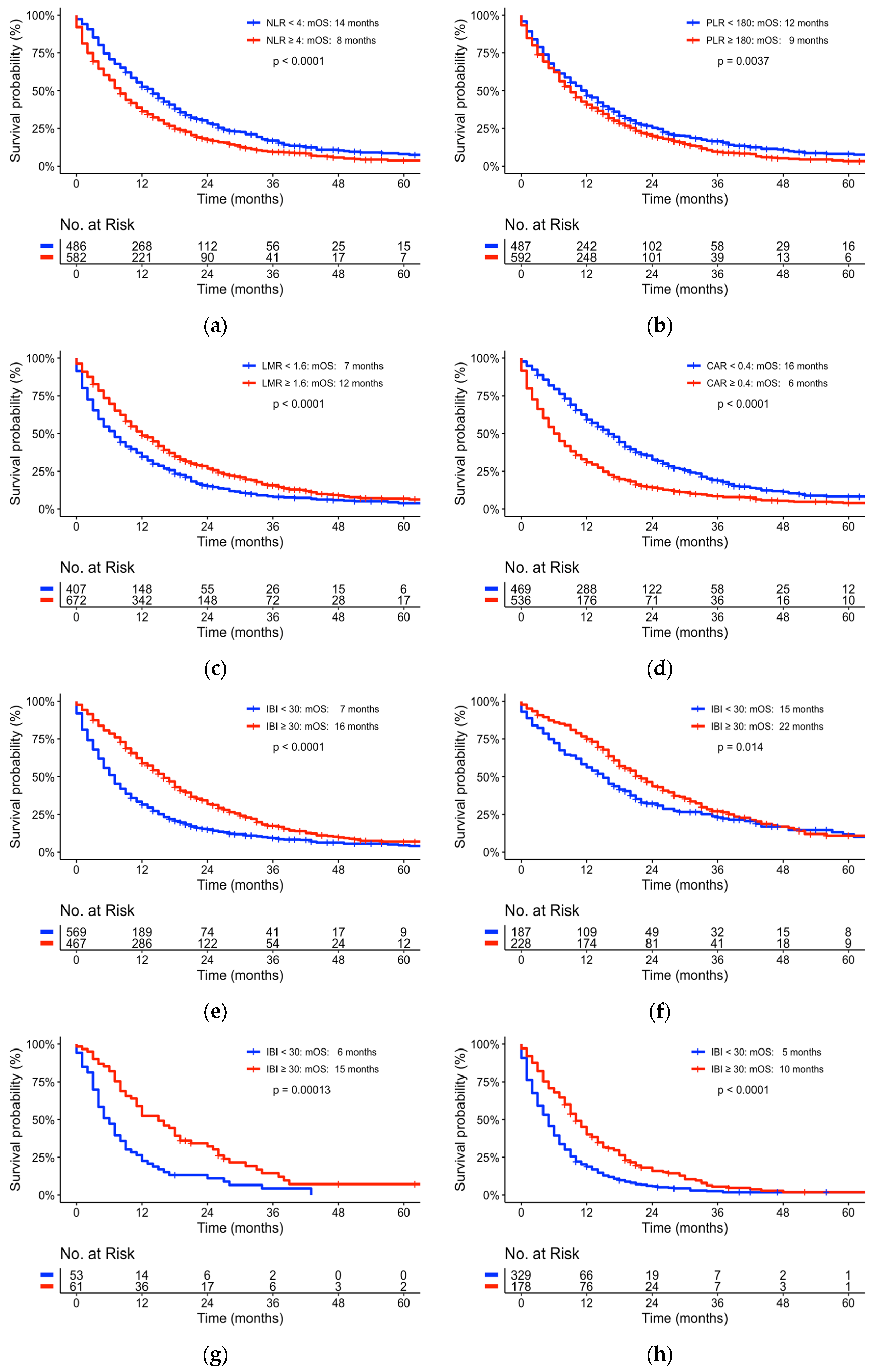
3.4. Assessing New Predictive Inflammatory Scores of Survival
3.5. Univariate and Multivariate Analyses of Patient Cohort Characteristics and Inflammatory-Based Markers
4. Discussion
| Author | Year | Country | Patient No | Parameter | Cut-Offs | Multivariate HR (95% CI) | Status |
|---|---|---|---|---|---|---|---|
| Sierzega [25] | 2017 | Poland | 54 | NLR | 5.0 | 1.66 [1.12–2.46]; p = 0.012 | Resected |
| Pointer [3] | 2020 | USA | 277 | NLR | 5.0 | 2.13 [1.41–3.22]; p = 0.002 | Resected |
| Giakoustidis [26] | 2018 | UK | 127 | NLR | 4.0 | 2.05 [1.11–3.78]; p = 0.020 | Resected |
| Iawi [27] | 2020 | Japan | 119 | NLR | 3.7 | 2.43 [1.48–3.98]; p < 0.001 | Non-Resectable |
| Ventriglia [4] | 2018 | Italy | 70 | NLR | 5.0 | 2.77 [1.30–5.70]; p = 0.006 | Metastasized |
| McLellan [5] | 2020 | France | 172 | NLR | 5.0 | 2.01 [1.33–3.05]; p = 0.001 | Metastasized |
| Giakoustidis [26] | 2018 | UK | 127 | PLR | 120.0 | 1.47 [0.88–2.45]; p = 0.138 | Resected |
| Martin [28] | 2015 | Australia | 124 | PLR | 200.0 | 1.158 [1.07–2.33]; p = 0.020 | Non-Resectable |
| Li [29] | 2019 | China | 134 | PLR | 123.0 | 1.72 [1.16–2.55]; p = 0.007 | Metastasized |
| Sierzega [25] | 2017 | Poland | 54 | LMR | 3.0 | 1.65 [1.06–2.58]; p = 0.026 | Resected |
| Li [30] | 2016 | China | 144 | LMR | 2.6 | 0.15 [0.09–0.25]; p < 0.001 | Resected |
| Stotz [31] | 2015 | Austria | 474 | LMR | 2.8 | 0.81 [0.66–0.99]; p = 0.040 | Mixed |
| Xue [32] | 2017 | Japan | 405 | LMR | 2.8 | 0.46 [0.31–0.69]; p < 0.001 | Non-Resectable + Metastasized |
| Van Wijk [33] | 2020 | Netherlands | 163 | CAR | 0.20 | 1.75 [1.20–2.54]; p = 0.004 | Resected |
| Funamizu [34] | 2022 | Japan | 203 | CAR | 0.09 | 34.51 [11.75–101.38]; p < 0.001 | Resected |
| Haruki [35] | 2016 | Japan | 113 | CAR | 0.03 | 1.73 [1.04–2.87]; p = 0.035 | Resected |
| Fan [36] | 2019 | China | 595 | CAR | 0.18 | 1.84 [1.51–2.24]; p < 0.001 | Non-Resectable + Metastasized |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the Outcomes of Pancreatic Cancer Surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Orhan, A.; Vogelsang, R.P.; Andersen, M.B.; Madsen, M.T.; Hölmich, E.R.; Raskov, H.; Gögenur, I. The Prognostic Value of Tumour-Infiltrating Lymphocytes (TILs) in Pancreatic Cancer: A Systematic Review and Meta-Analysis. Ann. Oncol. 2019, 30, xi2–xi3. [Google Scholar] [CrossRef]
- Pointer, D.T.; Roife, D.; Powers, B.D.; Murimwa, G.; Elessawy, S.; Thompson, Z.J.; Schell, M.J.; Hodul, P.J.; Pimiento, J.M.; Fleming, J.B.; et al. Neutrophil to Lymphocyte Ratio, Not Platelet to Lymphocyte or Lymphocyte to Monocyte Ratio, Is Predictive of Patient Survival after Resection of Early-Stage Pancreatic Ductal Adenocarcinoma. BMC Cancer 2020, 20, 750. [Google Scholar] [CrossRef] [PubMed]
- Ventriglia, J.; Petrillo, A.; Huerta Alváro, M.; Laterza, M.M.; Savastano, B.; Gambardella, V.; Tirino, G.; Pompella, L.; Diana, A.; Iovino, F.; et al. Neutrophil to Lymphocyte Ratio as a Predictor of Poor Prognosis in Metastatic Pancreatic Cancer Patients Treated with Nab-Paclitaxel plus Gemcitabine: A Propensity Score Analysis. Gastroenterol. Res. Pract. 2018, 2018, 2373868. [Google Scholar] [CrossRef]
- McLellan, P.; Henriques, J.; Ksontini, F.; Doat, S.; Hammel, P.; Desrame, J.; Trouilloud, I.; Louvet, C.; Pietrasz, D.; Vernerey, D.; et al. Prognostic Value of the Early Change in Neutrophil-to-Lymphocyte Ratio in Metastatic Pancreatic Adenocarcinoma. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101541. [Google Scholar] [CrossRef]
- Zang, Y.; Fan, Y.; Gao, Z. Pretreatment C-Reactive Protein/Albumin Ratio for Predicting Overall Survival in Pancreatic Cancer: A Meta-Analysis. Medicine 2020, 99, e20595. [Google Scholar] [CrossRef]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-Del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.-W.; Kishiwada, M.; et al. International Consensus on Definition and Criteria of Borderline Resectable Pancreatic Ductal Adenocarcinoma 2017. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al 2018, 18, 2–11. [Google Scholar] [CrossRef]
- Riley, R.D.; Moons, K.G.M.; Snell, K.I.E.; Ensor, J.; Hooft, L.; Altman, D.G.; Hayden, J.; Collins, G.S.; Debray, T.P.A. A Guide to Systematic Review and Meta-Analysis of Prognostic Factor Studies. BMJ 2019, 364, k4597. [Google Scholar] [CrossRef]
- Moons, K.G.M.; de Groot, J.A.H.; Bouwmeester, W.; Vergouwe, Y.; Mallett, S.; Altman, D.G.; Reitsma, J.B.; Collins, G.S. Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies: The CHARMS Checklist. PLoS Med. 2014, 11, e1001744. [Google Scholar] [CrossRef]
- Colloca, G. Performance Status as Prognostic Factor in Phase III Trials of First-Line Chemotherapy of Unresectable or Metastatic Pancreatic Cancer: A Trial-Level Meta-Analysis. Asia Pac. J. Clin. Oncol. 2022, 18, 232–239. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, M.G.; Passam, F.H.; Moschandrea, I.A.; Christophoridou, A.V.; Pappa, C.A.; Coulocheri, S.A.; Kyriakou, D.S. Levels of Serum Cytokines and Acute Phase Proteins in Patients With Essential and Cancer-Related Thrombocytosis. Am. J. Clin. Oncol. 2003, 26, 135. [Google Scholar] [CrossRef] [PubMed]
- Bellone, G.; Turletti, A.; Artusio, E.; Mareschi, K.; Carbone, A.; Tibaudi, D.; Robecchi, A.; Emanuelli, G.; Rodeck, U. Tumor-Associated Transforming Growth Factor-Beta and Interleukin-10 Contribute to a Systemic Th2 Immune Phenotype in Pancreatic Carcinoma Patients. Am. J. Pathol. 1999, 155, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Onfray, F.; López, M.N.; Mendoza-Naranjo, A. Paradoxical Effects of Cytokines in Tumor Immune Surveillance and Tumor Immune Escape. Cytokine Growth Factor Rev. 2007, 18, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, Q.; Fan, J.; Cheng, S.; Ding, W.; Hua, Z. Prognostic Role of the Neutrophil-to-Lymphocyte Ratio in Pancreatic Cancer: A Meta-Analysis Containing 8252 Patients. Clin. Chim. Acta 2018, 479, 181–189. [Google Scholar] [CrossRef]
- Chawla, A.; Huang, T.L.; Ibrahim, A.M.; Hardacre, J.M.; Siegel, C.; Ammori, J.B. Pretherapy Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio Do Not Predict Survival in Resectable Pancreatic Cancer. HPB 2018, 20, 398–404. [Google Scholar] [CrossRef]
- Jamieson, N.B.; Denley, S.M.; Logue, J.; MacKenzie, D.J.; Foulis, A.K.; Dickson, E.J.; Imrie, C.W.; Carter, R.; McKay, C.J.; McMillan, D.C. A Prospective Comparison of the Prognostic Value of Tumor- and Patient-Related Factors in Patients Undergoing Potentially Curative Surgery for Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2011, 18, 2318–2328. [Google Scholar] [CrossRef]
- Zhou, W.; Kuang, T.; Han, X.; Chen, W.; Xu, X.; Lou, W.; Wang, D. Prognostic Role of Lymphocyte-to-Monocyte Ratio in Pancreatic Neuroendocrine Neoplasms. Endocr. Connect. 2020, 9, 289–298. [Google Scholar] [CrossRef]
- Hu, R.-J.; Ma, J.-Y.; Hu, G. Lymphocyte-to-Monocyte Ratio in Pancreatic Cancer: Prognostic Significance and Meta-Analysis. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 481, 142–146. [Google Scholar] [CrossRef]
- Abe, T.; Nakata, K.; Kibe, S.; Mori, Y.; Miyasaka, Y.; Ohuchida, K.; Ohtsuka, T.; Oda, Y.; Nakamura, M. Prognostic Value of Preoperative Nutritional and Immunological Factors in Patients with Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2018, 25, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, S.; Fathy, A.H.; Qian, H.; Zhao, Y. Prognostic Value of Platelet-to-Lymphocyte Ratio in Pancreatic Cancer: A Comprehensive Meta-Analysis of 17 Cohort Studies. OncoTargets Ther. 2018, 11, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, K.; Liu, P.; Ma, D.; Lei, P.; Liu, Q. Deoxyribonuclease 1-like 3 Inhibits Hepatocellular Carcinoma Progression by Inducing Apoptosis and Reprogramming Glucose Metabolism. Int. J. Biol. Sci. 2022, 18, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, H.; Li, X.; Mo, X.; Zhang, H.; Yang, L.; Deng, Y.; Yan, Y.; Yang, G.; Liu, X.; et al. DNASE1L3 as an Indicator of Favorable Survival in Hepatocellular Carcinoma Patients Following Resection. Aging 2020, 12, 1171–1185. [Google Scholar] [CrossRef]
- Sierzega, M.; Lenart, M.; Rutkowska, M.; Surman, M.; Mytar, B.; Matyja, A.; Siedlar, M.; Kulig, J. Preoperative Neutrophil-Lymphocyte and Lymphocyte-Monocyte Ratios Reflect Immune Cell Population Rearrangement in Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2017, 24, 808–815. [Google Scholar] [CrossRef]
- Giakoustidis, A.; Neofytou, K.; Costa Neves, M.; Giakoustidis, D.; Louri, E.; Cunningham, D.; Mudan, S. Identifying the Role of Neutrophil-to-Lymphocyte Ratio and Platelets-to-Lymphocyte Ratio as Prognostic Markers in Patients Undergoing Resection of Pancreatic Ductal Adenocarcinoma. Ann. Hepato-Biliary-Pancreat. Surg. 2018, 22, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Okuda, T.; Sakagami, J.; Harada, T.; Ohara, T.; Taniguchi, M.; Sakai, H.; Oka, K.; Hara, T.; Tsuji, T.; et al. Neutrophil to Lymphocyte Ratio Predicts Prognosis in Unresectable Pancreatic Cancer. Sci. Rep. 2020, 10, 18758. [Google Scholar] [CrossRef]
- Martin, H.L.; Ohara, K.; Kiberu, A.; Van Hagen, T.; Davidson, A.; Khattak, M.A. Prognostic Value of Systemic Inflammation-Based Markers in Advanced Pancreatic Cancer. Intern. Med. J. 2014, 44, 676–682. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Wang, X.; Shi, Y.; Dai, G.; Li, X. Pretreatment Platelet to Lymphocyte Ratio Is Predictive of Overall Survival in Metastatic Pancreatic Ductal Adenocarcinoma. Transl. Cancer Res. 2019, 8, 17–22. [Google Scholar] [CrossRef]
- Li, G.-J.; Xu, H.-W.; Ji, J.-J.; Yang, F.; Gao, B.-Q. Prognostic Value of Preoperative Lymphocyte-to-Monocyte Ratio in Pancreatic Adenocarcinoma. OncoTargets Ther. 2016, 9, 1085–1092. [Google Scholar] [CrossRef]
- Stotz, M.; Szkandera, J.; Stojakovic, T.; Seidel, J.; Samonigg, H.; Kornprat, P.; Schaberl-Moser, R.; Seggewies, F.; Hoefler, G.; Gerger, A.; et al. The Lymphocyte to Monocyte Ratio in Peripheral Blood Represents a Novel Prognostic Marker in Patients with Pancreatic Cancer. Clin. Chem. Lab. Med. 2015, 53, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Hang, J.; Huang, W.; Li, S.; Li, N.; Kodama, Y.; Matsumoto, S.; Takaori, K.; Zhu, L.; Kanai, M. Validation of Lymphocyte-to-Monocyte Ratio as a Prognostic Factor in Advanced Pancreatic Cancer: An East Asian Cohort Study of 2 Countries. Pancreas 2017, 46, 1011. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, L.; de Klein, G.W.; Kanters, M.A.; Patijn, G.A.; Klaase, J.M. The Ultimate Preoperative C-Reactive Protein-to-Albumin Ratio Is a Prognostic Factor for Survival after Pancreatic Cancer Resection. Eur. J. Med. Res. 2020, 25, 46. [Google Scholar] [CrossRef]
- Funamizu, N.; Utsunomiya, T.; Honjo, M.; Ito, C.; Shine, M.; Uraoka, M.; Nagaoka, T.; Tamura, K.; Sakamoto, K.; Ogawa, K.; et al. Preoperative C-Reactive Protein-to-Albumin Ratio Predicts Postoperative Pancreatic Fistula Following Pancreatoduodenectomy: A Single-Center, Retrospective Study. Curr. Oncol. 2022, 29, 9867–9874. [Google Scholar] [CrossRef]
- Haruki, K.; Shiba, H.; Shirai, Y.; Horiuchi, T.; Iwase, R.; Fujiwara, Y.; Furukawa, K.; Misawa, T.; Yanaga, K. The C-Reactive Protein to Albumin Ratio Predicts Long-Term Outcomes in Patients with Pancreatic Cancer After Pancreatic Resection. World J. Surg. 2016, 40, 2254–2260. [Google Scholar] [CrossRef]
- Fan, Z.; Fan, K.; Gong, Y.; Huang, Q.; Yang, C.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; Luo, G.; et al. The CRP/Albumin Ratio Predicts Survival And Monitors Chemotherapeutic Effectiveness In Patients With Advanced Pancreatic Cancer. Cancer Manag. Res. 2019, 11, 8781–8788. [Google Scholar] [CrossRef] [PubMed]
| Factor | Total No. (%) |
|---|---|
| No. of patients Median Age (range) | 1294 66 (28–94) years |
| Sex | |
| Female | 576 (45.0) |
| Male | 718 (55.0) |
| ECOG | |
| ≥2 | 192 (14.8) |
| <2 Unknown | 616 (47.6) 486 (37.6) |
| Stage | |
| Resected | 537 (41.5) |
| Locally advanced | 134 (10.5) |
| Metastasized | 623 (48.0) |
| Localization | |
| Head | 675 (52.2) |
| Body | 125 (9.7) |
| Tail | 208 (16.1) |
| Overlap | 63 (4.9) |
| Not specified | 223 (17.1) |
| Treatment | |
| Curative~ | |
| R0 | 347 (26.7) |
| R1 | 147 (11.5) |
| R2 | 4 (0.3) |
| RX | 39 (3.0) |
| Palliative~ | 757 (58.5) |
| Factor | Cut-Off | mOS (Months) ≥Cut-Off | mOS (Months) <Cut-Off | p-Value |
|---|---|---|---|---|
| Neutrophils (/nL) | 5 | 9 | 15 | 0.001 |
| Lymphocytes (/nL) | 1.35 | 12 | 9 | 0.001 |
| Monocytes (/nL) | 0.60 | 9 | 13 | 0.001 |
| Platelets (/nL) | 235 | 11 | 9 | 0.190 |
| CRP (mg/L) | 15 | 6 | 15 | 0.001 |
| Albumin (g/L) | 38.5 | 15 | 8 | <0.0001 |
| NLR | 4.0 | 8 | 14 | 0.0001 |
| LMR | 1.6 | 12 | 7 | <0.0001 |
| PLR | 180 | 9 | 12 | 0.0037 |
| CAR | 0.4 | 6 | 16 | <0.0001 |
| IBI | 30 | 16 | 7 | 0.0001 |
| Factor | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (years) | |||||||
| ≥65 | 594 | ||||||
| <65 | 700 | 0.75 | 0.70–0.85 | <0.001 | 0.8 | 0.7–0.9 | <0.001 |
| Sex | |||||||
| Female | 576 | ||||||
| Male | 718 | 1.2 | 1.1–1.4 | <0.001 | 1.4 | 1.1–1.5 | 0.001 |
| Tumor stage | |||||||
| Resected | 537 | ||||||
| Locally advanced | 134 | 1.8 | 1.4–2.2 | <0.001 | 1.8 | 1.2–1.5 | <0.001 |
| Metastasized | 623 | 2.6 | 2.2–3.0 | <0.001 | 2.5 | 2.1–2.9 | <0.001 |
| Localization | |||||||
| Head | 675 | ||||||
| Body | 125 | 0.95 | 0.76–1.2 | 0.618 | 0.86 | 0.6–1.1 | 0.249 |
| Tail | 208 | 1.20 | 0.99–1.4 | 0.058 | 0.98 | 0.8–1.2 | 0.862 |
| Overlap | 63 | 1.49 | 1.12–2.0 | 0.007 | 1.01 | 0.7–1.4 | 0.970 |
| Not specified | 223 | 1.50 | 1.26–1.8 | 0.001 | 1.11 | 0.9–1.3 | 0.295 |
| CA19-9 (kU/L) | |||||||
| ≥300 | 611 | ||||||
| <300 | 524 | 0.55 | 0.48–0.64 | 0.001 | 1.5 | 1.2–1.7 | <0.001 |
| Unknown CA19-9 | 159 | ||||||
| NLR | |||||||
| <4 | 581 | ||||||
| ≥4 | 713 | 1.5 | 1.2–1.6 | 0.001 | 1.3 | 1.1–1.7 | 0.0011 |
| LMR | |||||||
| <1.6 | 491 | ||||||
| ≥1.6 | 803 | 0.69 | 0.61–0.79 | 0.001 | 0.8 | 0.7–0.99 | 0.0383 |
| PLR | |||||||
| <180 | 590 | ||||||
| ≥180 | 704 | 1.2 | 1.1–1.4 | 0.004 | 1.0 | 0.9–1.2 | 0.625 |
| CAR | |||||||
| <0.4 | 561 | ||||||
| ≥0.4 | 636 | 1.8 | 1.61–2.1 | 0.001 | 1.4 | 1.2–1.7 | <0.001 |
| Unknown CRP | 97 | ||||||
| IBI | |||||||
| <30 | 689 | ||||||
| ≥30 | 552 | 0.57 | 0.51–0.65 | 0.001 | 0.65 | 0.6–0.8 | <0.001 |
| Unknown IBI | 53 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, C.C.M.; Schneider, F.; Hilfenhaus, G.; Vecchione, L.; Felsenstein, M.; Ihlow, J.; Geisel, D.; Sander, S.; Pratschke, J.; Stintzing, S.; et al. Inflammation-Based Prognostic Scores in Pancreatic Cancer Patients—A Single-Center Analysis of 1294 Patients within the Last Decade. Cancers 2023, 15, 2367. https://doi.org/10.3390/cancers15082367
Neumann CCM, Schneider F, Hilfenhaus G, Vecchione L, Felsenstein M, Ihlow J, Geisel D, Sander S, Pratschke J, Stintzing S, et al. Inflammation-Based Prognostic Scores in Pancreatic Cancer Patients—A Single-Center Analysis of 1294 Patients within the Last Decade. Cancers. 2023; 15(8):2367. https://doi.org/10.3390/cancers15082367
Chicago/Turabian StyleNeumann, Christopher C. M., François Schneider, Georg Hilfenhaus, Loredana Vecchione, Matthäus Felsenstein, Jana Ihlow, Dominik Geisel, Steffen Sander, Johann Pratschke, Sebastian Stintzing, and et al. 2023. "Inflammation-Based Prognostic Scores in Pancreatic Cancer Patients—A Single-Center Analysis of 1294 Patients within the Last Decade" Cancers 15, no. 8: 2367. https://doi.org/10.3390/cancers15082367
APA StyleNeumann, C. C. M., Schneider, F., Hilfenhaus, G., Vecchione, L., Felsenstein, M., Ihlow, J., Geisel, D., Sander, S., Pratschke, J., Stintzing, S., Keilholz, U., & Pelzer, U. (2023). Inflammation-Based Prognostic Scores in Pancreatic Cancer Patients—A Single-Center Analysis of 1294 Patients within the Last Decade. Cancers, 15(8), 2367. https://doi.org/10.3390/cancers15082367








