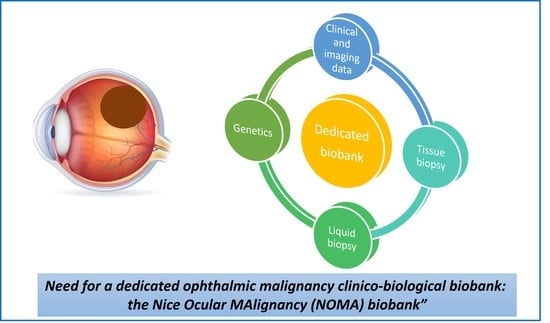
Need for a Dedicated Ophthalmic Malignancy Clinico-Biological Biobank: The Nice Ocular MAlignancy (NOMA) Biobank
Abstract
:Simple Summary
Abstract
1. Introduction
2. What Are the Specific Challenges Associated with Ophthalmic Malignancies?
2.1. A Wide Variety of Malignancies
2.2. A Wide Variety of Clinical Presentations and Aggressiveness
2.3. Tumor Rarity
2.4. Lack of Tissue Biopsy: The UM Example
2.5. Emergence of Liquid Biopsies
2.5.1. Venous Liquid Biopsies: The UM Example
2.5.2. Aqueous Humor (AH) Biopsies
3. Legal, Economic, and Technical Aspects
3.1. Legal Considerations
3.2. Funding Considerations
3.3. Technical, Space, and Computer Considerations
3.3.1. Biobanking Process for UM
3.3.2. Process for Other Ophthalmic Malignancies
4. Resources of the NOMA Biobank
4.1. All Tumors
4.2. Uveal Melanoma
5. Objectives of Setting Up a Dedicated Ophthalmic Malignancy Biobank
5.1. Diagnosis
5.2. Translational Research and Precision Medicine
5.3. Impact of Radiotherapy on UM Genetics
5.4. Pretreatment Screening
5.5. Scientific Output
5.6. National and International Collaborations
5.7. Information for Patients and Other Health Professionals
5.8. Education and Training
6. Strengths of the NOMA Biobank
- (i)
- The NOMA biobank is certified (ISO 9001, NF-96S-900) and accredited (ISO 20387) and belongs to an already well-established biobank (Cote d’Azur Biobank) (www.biobank-cotedazur.fr, accessed on 1 January 2023),
- (ii)
- It is stored in a university pathology laboratory (LPCE, Nice) accredited for clinical and molecular pathology according to the ISO 15189 standard,
- (iii)
- Clinical, imaging, histological, biological, and genetic data are collected for each patient,
- (iv)
- Its business model is supported by public funding programs,
- (v)
- It has allowed for an increase in the amount of translational research conducted,
- (vi)
- It allows for an increase in the number of scientific publications and national and international collaborations,
- (vii)
- A master’s degree entitled “Biobanks and Complex Data Management” has been set up by the Côte d’Azur University (Nice, France) to train students to become biobankers.
7. Limitations
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nahon-Estève, S.; Bertolotto, C.; Picard-Gauci, A.; Gastaud, L.; Baillif, S.; Hofman, P.; Groulier, A.; Maschi, C.; Caujolle, J.-P.; Lassalle, S.; et al. Small but Challenging Conjunctival Melanoma: New Insights, Paradigms and Future Perspectives. Cancers 2021, 13, 5691. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.-H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal Melanoma. Nat. Rev. Dis. Primer 2020, 6, 24. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal Melanoma: Relatively Rare but Deadly Cancer. Eye Lond. Engl. 2017, 31, 241–257. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic Disease from Uveal Melanoma: Treatment Options and Future Prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Uner, O.E.; Gandrakota, N.; Azarcon, C.P.; Grossniklaus, H.E. Animal Models of Uveal Melanoma. Ann. Eye Sci. 2022, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Lamas, N.J.; Martel, A.; Nahon-Estève, S.; Goffinet, S.; Macocco, A.; Bertolotto, C.; Lassalle, S.; Hofman, P. Prognostic Biomarkers in Uveal Melanoma: The Status Quo, Recent Advances and Future Directions. Cancers 2021, 14, 96. [Google Scholar] [CrossRef]
- Hewitt, R.; Watson, P. Defining Biobank. Biopreserv. Biobank 2013, 11, 309–315. [Google Scholar] [CrossRef]
- Clavreul, A.; Soulard, G.; Lemée, J.-M.; Rigot, M.; Fabbro-Peray, P.; Bauchet, L.; Figarella-Branger, D.; Menei, P. FGB network The French Glioblastoma Biobank (FGB): A National Clinicobiological Database. J. Transl. Med. 2019, 17, 133. [Google Scholar] [CrossRef]
- Gao, Z.; Tan, J.; Wang, S.; Yu, H.; Zhou, Z.; Zhang, Y.; Zhou, M.; Xia, X.; Yao, F.; Huang, J. The Xiangya Ocular Tumor Bank: A Disease-Specific Biobank for Advancing Translational Research into Ocular Tumors. Front. Med. 2021, 8, 774624. [Google Scholar] [CrossRef]
- Rodrigues, M.; de Koning, L.; Coupland, S.E.; Jochemsen, A.G.; Marais, R.; Stern, M.-H.; Valente, A.; Barnhill, R.; Cassoux, N.; Evans, A.; et al. So Close, yet so Far: Discrepancies between Uveal and Other Melanomas. A Position Paper from UM Cure 2020. Cancers 2019, 11, 1032. [Google Scholar] [CrossRef]
- Pandiani, C.; Béranger, G.E.; Leclerc, J.; Ballotti, R.; Bertolotto, C. Focus on Cutaneous and Uveal Melanoma Specificities. Genes Dev. 2017, 31, 724–743. [Google Scholar] [CrossRef]
- Reichstein, D.; Brock, A.; Lietman, C.; McKean, M. Treatment of Metastatic Uveal Melanoma in 2022: Improved Treatment Regimens and Improved Prognosis. Curr. Opin. Ophthalmol. 2022, 33, 585–590. [Google Scholar] [CrossRef]
- Komatsubara, K.M.; Carvajal, R.D. Immunotherapy for the Treatment of Uveal Melanoma: Current Status and Emerging Therapies. Curr. Oncol. Rep. 2017, 19, 45. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal Melanoma: Trends in Incidence, Treatment, and Survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Smit, K.N.; Jager, M.J.; de Klein, A.; Kiliç, E. Uveal Melanoma: Towards a Molecular Understanding. Prog. Retin. Eye Res. 2020, 75, 100800. [Google Scholar] [CrossRef]
- Stålhammar, G.; Grossniklaus, H.E. Intratumor Heterogeneity in Uveal Melanoma BAP-1 Expression. Cancers 2021, 13, 1143. [Google Scholar] [CrossRef]
- Trolet, J.; Hupé, P.; Huon, I.; Lebigot, I.; Decraene, C.; Delattre, O.; Sastre-Garau, X.; Saule, S.; Thiéry, J.-P.; Plancher, C.; et al. Genomic Profiling and Identification of High-Risk Uveal Melanoma by Array CGH Analysis of Primary Tumors and Liver Metastases. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2572–2580. [Google Scholar] [CrossRef]
- Onken, M.D.; Worley, L.A.; Char, D.H.; Augsburger, J.J.; Correa, Z.M.; Nudleman, E.; Aaberg, T.M.; Altaweel, M.M.; Bardenstein, D.S.; Finger, P.T.; et al. Collaborative Ocular Oncology Group Report Number 1: Prospective Validation of a Multi-Gene Prognostic Assay in Uveal Melanoma. Ophthalmology 2012, 119, 1596–1603. [Google Scholar] [CrossRef]
- Brouwer, N.J.; Verdijk, R.M.; Heegaard, S.; Marinkovic, M.; Esmaeli, B.; Jager, M.J. Conjunctival Melanoma: New Insights in Tumour Genetics and Immunology, Leading to New Therapeutic Options. Prog. Retin. Eye Res. 2022, 86, 100971. [Google Scholar] [CrossRef]
- Aronow, M.E.; Topham, A.K.; Singh, A.D. Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973–2013). Ocul. Oncol. Pathol. 2018, 4, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Höllhumer, R.; Williams, S.; Michelow, P. Ocular Surface Squamous Neoplasia: Management and Outcomes. Eye 2021, 35, 1562–1573. [Google Scholar] [CrossRef]
- Quigley, C.; Deady, S.; Hughes, E.; McElnea, E.; Zgaga, L.; Chetty, S. National Incidence of Eyelid Cancer in Ireland (2005–2015). Eye 2019, 33, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, L.; Midena, E.; Trainiti, S.; Londei, D.; Bonaldi, L.; Bini, S.; Parrozzani, R. Uveal Melanoma Biopsy: A Review. Cancers 2019, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, E.; Thariat, J.; Maschi, C.; Delas, J.; Schouver, E.D.; Hérault, J.; Baillif, S.; Caujolle, J.-P. Outcomes after Proton Beam Therapy for Large Choroidal Melanomas in 492 Patients. Am. J. Ophthalmol. 2016, 165, 78–87. [Google Scholar] [CrossRef]
- Martel, A.; Baillif, S.; Nahon-Esteve, S.; Gastaud, L.; Bertolotto, C.; Roméo, B.; Mograbi, B.; Lassalle, S.; Hofman, P. Liquid Biopsy for Solid Ophthalmic Malignancies: An Updated Review and Perspectives. Cancers 2020, 12, 3284. [Google Scholar] [CrossRef]
- Ulmer, A.; Beutel, J.; Süsskind, D.; Hilgers, R.-D.; Ziemssen, F.; Lüke, M.; Röcken, M.; Rohrbach, M.; Fierlbeck, G.; Bartz-Schmidt, K.-U.; et al. Visualization of Circulating Melanoma Cells in Peripheral Blood of Patients with Primary Uveal Melanoma. Clin. Cancer Res. 2008, 14, 4469–4474. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Madic, J.; Mariani, P.; Piperno-Neumann, S.; Rampanou, A.; Servois, V.; Cassoux, N.; Desjardins, L.; Milder, M.; Vaucher, I.; et al. Detection Rate and Prognostic Value of Circulating Tumor Cells and Circulating Tumor DNA in Metastatic Uveal Melanoma. Int. J. Cancer 2014, 134, 1207–1213. [Google Scholar] [CrossRef]
- Ghiam, B.K.; Xu, L.; Berry, J.L. Aqueous Humor Markers in Retinoblastoma, a Review. Transl. Vis. Sci. Technol. 2019, 8, 13. [Google Scholar] [CrossRef]
- Berry, J.L.; Xu, L.; Polski, A.; Jubran, R.; Kuhn, P.; Kim, J.W.; Hicks, J. Aqueous Humor Is Superior to Blood as a Liquid Biopsy for Retinoblastoma. Ophthalmology 2020, 127, 552–554. [Google Scholar] [CrossRef]
- Wierenga, A.P.A.; Cao, J.; Mouthaan, H.; van Weeghel, C.; Verdijk, R.M.; van Duinen, S.G.; Kroes, W.G.M.; Dogrusöz, M.; Marinkovic, M.; van der Burg, S.S.H.; et al. Aqueous Humor Biomarkers Identify Three Prognostic Groups in Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4740–4747. [Google Scholar] [CrossRef]
- Im, D.H.; Peng, C.-C.; Xu, L.; Kim, M.E.; Ostrow, D.; Yellapantula, V.; Bootwalla, M.; Biegel, J.A.; Gai, X.; Prabakar, R.K.; et al. Potential of Aqueous Humor as a Liquid Biopsy for Uveal Melanoma. Int. J. Mol. Sci. 2022, 23, 6226. [Google Scholar] [CrossRef]
- Washetine, K.; Heeke, S.; Bonnetaud, C.; Kara-Borni, M.; Ilié, M.; Lassalle, S.; Butori, C.; Long-Mira, E.; Marquette, C.H.; Cohen, C.; et al. Establishing a Dedicated Lung Cancer Biobank at the University Center Hospital of Nice (France). Why and How? Cancers 2018, 10, 220. [Google Scholar] [CrossRef]
- De Blasio, P.; Biunno, I. New Challenges for Biobanks: Accreditation to the New ISO 20387:2018 Standard Specific for Biobanks. BioTech 2021, 10, 13. [Google Scholar] [CrossRef]
- De Souza, Y.G. Sustainability of Biobanks in the Future. Adv. Exp. Med. Biol. 2015, 864, 29–35. [Google Scholar] [CrossRef]
- Vaught, J.; Rogers, J.; Carolin, T.; Compton, C. Biobankonomics: Developing a Sustainable Business Model Approach for the Formation of a Human Tissue Biobank. J. Natl. Cancer Inst. Monogr. 2011, 2011, 24–31. [Google Scholar] [CrossRef]
- Harati, M.D.; Williams, R.R.; Movassaghi, M.; Hojat, A.; Lucey, G.M.; Yong, W.H. An Introduction to Starting a Biobank. In Methods Molecular Biology; Humana Press: Clifton, NJ, USA, 2019; Volume 1897, pp. 7–16. [Google Scholar] [CrossRef]
- Hofman, P.; Bréchot, C.; Zatloukal, K.; Dagher, G.; Clément, B. Public-Private Relationships in Biobanking: A Still Underestimated Key Component of Open Innovation. Virchows Arch. Int. J. Pathol. 2014, 464, 3–9. [Google Scholar] [CrossRef]
- Clément, B.; Yuille, M.; Zaltoukal, K.; Wichmann, H.-E.; Anton, G.; Parodi, B.; Kozera, L.; Bréchot, C.; Hofman, P.; Dagher, G.; et al. Public Biobanks: Calculation and Recovery of Costs. Sci. Transl. Med. 2014, 6, 261fs45. [Google Scholar] [CrossRef]
- Mathis, T.; Cassoux, N.; Tardy, M.; Piperno, S.; Gastaud, L.; Dendale, R.; Maschi, C.; Nguyen, A.-M.; Meyer, L.; Bonnin, N.; et al. Management of uveal melanomas, guidelines for oncologists. Bull. Cancer 2018, 105, 967–980. [Google Scholar] [CrossRef]
- Hofman, V.; Ilie, M.; Long, E.; Lassalle, S.; Butori, C.; Bence, C.; Washetine, K.; Lalvee, S.; Hofman, P. Immunohistochemistry and personalised medicine in lung oncology: Advantages and limitations. Bull. Cancer 2014, 101, 958–965. [Google Scholar] [CrossRef]
- Jacotot, L.; Woodward, M.; de Montalier, A.; Vaglio, P. Utilizing Modular Biobanking Software in Different Types of Biobanking Activities. Biopreservation Biobanking 2022, 20, 417–422. [Google Scholar] [CrossRef] [PubMed]
- van Beek, J.G.M.; Koopmans, A.E.; Vaarwater, J.; de Rooi, J.J.; Paridaens, D.; Naus, N.C.; de Klein, A.; Verdijk, R.M.; Kiliç, E. The Prognostic Value of Extraocular Extension in Relation to Monosomy 3 and Gain of Chromosome 8q in Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, M.E.; Orloff, M.; Sato, R.; Chervoneva, I.; Shields, C.L.; Shields, J.A.; Mastrangelo, M.J.; Sato, T. Adjuvant Sunitinib in High-Risk Patients with Uveal Melanoma: Comparison with Institutional Controls. Ophthalmology 2018, 125, 210–217. [Google Scholar] [CrossRef]
- Dogrusöz, M.; Kroes, W.G.M.; van Duinen, S.G.; Creutzberg, C.L.; Versluis, M.; Bleeker, J.C.; Marinkovic, M.; Luyten, G.P.M.; Jager, M.J. Radiation Treatment Affects Chromosome Testing in Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5956–5964. [Google Scholar] [CrossRef] [PubMed]
- Coupland, S.E.; Kalirai, H.; Ho, V.; Thornton, S.; Damato, B.E.; Heimann, H. Concordant Chromosome 3 Results in Paired Choroidal Melanoma Biopsies and Subsequent Tumour Resection Specimens. Br. J. Ophthalmol. 2015, 99, 1444–1450. [Google Scholar] [CrossRef]
- Thornton, S.; Coupland, S.E.; Heimann, H.; Hussain, R.; Groenewald, C.; Kacperek, A.; Damato, B.; Taktak, A.; Eleuteri, A.; Kalirai, H. Effects of Plaque Brachytherapy and Proton Beam Radiotherapy on Prognostic Testing: A Comparison of Uveal Melanoma Genotyped by Microsatellite Analysis. Br. J. Ophthalmol. 2020, 104, 1462–1466. [Google Scholar] [CrossRef]
- Dhillon, S. Tebentafusp: First Approval. Drugs 2022, 82, 703–710. [Google Scholar] [CrossRef]
- Lassalle, S.; Nahon-Esteve, S.; Frouin, E.; Boulagnon-Rombi, C.; Josselin, N.; Cassoux, N.; Barnhill, R.; Scheller, B.; Baillif, S.; Hofman, P. PD-L1 Expression in 65 Conjunctival Melanomas and Its Association with Clinical Outcome. Int. J. Mol. Sci. 2020, 21, 9147. [Google Scholar] [CrossRef]
- Pandiani, C.; Strub, T.; Nottet, N.; Cheli, Y.; Gambi, G.; Bille, K.; Husser, C.; Dalmasso, M.; Béranger, G.; Lassalle, S.; et al. Single-Cell RNA Sequencing Reveals Intratumoral Heterogeneity in Primary Uveal Melanomas and Identifies HES6 as a Driver of the Metastatic Disease. Cell Death Differ. 2021, 28, 1990–2000. [Google Scholar] [CrossRef]
- Krossa, I.; Strub, T.; Martel, A.; Nahon-Esteve, S.; Lassalle, S.; Hofman, P.; Baillif, S.; Ballotti, R.; Bertolotto, C. Recent Advances in Understanding the Role of HES6 in Cancers. Theranostics 2022, 12, 4374–4385. [Google Scholar] [CrossRef]
- Martel, A.; Baillif, S.; Nahon-Esteve, S.; Gastaud, L.; Bertolotto, C.; Lassalle, S.; Lagier, J.; Hamedani, M.; Poissonnet, G. Orbital Exenteration: An Updated Review with Perspectives. Surv. Ophthalmol. 2021, 66, 856–876. [Google Scholar] [CrossRef]
- Martel, A.; Lassalle, S.; Picard-Gauci, A.; Gastaud, L.; Montaudie, H.; Bertolotto, C.; Nahon-Esteve, S.; Poissonnet, G.; Hofman, P.; Baillif, S. New Targeted Therapies and Immunotherapies for Locally Advanced Periocular Malignant Tumours: Towards a New “Eye-Sparing” Paradigm? Cancers 2021, 13, 2822. [Google Scholar] [CrossRef]
- Monjanel, B.; Baillif, S.; Lagier, J.; Gastaud, L.; Poissonnet, G.; Martel, A. Efficacy and Safety of an Artificial Dermal Graft for the Reconstruction of Exenterated Sockets: A Preliminary Report. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2827–2835. [Google Scholar] [CrossRef]
- Strub, T.; Martel, A.; Nahon-Esteve, S.; Baillif, S.; Ballotti, R.; Bertolotto, C. Translation of Single-Cell Transcriptomic Analysis of Uveal Melanomas to Clinical Oncology. Prog. Retin. Eye Res. 2021, 85, 100968. [Google Scholar] [CrossRef]
- Bellini, L.; Strub, T.; Habel, N.; Pandiani, C.; Marchetti, S.; Martel, A.; Baillif, S.; Bailly-Maitre, B.; Gual, P.; Ballotti, R.; et al. Endoplasmic Reticulum Stress Mediates Resistance to BCL-2 Inhibitor in Uveal Melanoma Cells. Cell Death Discov. 2020, 6, 22. [Google Scholar] [CrossRef]
- Thariat, J.; Martel, A.; Matet, A.; Loria, O.; Kodjikian, L.; Nguyen, A.-M.; Rosier, L.; Herault, J.; Nahon-Estève, S.; Mathis, T. Non-Cancer Effects Following Ionizing Irradiation Involving the Eye and Orbit. Cancers 2022, 14, 1194. [Google Scholar] [CrossRef]
- Martel, A.; Baillif, S.; Thomas, P.; Almairac, F.; Galatoire, O.; Hamedani, M.; Fontaine, D.; Lanteri-Minet, M. Phantom Vision after Eye Removal: Prevalence, Features and Related Risk Factors. Br. J. Ophthalmol. 2022, 106, 1603–1609. [Google Scholar] [CrossRef]
- Martel, A.; Oberic, A.; Moulin, A.; Tieulie, N.; Hamedani, M. Clinical, radiological, pathological features, treatment and follow-up of periocular and/or orbital amyloidosis: Report of 6 cases and literature review. J. Fr. Ophtalmol. 2018, 41, 492–506. [Google Scholar] [CrossRef]








| Tumor Histology | Incidence per Million Inhabitants (Reference) |
|---|---|
| UM | 6 [2] |
| CM | 0.8 [20] |
| Conjunctival squamous cell carcinoma | 0.3 [22] |
| Primary eyelid melanoma | 1 [23] |
| Year | Solid Biopsy Samples | Liquid Biopsy Samples | AH Puncture Samples |
|---|---|---|---|
| 2013 | 4 | 0 | 0 |
| 2014 | 5 | 0 | 0 |
| 2015 | 40 | 0 | 0 |
| 2016 | 131 | 0 | 0 |
| 2017 | 87 | 0 | 0 |
| 2018 | 94 | 710 | 0 |
| 2019 | 152 | 188 | 0 |
| 2020 | 97 | 307 | 11 |
| 2021 | 118 | 870 | 14 |
| 2022 | 108 | 647 | 14 |
| Type of Sample | Processing | Amount | Storage Condition |
|---|---|---|---|
| Tissue | |||
| Frozen tumor tissue | None | 5–50 mg | −80 °C |
| Frozen healthy choroid | None | 5–50 mg | −80 °C |
| Paraffin-embedded tumor tissue | Paraffin protocol (*) | 1–5 FFPE blocks | 4 °C |
| Paraffin-embedded healthy choroid | Paraffin protocol (*) | 1 FFPE blocks | 4 °C |
| Blood | |||
| Whole blood | None | 1 mL per aliquot | −80 °C |
| Plasma | Centrifugation (**) | 1 mL per aliquot | −80 °C |
| PBMC | Centrifugation (***) | 0.5–10 million per 1-mL aliquot | −196 °C (liquid nitrogen) |
| Aqueous humor | None | 10–50 µL per aliquot | −80 °C |
| Demographics | Clinical and Radiological Data | Tumor-Related Radiological Data | Histological Data | Genetic Data |
|---|---|---|---|---|
| Gender Age Personal and family history of cancer | Visual acuity Intraocular pressure Cataract Retinal detachment Optic nerve involvement Extraocular extent Intravitreal injections Follow-up | Diameter Thickness Ciliary body involvement | pTNM classification Cell type (epithelioid, fusiform, mixed) Mitoses Extraocular extent Necrosis Inflammatory infiltration Optic nerve involvement BAP 1 status Extent of resection (R0, R1, R2) | Chromosomal abnormalities Trolet classification |
| Demographics | Histological Data |
|---|---|
| Age Gender Date of surgery or collection | Histological diagnosis pTNM classification of the relevant tumor Tumor size % of tumor cells Cold ischemia time Primary vs. recurrent tumor Inflammatory infiltrate Ulceration Mitotic index Extent of resection (R0, R1, R2) |
| Tumor Location | Histological Subtype: Number (%) | Tissue Biopsy: Number (%) | Venous Liquid Biopsy: Number (%) |
|---|---|---|---|
| Intraocular N = 160 | Uveal melanoma: 160 (100) | 66 (41) | 124 (77.5) |
| Conjunctival N = 31 | Conjunctival melanoma: 21 (68) Conjunctival naevus: 8 (25.5) Squamous cell carcinoma: 2 (6.5) | 31 (100) | 11 (35.5) |
| Orbit N = 16 | Lymphoma: 8 (50) Carcinoma: 4 (25) Schwanoma: 3 (19) Solitary fibrous tumor: 1 (6) | 16 (100) | 0 (0) |
| Number of Enucleated Patients (%) | 66 (100) |
| Primary Enucleation | 62 (94) |
| Secondary Enucleation | 4 (6) |
| Tumor thickness in mm: mean (range) | 12.1 (0.2–20) |
| Epithelioid subtype: number (%) | 44 (67) |
| Extraocular extent: number (%) | 13 (19.7) |
| Ciliary body infiltration: number (%) | 22 (33) |
| Mitoses/mm2: mean number (range) | 1.9 (0–21) |
| % of tumor cells in frozen sample: mean (range) | 75.2 (0–95) |
| Cold ischemia time in min: mean (range) | 34.8 (15–180) |
| BAP 1 loss: number (%) * | 30 (75) |
| Institution | Type of Collaboration (Recent Related Articles) |
|---|---|
| Oncology Department, Antoine Lacassagne Cancer Centre, Nice, France | Fundamental and clinical [1,26,52,53,54] |
| Team 1, Molecular Mediterranean Medicine Centre (C3M), Nice, France | Fundamental [50,51,55,56] |
| Ophthalmology Department, Lyon University Hospital, France | Clinical [57] |
| Oculoplastic Department, Jules Gonin Eye Hospital, Lausanne, Switzerland | Clinical [52,58,59] |
| Anatomic Pathology Service, Pathology Department, Centro Hospitalar e Universitário do Porto, Portugal | Fundamental [6] |
| Liverpool Ocular Oncology Research Department, United Kingdom | Fundamental |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martel, A.; Gastaud, L.; Bonnetaud, C.; Nahon-Esteve, S.; Washetine, K.; Bordone, O.; Salah, M.; Tanga, V.; Fayada, J.; Lespinet, V.; et al. Need for a Dedicated Ophthalmic Malignancy Clinico-Biological Biobank: The Nice Ocular MAlignancy (NOMA) Biobank. Cancers 2023, 15, 2372. https://doi.org/10.3390/cancers15082372
Martel A, Gastaud L, Bonnetaud C, Nahon-Esteve S, Washetine K, Bordone O, Salah M, Tanga V, Fayada J, Lespinet V, et al. Need for a Dedicated Ophthalmic Malignancy Clinico-Biological Biobank: The Nice Ocular MAlignancy (NOMA) Biobank. Cancers. 2023; 15(8):2372. https://doi.org/10.3390/cancers15082372
Chicago/Turabian StyleMartel, Arnaud, Lauris Gastaud, Christelle Bonnetaud, Sacha Nahon-Esteve, Kevin Washetine, Olivier Bordone, Myriam Salah, Virginie Tanga, Julien Fayada, Virginie Lespinet, and et al. 2023. "Need for a Dedicated Ophthalmic Malignancy Clinico-Biological Biobank: The Nice Ocular MAlignancy (NOMA) Biobank" Cancers 15, no. 8: 2372. https://doi.org/10.3390/cancers15082372
APA StyleMartel, A., Gastaud, L., Bonnetaud, C., Nahon-Esteve, S., Washetine, K., Bordone, O., Salah, M., Tanga, V., Fayada, J., Lespinet, V., Allegra, M., Lalvee, S., Zahaf, K., Baillif, S., Bertolotto, C., Mograbi, B., Lassalle, S., & Hofman, P. (2023). Need for a Dedicated Ophthalmic Malignancy Clinico-Biological Biobank: The Nice Ocular MAlignancy (NOMA) Biobank. Cancers, 15(8), 2372. https://doi.org/10.3390/cancers15082372