Targeting Angiogenesis in the Era of Biliary Tract Cancer Immunotherapy: Biological Rationale, Clinical Implications, and Future Research Avenues
Abstract
Simple Summary
Abstract
1. Introduction
2. Tumor Microenvironment in Biliary Tract Tumors
3. Angiogenesis as a Hallmark of BTCs
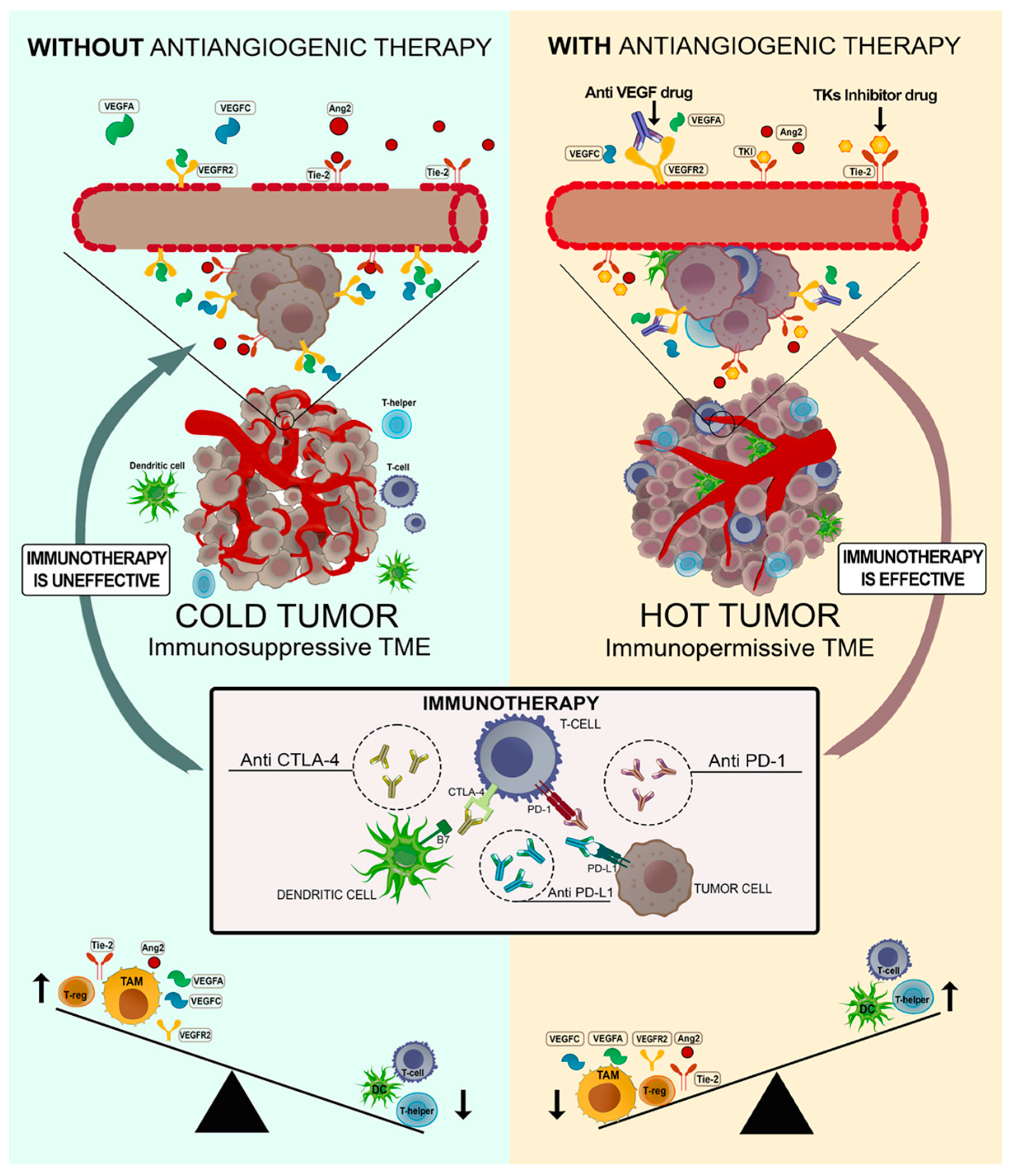
Blocking Angiogenesis to Induce an Immunoresponsive Microenvironment: A Winning Strategy?
4. Clinical Studies Targeting Both Angiogenesis and Immune Checkpoint Factors
4.1. Targeting the VEGF Pathway
4.2. Targeting TKs
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary Tract Cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Malenica, I.; Donadon, M.; Lleo, A. Molecular and Immunological Characterization of Biliary Tract Cancers: A Paradigm Shift Towards a Personalized Medicine. Cancers 2020, 12, 2190. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Lamarca, A.; Edeline, J.; Goyal, L. How I treat biliary tract cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef]
- Cillo, U.; Fondevila, C.; Donadon, M.; Gringeri, E.; Mocchegiani, F.; Schlitt, H.J.; Ijzermans, J.N.M.; Vivarelli, M.; Zieniewicz, K.; Olde Damink, S.W.M.; et al. Surgery for cholangiocarcinoma. Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39 (Suppl. S1), 143–155. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klumpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Oh, D.Y.; Lee, K.H.; Lee, D.W.; Yoon, J.; Kim, T.Y.; Bang, J.H.; Nam, A.R.; Oh, K.S.; Kim, J.M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; El-Rayes, B.F.; Akce, M. Evolving Role of Immunotherapy in Advanced Biliary Tract Cancers. Cancers 2022, 14, 1748. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Shroff, R.T. New Treatment Options for Advanced Biliary Tract Cancer. Curr. Treat. Options Oncol. 2020, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Frega, G.; Di Federico, A.; Brandi, G. FGFR inhibitors in elderly patients with advanced biliary tract cancer: An unsolved issue. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Ayasun, R.; Sahin, I. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer. Lancet Gastroenterol. Hepatol. 2023, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Barriuso, J.; McNamara, M.G.; Valle, J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020, 73, 170–185. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Elez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Harding, J.J.; Cleary, J.M.; Quinn, D.I.; Braña, I.; Moreno, V.; Borad, M.J.; Loi, S.; Spanggaard, I.; Park, H.; Ford, J.M.; et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: Results from the phase II SUMMIT ‘basket’ trial. J. Clin. Oncol. 2021, 39 (Suppl. S3), 320. [Google Scholar] [CrossRef]
- Di Federico, A.; Rizzo, A.; Ricci, A.D.; Frega, G.; Palloni, A.; Tavolari, S.; Brandi, G. Nivolumab: An investigational agent for the treatment of biliary tract cancer. Expert Opin. Investig. Drugs 2021, 30, 325–332. [Google Scholar] [CrossRef]
- Cheng, B.; Yu, Q.; Wang, W. Intimate communications within the tumor microenvironment: Stromal factors function as an orchestra. J. Biomed. Sci. 2023, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.; Edeline, J.; Coulouarn, C. Targeting the tumor microenvironment in cholangiocarcinoma: Implications for therapy. Expert Opin. Ther. Targets 2021, 25, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Di Federico, A.; Frega, G.; Palloni, A.; Tavolari, S.; Brandi, G. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy in Hepatocellular Carcinoma: Where Do We Stand? Front. Oncol. 2021, 11, 803133. [Google Scholar] [CrossRef] [PubMed]
- Rihawi, K.; Ricci, A.D.; Rizzo, A.; Brocchi, S.; Marasco, G.; Pastore, L.V.; Llimpe, F.L.R.; Golfieri, R.; Renzulli, M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int. J. Mol. Sci. 2021, 22, 3805. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huang, T.; Dai, M.; Kong, X.; Liu, H.; Zheng, Z.; Sun, G.; Rong, D.; Jin, Z.; Tang, W.; et al. Tumor Microenvironment and its Implications for Antitumor Immunity in Cholangiocarcinoma: Future Perspectives for Novel Therapies. Int. J. Biol. Sci. 2022, 18, 5369–5390. [Google Scholar] [CrossRef]
- Gambardella, V.; Castillo, J.; Tarazona, N.; Gimeno-Valiente, F.; Martinez-Ciarpaglini, C.; Cabeza-Segura, M.; Rosello, S.; Roda, D.; Huerta, M.; Cervantes, A.; et al. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat. Rev. 2020, 86, 102015. [Google Scholar] [CrossRef]
- Bonecchi, R.; Mantovani, A.; Jaillon, S. Chemokines as Regulators of Neutrophils: Focus on Tumors, Therapeutic Targeting, and Immunotherapy. Cancers 2022, 14, 680. [Google Scholar] [CrossRef]
- Mantovani, A. Cancer-Related Inflammation in Tumor Progression. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128012383. [Google Scholar]
- Kopecka, J.; Salaroglio, I.C.; Perez-Ruiz, E.; Sarmento-Ribeiro, A.B.; Saponara, S.; De Las Rivas, J.; Riganti, C. Hypoxia as a driver of resistance to immunotherapy. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2021, 59, 100787. [Google Scholar] [CrossRef]
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ntellas, P.; Mavroeidis, L.; Gkoura, S.; Gazouli, I.; Amylidi, A.L.; Papadaki, A.; Zarkavelis, G.; Mauri, D.; Karpathiou, G.; Kolettas, E.; et al. Old Player-New Tricks: Non Angiogenic Effects of the VEGF/VEGFR Pathway in Cancer. Cancers 2020, 12, 3145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sprengers, D.; Mancham, S.; Erkens, R.; Boor, P.P.C.; van Beek, A.A.; Doukas, M.; Noordam, L.; Campos Carrascosa, L.; de Ruiter, V.; et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J. Hepatol. 2019, 71, 753–762. [Google Scholar] [CrossRef]
- Job, S.; Rapoud, D.; Dos Santos, A.; Gonzalez, P.; Desterke, C.; Pascal, G.; Elarouci, N.; Ayadi, M.; Adam, R.; Azoulay, D.; et al. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 72, 965–981. [Google Scholar] [CrossRef]
- Alvisi, G.; Termanini, A.; Soldani, C.; Portale, F.; Carriero, R.; Pilipow, K.; Costa, G.; Polidoro, M.; Franceschini, B.; Malenica, I.; et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated Tregs as a potential therapeutic target. J. Hepatol. 2022, 77, 1359–1372. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Wan, L.; Wang, Z.; Wang, H.; Ge, C.; Liu, Y.; Hao, Y.; Zhang, D.; Shi, G.; et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 1118–1130. [Google Scholar] [CrossRef]
- Ma, L.; Hernandez, M.O.; Zhao, Y.; Mehta, M.; Tran, B.; Kelly, M.; Rae, Z.; Hernandez, J.M.; Davis, J.L.; Martin, S.P.; et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell 2019, 36, 418–430.e6. [Google Scholar] [CrossRef]
- Rahma, O.E.; Hodi, F.S. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 5449–5457. [Google Scholar] [CrossRef]
- Refolo, M.G.; Lotesoriere, C.; Messa, C.; Caruso, M.G.; D’Alessandro, R. Integrated immune gene expression signature and molecular classification in gastric cancer: New insights. J. Leukoc. Biol. 2020, 108, 633–646. [Google Scholar] [CrossRef]
- Hack, S.P.; Zhu, A.X.; Wang, Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front. Immunol. 2020, 11, 598877. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xiong, A.; Wu, F.; Li, X.; Wang, J.; Jiang, T.; Chen, P.; Zhang, X.; Zhao, Z.; Liu, H.; et al. Spatial multi-omics revealed the impact of tumor ecosystem heterogeneity on immunotherapy efficacy in patients with advanced non-small cell lung cancer treated with bispecific antibody. J. Immunother. Cancer 2023, 11, e006234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, T.; Li, K.; Mu, W.; Liu, Z.; Shi, A.; Liu, J.; Zhao, W.; Lian, S.; Huang, S.; et al. Recent Advances in the Mechanism Research and Clinical Treatment of Anti-Angiogenesis in Biliary Tract Cancer. Front. Oncol. 2021, 11, 777617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, X.; Wang, D.; Long, J.; Zhou, J.; Lu, Z.; Mao, Y.; Sang, X.; Guan, M.; Zhao, H. Lenvatinib Beyond First-Line Therapy in Patients with Advanced Biliary Tract Carcinoma. Front. Oncol. 2022, 12, 785535. [Google Scholar] [CrossRef]
- Simone, V.; Brunetti, O.; Lupo, L.; Testini, M.; Maiorano, E.; Simone, M.; Longo, V.; Rolfo, C.; Peeters, M.; Scarpa, A.; et al. Targeting Angiogenesis in Biliary Tract Cancers: An Open Option. Int. J. Mol. Sci. 2017, 18, 418. [Google Scholar] [CrossRef]
- Sun, X.N.; Cao, W.G.; Wang, X.; Wang, Q.; Gu, B.X.; Yang, Q.C.; Hu, J.B.; Liu, H.; Zheng, S. Prognostic impact of vascular endothelial growth factor-A expression in resected gallbladder carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2011, 32, 1183–1190. [Google Scholar] [CrossRef]
- Boonjaraspinyo, S.; Boonmars, T.; Wu, Z.; Loilome, W.; Sithithaworn, P.; Nagano, I.; Pinlaor, S.; Yongvanit, P.; Nielsen, P.S.; Pairojkul, C.; et al. Platelet-derived growth factor may be a potential diagnostic and prognostic marker for cholangiocarcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2012, 33, 1785–1802. [Google Scholar] [CrossRef]
- Cadamuro, M.; Nardo, G.; Indraccolo, S.; Dall’olmo, L.; Sambado, L.; Moserle, L.; Franceschet, I.; Colledan, M.; Massani, M.; Stecca, T.; et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013, 58, 1042–1053. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef]
- Sia, D.; Hoshida, Y.; Villanueva, A.; Roayaie, S.; Ferrer, J.; Tabak, B.; Peix, J.; Sole, M.; Tovar, V.; Alsinet, C.; et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 2013, 144, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Feng, F.; Zhu, L.; Zheng, Y.; Luo, X.; Liu, C.; Yi, B.; Jiang, X. Circulating miR-106a is a Novel Prognostic and Lymph Node Metastasis Indicator for Cholangiocarcinoma. Sci. Rep. 2015, 5, 16103. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Thongprasert, S.; Lee, J.; Doval, D.C.; Park, S.H.; Park, J.O.; Park, Y.S.; Kang, W.K.; Lim, H.Y. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: A multicentre, multinational study. Eur. J. Cancer 2012, 48, 196–201. [Google Scholar] [CrossRef]
- Takahashi, H.; Ojima, H.; Shimizu, H.; Furuse, J.; Furukawa, H.; Shibata, T. Axitinib (AG-013736), an oral specific VEGFR TKI, shows potential therapeutic utility against cholangiocarcinoma. Jpn. J. Clin. Oncol. 2014, 44, 570–578. [Google Scholar] [CrossRef]
- Goyal, L.; Zheng, H.; Yurgelun, M.B.; Abrams, T.A.; Allen, J.N.; Cleary, J.M.; Knowles, M.; Regan, E.; Reardon, A.; Khachatryan, A.; et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 2017, 123, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.V.; Pokuri, V.K.; Groman, A.; Ma, W.W.; Malhotra, U.; Iancu, D.M.; Grande, C.; Saab, T.B. A Multicenter Phase II Study of Gemcitabine, Capecitabine, and Bevacizumab for Locally Advanced or Metastatic Biliary Tract Cancer. Am. J. Clin. Oncol. 2018, 41, 649–655. [Google Scholar] [CrossRef]
- Krook, M.A.; Lenyo, A.; Wilberding, M.; Barker, H.; Dantuono, M.; Bailey, K.M.; Chen, H.Z.; Reeser, J.W.; Wing, M.R.; Miya, J.; et al. Efficacy of FGFR Inhibitors and Combination Therapies for Acquired Resistance in FGFR2-Fusion Cholangiocarcinoma. Mol. Cancer Ther. 2020, 19, 847–857. [Google Scholar] [CrossRef]
- Torrens, L.; Montironi, C.; Puigvehi, M.; Mesropian, A.; Leslie, J.; Haber, P.K.; Maeda, M.; Balaseviciute, U.; Willoughby, C.E.; Abril-Fornaguera, J.; et al. Immunomodulatory Effects of Lenvatinib Plus Anti-Programmed Cell Death Protein 1 in Mice and Rationale for Patient Enrichment in Hepatocellular Carcinoma. Hepatology 2021, 74, 2652–2669. [Google Scholar] [CrossRef]
- Arkenau, H.T.; Martin-Liberal, J.; Calvo, E.; Penel, N.; Krebs, M.G.; Herbst, R.S.; Walgren, R.A.; Widau, R.C.; Mi, G.; Jin, J.; et al. Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF). Oncologist 2018, 23, 1407-e136. [Google Scholar] [CrossRef]
- Cousin, S.; Cantarel, C.; Guegan, J.P.; Mazard, T.; Gomez-Roca, C.; Metges, J.P.; Bellera, C.; Adenis, A.; Korakis, I.; Poureau, P.G.; et al. Regorafenib-avelumab combination in patients with biliary tract cancer (REGOMUNE): A single-arm, open-label, phase II trial. Eur. J. Cancer 2022, 162, 161–169. [Google Scholar] [CrossRef]
- Lin, J.; Yang, X.; Long, J.; Zhao, S.; Mao, J.; Wang, D.; Bai, Y.; Bian, J.; Zhang, L.; Wang, A.; et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg. Nutr. 2020, 9, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Wei, S.; Zhang, L.; Tian, Y.; Gao, Z.; Jin, M.; Yan, S. Lenvatinib Plus PD-1 Inhibitors as First-Line Treatment in Patients with Unresectable Biliary Tract Cancer: A Single-Arm, Open-Label, Phase II Study. Front. Oncol. 2021, 11, 751391. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Li, Y.; Yang, C.; Qiao, L.; Tang, L.; Zheng, Y.; Chen, X.; Qian, Y.; Yang, J.; Wu, D.; et al. Lenvatinib Plus Programmed Cell Death Protein-1 Inhibitor Beyond First-Line Systemic Therapy in Refractory Advanced Biliary Tract Cancer: A Real-World Retrospective Study in China. Front. Immunol. 2022, 13, 946861. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Y.; Zhang, W.; Yuan, J.; Peng, Z.; Wang, W.; Gong, J.; Yang, L.; Cao, Y.; Zhao, H.; et al. Phase Ib study of anlotinib combined with TQB2450 in pretreated advanced biliary tract cancer and biomarker analysis. Hepatology 2023, 77, 65–76. [Google Scholar] [CrossRef]
- Jin, S.; Zhao, R.; Zhou, C.; Zhong, Q.; Shi, J.; Su, C.; Li, Q.; Su, X.; Chi, H.; Lu, X.; et al. Feasibility and tolerability of sintilimab plus anlotinib as the second-line therapy for patients with advanced biliary tract cancers: An open-label, single-arm, phase II clinical trial. Int. J. Cancer 2023, 152, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Cope, L.; Ruggieri, A.N.; Anders, R.A.; Noonan, A.M.; Goff, L.W.; Goyal, L.; Lacy, J.; Li, D.; Patel, A.K.; et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J. Clin. Investig. 2021, 131, e152670. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Moore, K.N.; Pignata, S. Trials in progress: IMagyn050/GOG 3015/ENGOT-OV39. A Phase III, multicenter, randomized study of atezolizumab versus placebo administered in combination with paclitaxel, carboplatin, and bevacizumab to patients with newly-diagnosed stage III or stage IV ovarian, fallopian tube, or primary peritoneal cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2019, ijgc-2018-000071. [Google Scholar] [CrossRef]
- Hack, S.P.; Verret, W.; Mulla, S.; Liu, B.; Wang, Y.; Macarulla, T.; Ren, Z.; El-Khoueiry, A.B.; Zhu, A.X. IMbrave 151: A randomized phase II trial of atezolizumab combined with bevacizumab and chemotherapy in patients with advanced biliary tract cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211036544. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Arora, S.; Balasubramaniam, S.; Zhang, W.; Zhang, L.; Sridhara, R.; Spillman, D.; Mathai, J.P.; Scott, B.; Golding, S.J.; Coory, M.; et al. FDA Approval Summary: Pembrolizumab plus Lenvatinib for Endometrial Carcinoma, a Collaborative International Review under Project Orbis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 5062–5067. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Powell, M.A.; Teneriello, M.G.; Miller, D.S.; Garcia, A.A.; Mikheeva, O.N.; Bidzinski, M.; Cebotaru, C.L.; Dutcus, C.E.; Ren, M.; et al. Second-line lenvatinib in patients with recurrent endometrial cancer. Gynecol. Oncol. 2020, 156, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.H.; Lee, C.H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients with Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Han, X.; Sun, Z.; Tang, J.; Wu, Y.; Wang, W. Systemic Sequential Therapy of CisGem, Tislelizumab, and Lenvatinib for Advanced Intrahepatic Cholangiocarcinoma Conversion Therapy. Front. Oncol. 2021, 11, 691380. [Google Scholar] [CrossRef]
- Chu, T.; Zhong, R.; Zhong, H.; Zhang, B.; Zhang, W.; Shi, C.; Qian, J.; Zhang, Y.; Chang, Q.; Zhang, X.; et al. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients with Advanced NSCLC. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 643–652. [Google Scholar] [CrossRef]
- Liu, L.; Mayes, P.A.; Eastman, S.; Shi, H.; Yadavilli, S.; Zhang, T.; Yang, J.; Seestaller-Wehr, L.; Zhang, S.Y.; Hopson, C.; et al. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 1639–1651. [Google Scholar] [CrossRef]
- Dushyanthen, S.; Teo, Z.L.; Caramia, F.; Savas, P.; Mintoff, C.P.; Virassamy, B.; Henderson, M.A.; Luen, S.J.; Mansour, M.; Kershaw, M.H.; et al. Agonist immunotherapy restores T cell function following MEK inhibition improving efficacy in breast cancer. Nat. Commun. 2017, 8, 606. [Google Scholar] [CrossRef]
- Ebert, P.J.R.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef]
- Eng, C.; Kim, T.W.; Bendell, J.; Argiles, G.; Tebbutt, N.C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H.; et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef]
- Shroff, R.T.; Guthrie, K.A.; Scott, A.J.; Borad, M.J.; Goff, L.W.; Matin, K.; Mahipal, A.; Kalyan, A.; Javle, M.M.; Aghajanian, C.; et al. SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. Late-breaking abstract 490. In Proceedings of the ASCO GI 2023, San Francisco, CA, USA, 19–21 January 2023. [Google Scholar]

| Phase | Setting | Treatment | Number of Patients | Type of Tumors | Results |
|---|---|---|---|---|---|
| I [60] | Second line | Ramucirumab + Pembrolizumab vs. Pembrolizumab | 26 patients | aBTC | No adverse events; PFS of 6.4 months vs. 1.6 months |
| II [61] | Second line | Regorafenib + Avelumab | 34 patients | BTC | 13.8% partial response, 37.9% stable disease, and 48.3% progressive disease. Median PFS: 2.5 months; median OS: 11.9 months |
| II [62] | Second line | Lenvatinib + Pembrolizumab | 50 patients | Advanced hepatobiliary malignancies | ORR of 25%, CBR of 40.5%, mPFS of 4.9 months, and mOS of 11.0 months |
| II [63] | First line | Lenvatinib +PD-1 inhibitors | 38 patients | Unresectable BTC | ORR of 42.1% and DCR of 76.3% |
| Retrospective study [64] | Second line | Lenvatinib + Programmed Cell Death Protein-1 inhibitor | 74 patients | BTC | ORR of 20.27%, DCR of 71.62%, mPFS of 4.0 months, and OS of 9.50 months |
| Ib [65] | Second line | TQB2450 + Anlotinib | 66 patients | Advanced CCA | ORR of 21.21%, DCR of 72.73%, CBR of 42.42%, mPFS of 6.24 months, and OS of 15.77 months |
| II [66] | Second line | Anlotinib + Sintlimab | 20 patients | aBTC | mOS of 12.3 months, 12-month OS rate of 55.0%, mPFS of 6.5 months, ORR of 30%, and DCR of 95% |
| II [67] | Second or later line | Atezolizumab + Cobimetinib vs. Atezolizumab | 77 patients | BTC | mPFS of 3.65 months vs. 1.87 months |
| NCT (ClinicalTrial.gov) | Phase | Setting | Treatment | Number of Patients | Types of Tumors | Primary Outcomes | Status | Estimated Study Completion Date |
|---|---|---|---|---|---|---|---|---|
| NCT04677504 (IMbrave 151) | II | First line | Atezolizumab and GemCis + Bevacizumab vs. Atezolizumab, GemCis | 162 patients | aBTC | PFS | Active, not recruiting | 24 April 2023 |
| NCT04217954 | II | First line | HAIC with Oxaliplatin, 5-FU, and Bevacizumab plus intravenous Toripalimab | 32 patients | aBTC | PFS, ORR | Recruiting | 28 July 2023 |
| NCT05052099 | I/II | Second line | mFOLFOX6, Bevacizumab, and Atezolizumab | 35 patients | aBTC | ORR | Recruiting | June 2024 |
| NCT03937830 | II | Second or later line | Arm A: Durvalumab + bevacizumab + Tremelimumab; Arm B: Durvalumab + bevacizumab + Tremelimumab + TACE | 39 patients | HCC, BTC | PFS | Recruiting | 31 December 2023 |
| NCT03475953 | I/II | Second or later line | Regorafenib + Avelumab | 482 patients | Not MSI-H or MMR-deficient CRC, GIST, GOJ or GC, BTC, HCC, STS, RR-DTC, GEP-NETs, NSCLC, solid tumor with immune signature (TLS+) | ORR | Recruiting | 31 December 2022 |
| NCT04781192 | I/II | Second or later line | Durvalumab + Regorafenib | 40 patients | aBTC | AEs, PFS | Recruiting | December 2023 |
| NCT05620498 | II | Conversion therapy | Tislelizumab + Lenvatinib and GEMOX vs. Tislelizumab plus GEMOX | 60 patients | ICC, GBC | ORR | Recruiting | 31 March 2024 |
| NCT03797326 | II | Second line | Pembrolizumab + lenvatinib | 590 patients | TNBC, OVC, GC, CRC, GBM, PAC | ORR, AEs | Active, not recruiting | 22 December 2023 |
| NCT04211168 | II | Second line | Toripalimab + Lenvatinib | 44 patients | aBTC | ORR, AE | Unknown | December 2022 |
| NCT05327582 | I/II | First or later line | Durvalumab + Lenvatinib plus Nab paclitaxel | 65 patients | PC, BTC | AEs, PFS | Recruiting | 30 April 2024 |
| NCT04010071 | II | Second line | Axitinib + Toripalimab | 60 patients | Hepatobiliary neoplasm, liver neoplasm, BTC | ORR, PFS | Recruiting | 18 August 2021 |
| NCT04941287 | II | Second line | Atezolizumab and CDX-1127 (Varlilumab) + Cobimetinib vs. Atezolizumab and CDX-1127 | 64 patients | aBTC | ORR, PFS | Recruiting | 1 September 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirizzi, A.; De Leonardis, G.; Lorusso, V.; Donghia, R.; Rizzo, A.; Vallarelli, S.; Ostuni, C.; Troiani, L.; Lolli, I.R.; Giannelli, G.; et al. Targeting Angiogenesis in the Era of Biliary Tract Cancer Immunotherapy: Biological Rationale, Clinical Implications, and Future Research Avenues. Cancers 2023, 15, 2376. https://doi.org/10.3390/cancers15082376
Schirizzi A, De Leonardis G, Lorusso V, Donghia R, Rizzo A, Vallarelli S, Ostuni C, Troiani L, Lolli IR, Giannelli G, et al. Targeting Angiogenesis in the Era of Biliary Tract Cancer Immunotherapy: Biological Rationale, Clinical Implications, and Future Research Avenues. Cancers. 2023; 15(8):2376. https://doi.org/10.3390/cancers15082376
Chicago/Turabian StyleSchirizzi, Annalisa, Giampiero De Leonardis, Vincenza Lorusso, Rossella Donghia, Alessandro Rizzo, Simona Vallarelli, Carmela Ostuni, Laura Troiani, Ivan Roberto Lolli, Gianluigi Giannelli, and et al. 2023. "Targeting Angiogenesis in the Era of Biliary Tract Cancer Immunotherapy: Biological Rationale, Clinical Implications, and Future Research Avenues" Cancers 15, no. 8: 2376. https://doi.org/10.3390/cancers15082376
APA StyleSchirizzi, A., De Leonardis, G., Lorusso, V., Donghia, R., Rizzo, A., Vallarelli, S., Ostuni, C., Troiani, L., Lolli, I. R., Giannelli, G., Ricci, A. D., D’Alessandro, R., & Lotesoriere, C. (2023). Targeting Angiogenesis in the Era of Biliary Tract Cancer Immunotherapy: Biological Rationale, Clinical Implications, and Future Research Avenues. Cancers, 15(8), 2376. https://doi.org/10.3390/cancers15082376









