Non-Invasive Imaging Modalities in Intravesical Murine Models of Bladder Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Murine Models of BCa
Categorization of Intravesical Murine Models of BCa
3. Requirement of Non-Invasive Imaging Modalities to Confirm and Evaluate Tumor Progression in an Intravesical Murine Model of BCa
4. Non-Invasive Imaging Modalities
4.1. Bioluminescence Imaging (BLI)
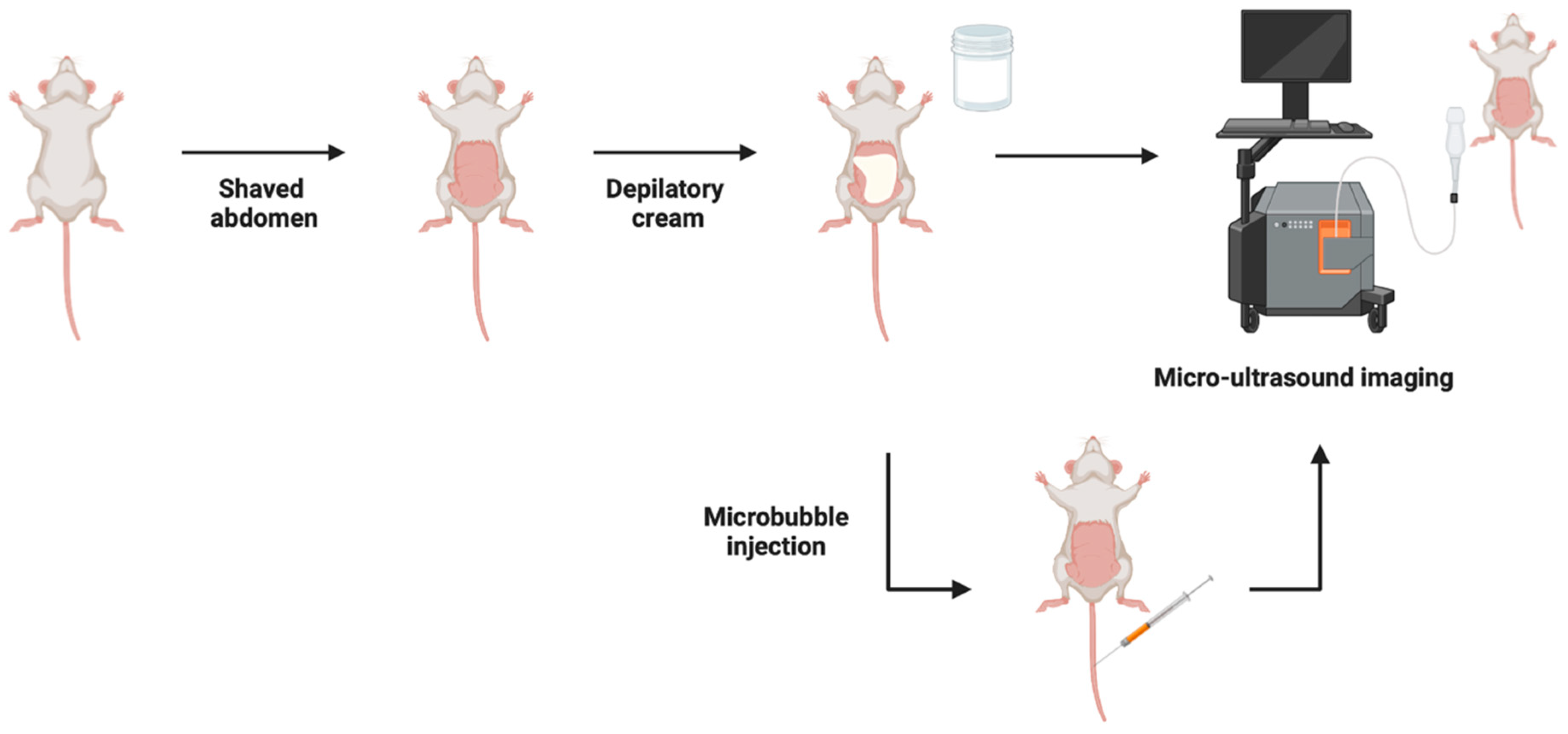
4.2. Micro-Ultrasound Imaging (MUI)
4.3. Magnetic Resonance Imaging (MRI)
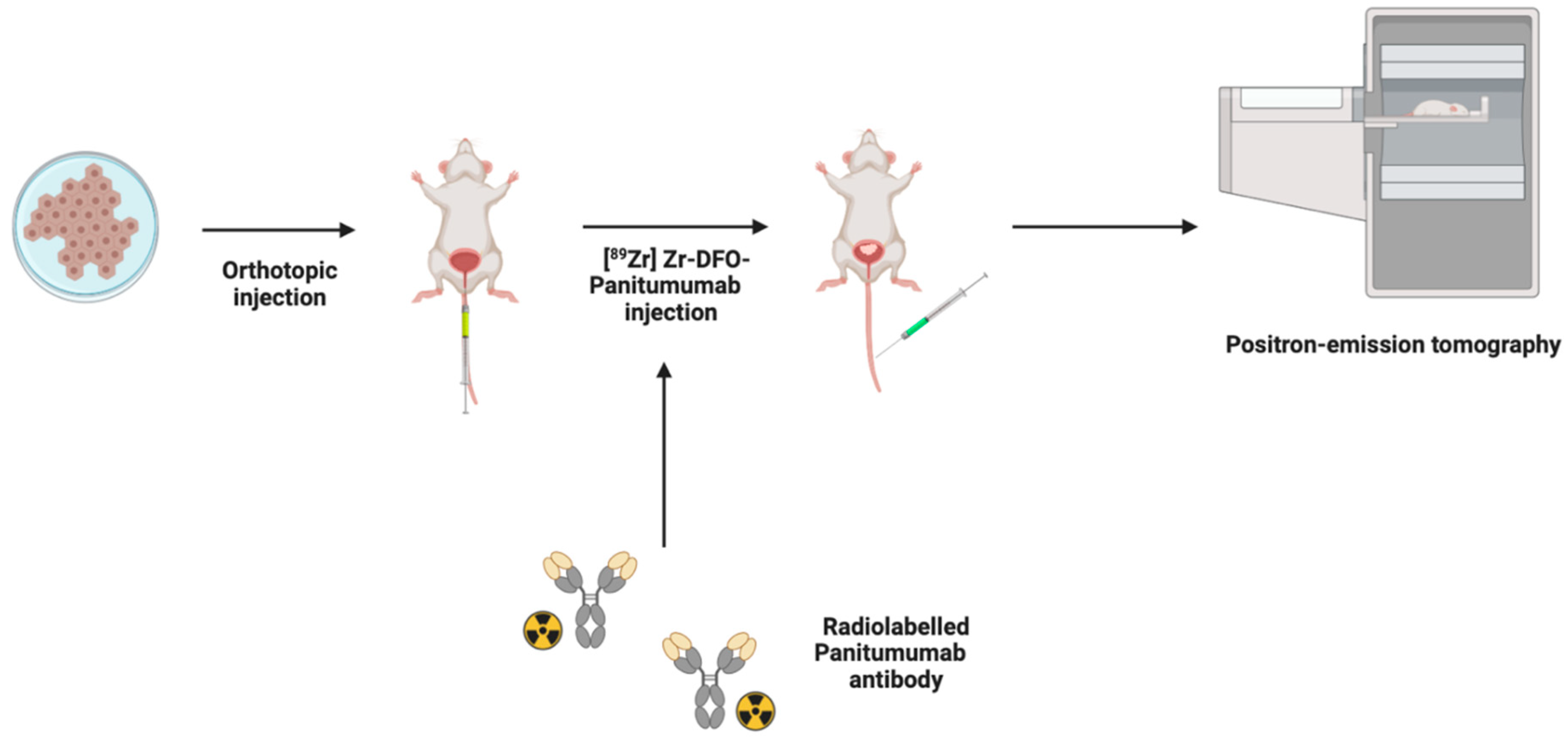
4.4. Positron Emission Tomography (PET)
4.5. Imaging Modalities in Combination for BCa
5. Urinary Analysis for BCa Detection and Progression
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- Sylvester, R.J. Bacillus Calmette-Guérin treatment of non-muscle invasive bladder cancer. Int. J. Urol. 2011, 18, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zlotta, A.R.; Fleshner, N.E.; Jewett, M.A. The management of BCG failure in non-muscle-invasive bladder cancer: An update. Can. Urol. Assoc. J. 2009, 3 (Suppl. 4), S199–S205. [Google Scholar] [CrossRef]
- Meng, M.V.; Gschwend, J.E.; Shore, N.; Grossfeld, G.D.; Mostafid, H.; Black, P.C. Emerging Immunotherapy Options for bacillus Calmette-Guérin Unresponsive Nonmuscle Invasive Bladder Cancer. J. Urol. 2019, 202, 1111–1119. [Google Scholar] [CrossRef]
- Sievert, K.D.; Amend, B.; Nagele, U.; Schilling, D.; Bedke, J.; Horstmann, M.; Hennenlotter, J.; Kruck, S.; Stenzl, A. Economic aspects of bladder cancer: What are the benefits and costs? World J. Urol. 2009, 27, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.A.; Gil da Costa, R.M.; Vasconcelos-Nóbrega, C.; Arantes-Rodrigues, R.; Pinto-Leite, R. Challenges with in vitro and in vivo experimental models of urinary bladder cancer for novel drug discovery. Expert Opin. Drug Discov. 2016, 11, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, D.; Shao, J.; Wang, X. Animal models for bladder cancer: The model establishment and evaluation (Review). Oncol. Lett. 2015, 9, 1515–1519. [Google Scholar] [CrossRef]
- John, B.A.; Said, N. Insights from animal models of bladder cancer: Recent advances, challenges, and opportunities. Oncotarget 2017, 8, 57766–57781. [Google Scholar] [CrossRef]
- Raven, P.A.; D’Costa, N.M.; Moskalev, I.; Tan, Z.; Frees, S.; Chavez-Munoz, C.; So, A.I. Development of murine intravesical orthotopic human bladder cancer (mio-hBC) model. Am. J. Clin. Exp. Urol. 2018, 6, 245. [Google Scholar] [PubMed]
- Seo, H.K.; Shin, S.-P.; Jung, N.-R.; Kwon, W.-A.; Jeong, K.-C.; Lee, S.-J. The establishment of a growth-controllable orthotopic bladder cancer model through the down-regulation of c-myc expression. Oncotarget 2016, 8, 50500–50509. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.S.; Streeter, E.H.; Jones, A.; Harris, A.L.; Bicknell, R. Cooperative stimulation of vascular endothelial growth factor expression by hypoxia and reactive oxygen species: The effect of targeting vascular endothelial growth factor and oxidative stress in an orthotopic xenograft model of bladder carcinoma. Br. J. Cancer 2005, 92, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Parada, B.; Reis, F.; Pinto, A.; Sereno, J.; Xavier-Cunha, M.; Neto, P.; Rocha-Pereira, P.; Mota, A.; Figueiredo, A.; Teixeira, F. Chemopreventive Efficacy of Atorvastatin against Nitrosamine-Induced Rat Bladder Cancer: Antioxidant, Anti-Proliferative and Anti-Inflammatory Properties. Int. J. Mol. Sci. 2012, 13, 8482–8499. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ma, A.-H.; Zhang, H.; Lin, T.-Y.; Xue, X.; Farrukh, H.; Zhu, S.; Shi, W.; Yuan, R.; Cao, Z.; et al. Phototherapy with Cancer-Specific Nanoporphyrin Potentiates Immunotherapy in Bladder Cancer. Clin. Cancer Res. 2022, 28, 4820–4831. [Google Scholar] [CrossRef]
- Garris, C.S.; Wong, J.L.; Ravetch, J.V.; Knorr, D.A. Intravesical dendritic cell targeting with Fc-enhanced CD40 agonistic antibodies induced durable bladder cancer immunity. Sci. Transl. Med. 2021, 13, eabd1346. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Teixeira, J.C.; Vasconcelos-Nóbrega, C.; Felix, L.M.; Sardão, V.A.; Colaço, A.A.; Oliveira, P.A.; Peixoto, F.P. Mitochondrial and liver oxidative stress alterations induced by N-butyl-N-(4-hydroxybutyl)nitrosamine: Relevance for hepatotoxicity. J. Appl. Toxicol. 2013, 33, 434–443. [Google Scholar] [CrossRef]
- Afify, H.; Ghoneum, A.; Almousa, S.; Abdulfattah, A.Y.; Warren, B.; Langsten, K.; Gonzalez, D.; Casals, R.; Bharadwaj, M.; Kridel, S.; et al. Metabolomic credentialing of murine carcinogen-induced urothelial cancer. Sci. Rep. 2021, 11, 22085. [Google Scholar] [CrossRef]
- Fantini, D.; Meeks, J.J. The BBN model: A mouse bladder cancer model featuring basalsubtype gene expression and MLL3/MLL4 genetic disruption. Oncoscience 2018, 5, 172–173. [Google Scholar] [CrossRef]
- Smilowitz, H.M.; Tarmu, L.J.; Sanders, M.M.; Taylor, J.A.T., III; Choudhary, D.; Xue, C.; Dyment, N.A.; Sasso, D.; Deng, X.; Hainfeld, J.F. Biodistribution of gold nanoparticles in BBN-induced muscle-invasive bladder cancer in mice. Int. J. Nanomed. 2017, 12, 7937–7946. [Google Scholar] [CrossRef]
- He, C.; Huang, L.; Lei, P.; Liu, X.; Li, B.; Shan, Y. Sulforaphane Normalizes Intestinal Flora and Enhances Gut Barrier in Mice with BBN-Induced Bladder Cancer. Mol. Nutr. Food Res. 2018, 62, e1800427. [Google Scholar] [CrossRef]
- Chan, E.; Patel, A.; Heston, W.; Larchian, W. Mouse orthotopic models for bladder cancer research. BJU Int. 2009, 104, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Sansom, O.J.; Leung, H.Y. Exploring molecular genetics of bladder cancer: Lessons learned from mouse models. Dis. Model. Mech. 2012, 5, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Lyu, Y.; Yang, Y.-G.; Hu, Z. Humanized Rodent Models for Cancer Research. Front. Oncol. 2020, 10, 01696. [Google Scholar] [CrossRef] [PubMed]
- De La Rochere, P.; Guil-Luna, S.; Decaudin, D.; Azar, G.; Sidhu, S.S.; Piaggio, E. Humanized Mice for the Study of Immuno-Oncology. Trends Immunol. 2018, 39, 748–763. [Google Scholar] [CrossRef]
- Fodor, I.; Timiryasova, T.; Denes, B.; Yoshida, J.; Ruckle, H.; Lilly, M. Vaccinia virus mediated p53 gene therapy for bladder cancer in an orthotopic murine model. J. Urol. 2005, 173, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, Y.; Larchian, W.A.; Kaplinsky, R.; Fair, W.R.; Heston, W.D. Intravesical liposome-mediated interleukin-2 gene therapy in orthotopic murine bladder cancer model. Gene Ther. 2000, 7, 844–851. [Google Scholar] [CrossRef]
- Ohtani, M.; Kakizoe, T.; Nishio, Y.; Sato, S.; Sugimura, T.; Fukushima, S.; Niijima, T. Sequential changes of mouse bladder epithelium during induction of invasive carcinomas by N-butyl-N-(4-hydroxybutyl)nitrosamine. Cancer Res. 1986, 46 Pt 2, 2001–2004. [Google Scholar]
- Ravoori, M.K.; Margalit, O.; Singh, S.; Kim, S.-H.; Wei, W.; Menter, D.G.; DuBois, R.N.; Kundra, V. Magnetic Resonance Imaging and Bioluminescence Imaging for Evaluating Tumor Burden in Orthotopic Colon Cancer. Sci. Rep. 2019, 9, 6100. [Google Scholar] [CrossRef]
- Bachawal, S.V.; Jensen, K.C.; Lutz, A.M.; Gambhir, S.S.; Tranquart, F.; Tian, L.; Willmann, J.K. Earlier Detection of Breast Cancer with Ultrasound Molecular Imaging in a Transgenic Mouse Model. Cancer Res. 2013, 73, 1689–1698. [Google Scholar] [CrossRef]
- Raes, F.; Sobilo, J.; Le Mée, M.; Rétif, S.; Natkunarajah, S.; Lerondel, S.; Le Pape, A. High Resolution Ultrasound and Photoacoustic Imaging of Orthotopic Lung Cancer in Mice: New Perspectives for Onco-Pharmacology. PLoS ONE 2016, 11, e0153532. [Google Scholar] [CrossRef]
- Latgé, A.; Boisson, F.; Ouadi, A.; Averous, G.; Thomas, L.; Imperiale, A.; Brasse, D. 64CuCl2 PET Imaging of 4T1-Related Allograft of Triple-Negative Breast Cancer in Mice. Molecules 2022, 27, 4869. [Google Scholar] [CrossRef] [PubMed]
- Rix, A.; Piepenbrock, M.; Flege, B.; von Stillfried, S.; Koczera, P.; Opacic, T.; Simons, N.; Boor, P.; Thoröe-Boveleth, S.; Deckers, R.; et al. Effects of contrast-enhanced ultrasound treatment on neoadjuvant chemotherapy in breast cancer. Theranostics 2021, 11, 9557–9570. [Google Scholar] [CrossRef]
- Ravoori, M.K.; Singh, S.; Yang, P.; Wei, W.; Chen, H.; Ma, J.; Bankson, J.A.; Kundra, V. In vivo magnetic resonance imaging of orthotopic prostate cancer. Biotechniques 2020, 69, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Black, P.C.; Shetty, A.; Brown, G.A.; Esparza-Coss, E.; Metwalli, A.R.; Agarwal, P.K.; McConkey, D.J.; Hazle, J.D.; Dinney, C.P. Validating bladder cancer xenograft bioluminescence with magnetic resonance imaging: The significance of hypoxia and necrosis. BJU Int. 2010, 106, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kessler, E.; Su, L.-J.; Thorburn, A.; Frankel, A.E.; Li, Y.; La Rosa, F.G.; Shen, J.; Li, C.-Y.; Varella-Garcia, M.; et al. Diphtheria Toxin–Epidermal Growth Factor Fusion Protein DAB389EGF for the Treatment of Bladder Cancer. Clin. Cancer Res. 2013, 19, 148–157. [Google Scholar] [CrossRef]
- Jurczok, A.; Fornara, P.; Söling, A. Bioluminescence imaging to monitor bladder cancer cell adhesion in vivo: A new approach to optimize a syngeneic, orthotopic, murine bladder cancer model. BJU Int. 2008, 101, 120–124. [Google Scholar] [CrossRef]
- Huebner, D.; Rieger, C.; Bergmann, R.; Ullrich, M.; Meister, S.; Toma, M.; Wiedemuth, R.; Temme, A.; Novotny, V.; Wirth, M.P.; et al. An orthotopic xenograft model for high-risk non-muscle invasive bladder cancer in mice: Influence of mouse strain, tumor cell count, dwell time and bladder pretreatment. BMC Cancer 2017, 17, 790. [Google Scholar] [CrossRef]
- Scheepbouwer, C.; Meyer, S.; Burggraaf, M.J.; Jose, J.; Molthoff, C.F.M. A Multimodal Imaging Approach for Longitudinal Evaluation of Bladder Tumor Development in an Orthotopic Murine Model. PLoS ONE 2016, 11, e0161284. [Google Scholar] [CrossRef]
- Jager, W.; Moskalev, I.; Janssen, C.; Hayashi, T.; Awrey, S.; Gust, K.M.; So, A.I.; Zhang, K.; Fazli, L.; Li, E.; et al. Ultrasound-Guided Intramural Inoculation of Orthotopic Bladder Cancer Xenografts: A Novel High-Precision Approach. PLoS ONE 2013, 8, e59536. [Google Scholar] [CrossRef]
- Chan, E.S.; Patel, A.R.; Larchian, W.A.; Heston, W.D. In Vivo Targeted Contrast Enhanced Micro-Ultrasound to Measure Intratumor Perfusion and Vascular Endothelial Growth Factor Receptor 2 Expression in a Mouse Orthotopic Bladder Cancer Model. J. Urol. 2011, 185, 2359–2365. [Google Scholar] [CrossRef]
- Yang, X.; Su, L.-J.; La Rosa, F.G.; Smith, E.E.; Schlaepfer, I.R.; Cho, S.K.; Kavanagh, B.; Park, W.; Flaig, T.W. The Antineoplastic Activity of Photothermal Ablative Therapy with Targeted Gold Nanorods in an Orthotopic Urinary Bladder Cancer Model. Bladder Cancer 2017, 3, 201–210. [Google Scholar] [CrossRef]
- Glaser, A.P.; Procissi, D.; Yu, Y.; Meeks, J.J. Magnetic Resonance Imaging Assessment of Carcinogen-induced Murine Bladder Tumors. J. Vis. Exp. 2019, 145, e59101. [Google Scholar] [CrossRef]
- Patel, A.R.; Chan, E.S.; Hansel, D.E.; Powell, C.T.; Heston, W.D.; Larchian, W.A. Transabdominal Micro-ultrasound Imaging of Bladder Cancer in a Mouse Model: A Validation Study. Urology 2010, 75, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xie, Z.; Yan, Y.; Huang, Z.; Tang, P.; Cao, X.; Wang, Z.; Yang, C.; Tan, M.; Zhang, F.; et al. Establishment of an optimized orthotopic bladder cancer model in mice. BMC Urol. 2022, 22, 142. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, E.; Xu, S.; Ohori, M.; Matei, C.; Lupu, M.; Menendez, S.; Koutcher, J.A.; Bochner, B.H. Detection and Quantitative Analysis of Early Stage Orthotopic Murine Bladder Tumor Using In Vivo Magnetic Resonance Imaging. J. Urol. 2003, 170 Pt 1, 1375–1378. [Google Scholar] [CrossRef]
- Sai, K.K.S.; Zachar, Z.; Bingham, P.M.; Mintz, A. Metabolic PET Imaging in Oncology. Am. J. Roentgenol. 2017, 209, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.; Roberts, S.; Figueira, F.; Tomé, J.P.; Reiner, T.; Lewis, J.S. PET/CT Imaging with an 18F-Labeled Galactodendritic Unit in a Galectin-1–Overexpressing Orthotopic Bladder Cancer Model. J. Nucl. Med. 2020, 61, 1369–1375. [Google Scholar] [CrossRef]
- Hoang, T.T.; Mandleywala, K.; Viray, T.; Tan, K.V.; Lewis, J.S.; Pereira, P.M.R. EGFR-Targeted ImmunoPET of UMUC3 Orthotopic Bladder Tumors. Mol. Imaging Biol. 2022, 24, 511–518. [Google Scholar] [CrossRef]
- Vasireddi, A.; Nguyen, N.C. PET/CT Limitations and Pitfalls in Urogenital Cancers. Semin. Nucl. Med. 2021, 51, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, I.; Hanaoka, H.; Yamaguchi, A.; Amartuvshin, T.; Tsushima, Y. Diagnosis of bladder cancer using 18F-labeled α-methyl-phenylalanine tracers in a mouse model. Ann. Nucl. Med. 2020, 34, 329–336. [Google Scholar] [CrossRef]
- Sato, K.; Yuasa, T.; Nogawa, M.; Kimura, S.; Segawa, H.; Yokota, A.; Maekawa, T. A third-generation bisphosphonate, minodronic acid (YM529), successfully prevented the growth of bladder cancer in vitro and in vivo. Br. J. Cancer 2006, 95, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, M.; Yuasa, T.; Kimura, S.; Tanaka, M.; Kuroda, J.; Sato, K.; Yokota, A.; Segawa, H.; Toda, Y.; Kageyama, S.; et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J. Clin. Investig. 2005, 115, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.K.; Luo, Y.; O’donnell, M.A.; Assouline, J. Nanotechnology and cancer: Improving real-time monitoring and staging of bladder cancer with multimodal mesoporous silica nanoparticles. Cancer Nanotechnol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Pellini, B.; Pejovic, N.; Chauhan, P.S.; Harris, P.K.; Szymanski, J.J.; Smith, Z.L.; Arora, V.K. Emerging Roles of Urine-Based Tumor DNA Analysis in Bladder Cancer Management. JCO Precis. Oncol. 2020, 4, 806–817. [Google Scholar] [CrossRef]
- Tan, X.; Broses, L.J.; Zhou, M.; Day, K.C.; Liu, W.; Li, Z.; Weizer, A.Z.; Munson, K.A.; Oo, M.K.K.; Day, M.L.; et al. Multiparameter urine analysis for quantitative bladder cancer surveillance of orthotopic xenografted mice. Lab Chip 2020, 20, 634–646. [Google Scholar] [CrossRef]
- Kawano, T.; Tachibana, Y.; Inokuchi, J.; Kang, J.-H.; Murata, M.; Eto, M. Identification of Activated Protein Kinase Cα (PKCα) in the Urine of Orthotopic Bladder Cancer Xenograft Model as a Potential Biomarker for the Diagnosis of Bladder Cancer. Int. J. Mol. Sci. 2021, 22, 9276. [Google Scholar] [CrossRef]

| Imaging Modality | Reported Advantages | Reported Limitations | Imaging Time *ϕ (Minutes) | Earliest Detection Time (Days) | Detectable Tumor Size | Confirmation Rate (%) |
|---|---|---|---|---|---|---|
| BLI | Linearly correlated with cell quantity [10,34] and ex vivo tumor size [10] | Loss of signal overtime [35,36] Cannot detect tumor size or location [37] | 12–18 [10,35,38] | 4 [39] | - | 91.6 [36] |
| MUI | MUI: Accurate detection of tumor location [40] Linearly correlated with tumor size [40] Contrast-enhanced MUI: evaluation of tumor vasculature [41] | Lack of 3-dimensional imaging [42] | MUI: ~5 [43] Contrast-enhanced MUI: ~60 [40] | 4 [39] | 0.4 mm3 [38] | 87 [43] |
| MRI | 3-dimensional imaging [44] Provide accurate tumor measurements that correlate with tumor stage and size [34,42,45] | Long examination time and high cost [42] Unable to detect tumors less than 1 mm in diameter [45] | <10 [42] | - | 1.5 mm in diameter [45] | 86.4 [45] |
| PET | Evaluates metabolic activity, identifies genomic aberrations, and protein dysregulation [46] Detects metastatic disease [47] Sensitive, quantitative, and high target selectivity [48] | Obstructed by renal excretion [47,49] | 10 [50] | PET: 1 and 3 h [47,49] ImmunoPET: 72 h [48] | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Relouw, S.; Dugbartey, G.J.; Sener, A. Non-Invasive Imaging Modalities in Intravesical Murine Models of Bladder Cancer. Cancers 2023, 15, 2381. https://doi.org/10.3390/cancers15082381
Relouw S, Dugbartey GJ, Sener A. Non-Invasive Imaging Modalities in Intravesical Murine Models of Bladder Cancer. Cancers. 2023; 15(8):2381. https://doi.org/10.3390/cancers15082381
Chicago/Turabian StyleRelouw, Sydney, George J. Dugbartey, and Alp Sener. 2023. "Non-Invasive Imaging Modalities in Intravesical Murine Models of Bladder Cancer" Cancers 15, no. 8: 2381. https://doi.org/10.3390/cancers15082381
APA StyleRelouw, S., Dugbartey, G. J., & Sener, A. (2023). Non-Invasive Imaging Modalities in Intravesical Murine Models of Bladder Cancer. Cancers, 15(8), 2381. https://doi.org/10.3390/cancers15082381





