Characterization of Tumor and Immune Tumor Microenvironment of Primary Tumors and Metastatic Sites in Advanced Renal Cell Carcinoma Patients Based on Response to Nivolumab Immunotherapy: Preliminary Results from the Meet-URO 18 Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Procedures
2.3. Study Objectives
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
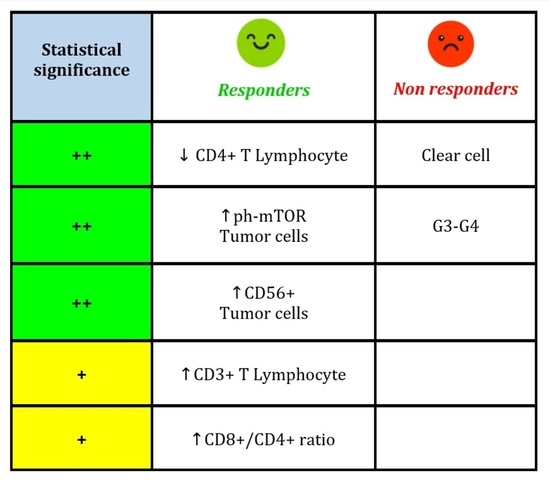
3.2. IHC Analysis of Primary Tumors and Metastases (Responders vs. Non-Responders)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhuang, T.Z.; Case, K.; Olsen, T.A.; Brown, J.T.; Carthon, B.C.; Kucuk, O.; Nazha, B. Metastatic Clear-Cell Renal Cell Carcinoma in the Era of Immune Checkpoint Inhibitors: Therapies and Ongoing Trials. Cancers 2022, 14, 2867. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Sharma, P. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Rebuzzi, S.E.; Perrone, F.; Bersanelli, M.; Bregni, G.; Milella, M.; Buti, S. Prognostic and predictive molecular biomarkers in metastatic renal cell carcinoma patients treated with immune checkpoint inhibitors: A systematic review. Expert Rev. Mol. Diagn. 2019, 20, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Simonaggio, A.; Epaillard, N.; Pobel, C.; Moreira, M.; Oudard, S.; Vano, Y.A. Tumor Microenvironment Features as Predictive Biomarkers of Response to Immune Checkpoint Inhibitors (ICI) in Metastatic Clear Cell Renal Cell Carcinoma (mccRCC). Cancers 2021, 13, 231. [Google Scholar] [CrossRef]
- Houshyari, M.; Taghizadeh-Hesary, F. Is Mitochondrial Metabolism a New Predictive Biomarker for Antiprogrammed Cell Death Protein-1 Immunotherapy? JCO Oncol. Pract. 2023, 19, 123–124. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Mori, K.; Katayama, S.; Mostafaei, H.; Quhal, F.; Laukhtina, E.; Shariat, S.F. Hematological prognosticators in metastatic renal cell cancer treated with immune checkpoint inhibitors: A meta-analysis. Immunotherapy 2022, 14, 709–725. [Google Scholar] [CrossRef]
- Li, J.; Byrne, K.T.; Yan, F.; Yamazoe, T.; Chen, Z.; Baslan, T.; Stanger, B.Z. Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy. Immunity 2018, 49, 178–193.e7. [Google Scholar] [CrossRef]
- Zhu, G.; Pei, L.; Yin, H.; Lin, F.; Li, X.; Zhu, X.; Gou, X. Profiles of tumor-infiltrating immune cells in renal cell carcinoma and their clinical implications. Oncol Lett. 2019, 18, 5235–5242. [Google Scholar] [CrossRef]
- Anker, J.; Miller, J.; Taylor, N.; Kyprianou, N.; Tsao, C.K. From Bench to Bedside: How the Tumor Microenvironment Is Impacting the Future of Immunotherapy for Renal Cell Carcinoma. Cells 2021, 10, 3231. [Google Scholar] [CrossRef]
- Lin, E.; Liu, X.; Liu, Y.; Zhang, Z.; Xie, L.; Tian, K.; Liu, J.; Yu, Y. Roles of the Dynamic Tumor Immune Microenvironment in the Individualized Treatment of Advanced Clear Cell Renal Cell Carcinoma. Front. Immunol. 2021, 12, 653358. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Signori, A.; Banna, G.L.; Maruzzo, M.; De Giorgi, U.; Pedrazzoli, P.; Fornarini, G. Inflammatory indices and clinical factors in metastatic renal cell carcinoma patients treated with nivolumab: The development of a novel prognostic score (Meet-URO 15 study). Ther. Adv. Med. Oncol. 2021, 13, 17588359211019642. [Google Scholar] [CrossRef] [PubMed]
- Ged, Y.; Voss, M.H. Novel emerging biomarkers to immunotherapy in kidney cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211059367. [Google Scholar] [CrossRef] [PubMed]
- Naser, R.; Fakhoury, I.; El-Fouani, A.; Abi-Habib, R.; El-Sibai, M. Role of the tumor microenvironment in cancer hallmarks and targeted therapy (Review). Int. J. Oncol. 2023, 62, 23. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, G.; Fanale, D.; Incorvaia, L.; Barraco, N.; Listi, A.; Maragliano, R.; Russo, A. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell Immunol. 2019, 343, 103753. [Google Scholar] [CrossRef]
- Presti, D.; Dall’Olio, F.G.; Besse, B.; Ribeiro, J.M.; Di Meglio, A.; Soldato, D. Tumor infiltrating lymphocytes (TILs) as a predictive biomarker of response to checkpoint blockers in solid tumors: A systematic review. Crit. Rev. Oncol. Hematol. 2022, 177, 103773. [Google Scholar] [CrossRef]
- Braun, D.A.; Street, K.; Burke, K.P.; Cookmeyer, D.L.; Denize, T.; Pedersen, C.B.; Wu, C.J. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell 2021, 39, 632–648.e8. [Google Scholar] [CrossRef]
- Basu, A.; Ramamoorthi, G.; Albert, G.; Gallen, C.; Beyer, A.; Snyder, C.; Kodumudi, K. Differentiation and Regulation of TH Cells: A Balancing Act for Cancer Immunotherapy. Front. Immunol. 2021, 12, 669474. [Google Scholar] [CrossRef]
- Ćirović, S.; Vještica, J.; Mueller, C.A.; Tatić, S.; Vasiljević, J.; Milenković, S.; Marković-Lipkovski, J. NCAM and FGFR1 coexpression and colocalization in renal tumors. Int. J. Clin. Exp. Pathol. 2014, 7, 1402–1414. [Google Scholar]
- Van Acker, H.H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Van Acker, Z.P.; Versteven, M.; Ponsaerts, P.; Pende, D.; Berneman, Z.N.; Smits, E.L. CD56 Homodimerization and Participation in Anti-Tumor Immune Effector Cell Functioning: A Role for Interleukin-15. Cancers 2019, 11, 1029. [Google Scholar] [CrossRef]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Pilotto, S.; Bria, E.; Cognetti, F.; Ciuffreda, L. Role of mTOR Signaling in Tumor Microenvironment: An Overview. Int. J. Mol. Sci. 2018, 19, 2453. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.H.; Molina, A.M.; Motzer, R.J. mTOR inhibitors in advanced renal cell carcinoma. Hematol. Oncol. Clin. N. Am. 2011, 25, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Caforio, M.; de Billy, E.; De Angelis, B.; Iacovelli, S.; Quintarelli, C.; Paganelli, V.; Folgiero, V. PI3K/Akt Pathway: The Indestructible Role of a Vintage Target as a Support to the Most Recent Immunotherapeutic Approaches. Cancers 2021, 13, 4040. [Google Scholar] [CrossRef] [PubMed]
- Braun, C.; Weichhart, T. mTOR-dependent immunometabolism as Achilles’ heel of anticancer therapy. Eur. J. Immunol. 2021, 51, 3161–3175. [Google Scholar] [CrossRef] [PubMed]
- Carretero-González, A.; Lora, D.; Martin Sobrino, I.; Saez Sanz, I.; Bourlon, M.T.; Anido Herranz, U.; de Velasco, G. The Value of PD-L1 Expression as Predictive Biomarker in Metastatic Renal Cell Carcinoma Patients: A Meta-Analysis of Randomized Clinical Trials. Cancers 2020, 12, 1945. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef]
- Petitprez, F.; Ayadi, M.; de Reyniès, A.; Fridman, W.H.; Sautès-Fridman, C.; Job, S. Review of Prognostic Expression Markers for Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 643065. [Google Scholar] [CrossRef]
- Vano, Y.A.; Elaidi, R.; Bennamoun, M.; Chevreau, C.; Borchiellini, D.; Pannier, D.; Oudard, S. Nivolumab, nivolumab-ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): A biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol. 2022, 23, 612–624. [Google Scholar] [CrossRef]

| Characteristic | N (%) | p-Value | ||
|---|---|---|---|---|
| Patients N = 84 | All Patients | Responders (N = 37, 42.5%) | Non-Responders (N = 47, 57.5%) | |
| Gender | ||||
| Male | 62 (73.8) | 25 (67.6) | 37 (78.7) | 0.25 |
| Female | 22 (26.2) | 12 (32.4) | 10 (21.3) | |
| Age (median, range) | 69, 27–84 | 73, 50–84 | 66, 27–82 | 0.004 |
| Histology | ||||
| ccRCC | 65 (77.4) | 25 (67.6) | 40 (85.1) | 0.056 |
| Other | 19 (22.6) | 12 (32.4) | 7 (14.9) | |
| Nephrectomy | ||||
| Yes | 72 (86.8) | 34 (94.4) | 38 (80.9) | 0.070 |
| No | 11 (13.2) | 2 (5.6) | 9 (19.2) | |
| IMDC score | ||||
| Favorable | 17 (20.2) | 12 (32.4) | 5 (10.6) | 0.030 |
| Intermediate | 52 (61.9) | 21 (56.8) | 31 (66.0) | |
| Poor | 15 (17.9) | 4 (10.8) | 11 (23.4) | |
| Meet-URO score | ||||
| 1 | 18 (22.5) | 8 (44.4) | 10 (55.6) | 0.21 |
| 2 | 28 (35.0) | 14 (50) | 14 (50) | |
| 3 | 17 (21.3) | 7 (41.2) | 10 (58.8) | |
| 4 | 10 (12.5) | 4 (40.0) | 6 (60.0) | |
| 5 | 7 (8.7) | 0 (0) | 7 (100.0) | |
| Treatment line | ||||
| 2nd line | 60 (71.4) | 26 (70.3) | 34 (72.3) | 0.84 |
| ≥3rd line | 24 (28.6) | 11 (29.7) | 13 (27.7) | |
| Samples N = 116 | All samples | Samples of Responders (N = 55, 47%) | Samples of Non-responders (N = 61, 53%) | |
| Type of tumor sample | ||||
| Primitive tumor | 68 (59) | 32 (58.2) | 36 (59.0) | 0.93 |
| Metastasis | 48 (41) | 23 (41.8) | 25 (41.0) | |
| Parameter (Cut-Off) | Responders N (%) | Non-Responders N (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Histology | ||||
| ccRCC | 34 (61.8) | 52 (85.3) | 3.57 (1.46–8.71) | 0.005 |
| Other | 21 (38.2) | 9 (14.7) | 1.00 (ref.) | |
| Grading | ||||
| 1–2 | 17 (48.6) | 10 (19.6) | 1.00 (ref.) | |
| 3 | 12 (34.3) | 26 (51.0) | 3.68 (1.30–10.40) | 0.014 |
| 4 | 6 (17.1) | 15 (29.4) | 4.25 (1.25–14.50) | 0.021 |
| CD3+ IC | ||||
| Median (IQR) | 90 (34–200) | 45 (25–210) | - | 0.77 |
| <40 | 15 (27.3) | 27 (44.3) | 1.00 (ref.) | 0.059 |
| ≥40 | 40 (72.7) | 34 (55.7) | 0.47 (0.22–1.03) | |
| CD8+ IC | ||||
| Median (IQR) | 100 (25–150) | 105 (25–139) | - | 0.76 |
| CD4+ IC | ||||
| Median (IQR) | 45 (12–70) | 60 (15–88) | - | 0.22 |
| <70 | 41 (74.6) | 32 (52.5) | 1.00 (ref.) | 0.015 |
| ≥70 | 14 (25.5) | 29 (47.5) | 2.65 (1.21–5.83) | |
| CD8+/CD4+ ratio Median, IQR | 1.74 (0.54–3.71) | 1.20 (0.32–2.39) | - | 0.084 |
| Peri/intra-tumoral T cells | ||||
| Absent | 24 (43.6) | 26 (42.6) | 1.00 (ref.) | 0.91 |
| Present | 31 (56.4) | 35 (57.4) | 1.04 (0.50–2.18) | |
| CD56 TC | ||||
| Median (IQR) | 0 (0–40) | 0 (0–10) | - | 0.23 |
| <40 | 40 (72.7) | 54 (88.5) | 1.00 (ref.) | 0.035 |
| ≥40 | 15 (27.3) | 7 (11.5) | 0.35 (0.13–0.93) | |
| CD15 TC | ||||
| Median (IQR) | 1 (0–10) | 1 (0–5) | - | 0.70 |
| <30 | 48 (87.3) | 48 (78.7) | 1.00 (ref.) | 0.23 |
| ≥30 | 7 (12.7) | 13 (21.3) | 1.86 (0.68–5.06) | |
| CD68 TC | ||||
| Median (IQR) | 0 (0–40) | 0 (0–10) | - | 0.77 |
| ph-mTOR TC | ||||
| Median (IQR) | 20 (10–70) | 15 (0–70) | - | 0.25 |
| <15 | 16 (29.1) | 30 (49.2) | 1.00 (ref.) | 0.029 |
| ≥15 | 39 (70.9) | 31 (50.8) | 0.42 (0.20–0.91) | |
| PD-L1 TC/IC | ||||
| Median (IQR) | 3 (0–10) | 0 (0–5) | - | 0.46 |
| <10 | 40 (72.7) | 51 (83.6) | 1.00 (ref.) | 0.16 |
| ≥10 | 15 (27.3) | 10 (16.4) | 0.52 (0.21–1.29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebuzzi, S.E.; Brunelli, M.; Galuppini, F.; Vellone, V.G.; Signori, A.; Catalano, F.; Damassi, A.; Gaggero, G.; Rescigno, P.; Maruzzo, M.; et al. Characterization of Tumor and Immune Tumor Microenvironment of Primary Tumors and Metastatic Sites in Advanced Renal Cell Carcinoma Patients Based on Response to Nivolumab Immunotherapy: Preliminary Results from the Meet-URO 18 Study. Cancers 2023, 15, 2394. https://doi.org/10.3390/cancers15082394
Rebuzzi SE, Brunelli M, Galuppini F, Vellone VG, Signori A, Catalano F, Damassi A, Gaggero G, Rescigno P, Maruzzo M, et al. Characterization of Tumor and Immune Tumor Microenvironment of Primary Tumors and Metastatic Sites in Advanced Renal Cell Carcinoma Patients Based on Response to Nivolumab Immunotherapy: Preliminary Results from the Meet-URO 18 Study. Cancers. 2023; 15(8):2394. https://doi.org/10.3390/cancers15082394
Chicago/Turabian StyleRebuzzi, Sara Elena, Matteo Brunelli, Francesca Galuppini, Valerio Gaetano Vellone, Alessio Signori, Fabio Catalano, Alessandra Damassi, Gabriele Gaggero, Pasquale Rescigno, Marco Maruzzo, and et al. 2023. "Characterization of Tumor and Immune Tumor Microenvironment of Primary Tumors and Metastatic Sites in Advanced Renal Cell Carcinoma Patients Based on Response to Nivolumab Immunotherapy: Preliminary Results from the Meet-URO 18 Study" Cancers 15, no. 8: 2394. https://doi.org/10.3390/cancers15082394
APA StyleRebuzzi, S. E., Brunelli, M., Galuppini, F., Vellone, V. G., Signori, A., Catalano, F., Damassi, A., Gaggero, G., Rescigno, P., Maruzzo, M., Merler, S., Vignani, F., Cavo, A., Basso, U., Milella, M., Panepinto, O., Mencoboni, M., Sbaraglia, M., Dei Tos, A. P., ... Fornarini, G. (2023). Characterization of Tumor and Immune Tumor Microenvironment of Primary Tumors and Metastatic Sites in Advanced Renal Cell Carcinoma Patients Based on Response to Nivolumab Immunotherapy: Preliminary Results from the Meet-URO 18 Study. Cancers, 15(8), 2394. https://doi.org/10.3390/cancers15082394