Resected Tumor Outcome and Recurrence (RESTORE) Index for Hepatocellular Carcinoma Recurrence after Resection
Abstract
:Simple Summary
Abstract: Importance
1. Introduction
2. Methods
2.1. Study Design and Patient Population
2.2. Data Source and Variables
2.3. Statistical Analysis and Generation of the RESTORE Index
2.4. Evaluation of Risk Score Performance
3. Results
3.1. Baseline Characteristics of Study Cohort
3.2. Post-Resection Outcomes
3.3. Recurrence Prediction
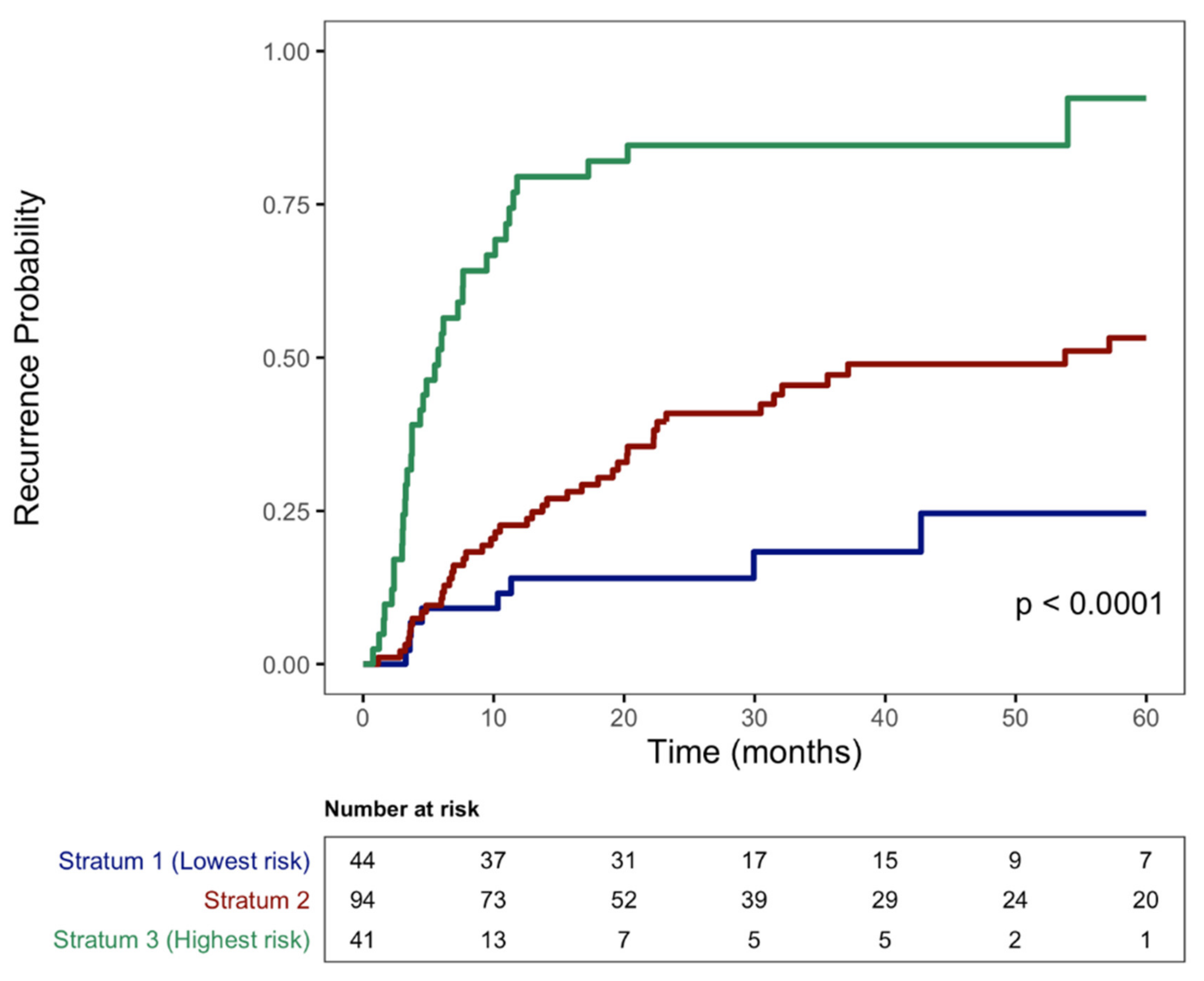
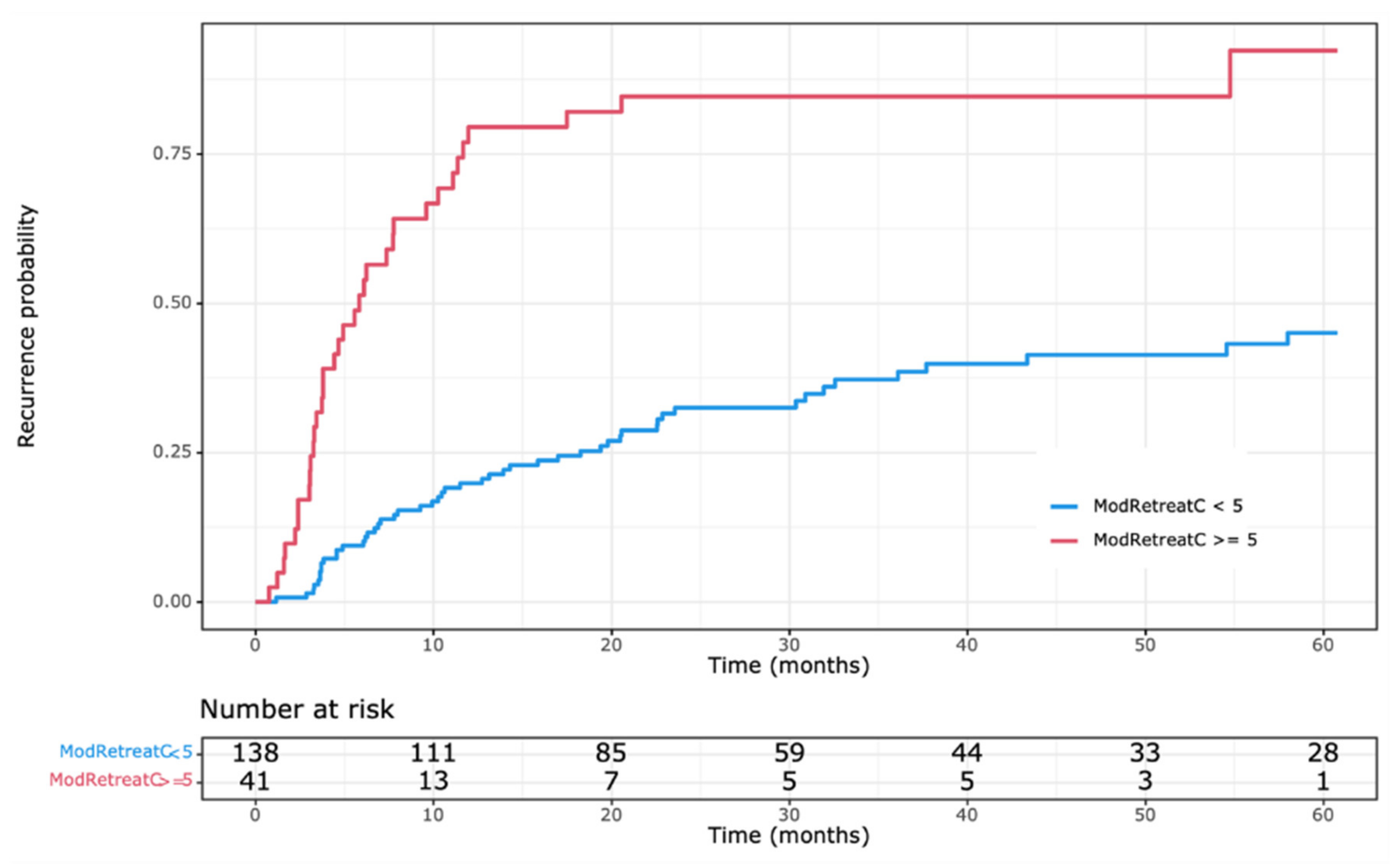
3.4. Construction of RESTORE Index and Recurrence Estimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzaferro, V.; Rondinara, G.F.; Rossi, G.; Regalia, E.; De Carlis, L.; Caccamo, L.; Doci, R.; Sansalone, C.V.; Belli, L.S.; Armiraglio, E. Milan multicenter experience in liver transplantation for hepatocellular carcinoma. Transplant. Proc. 1994, 26, 3557–3560. [Google Scholar] [PubMed]
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009, 27, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef]
- Shah, S.A.; Cleary, S.P.; Wei, A.C.; Yang, I.; Taylor, B.R.; Hemming, A.W.; Langer, B.; Grant, D.R.; Greig, P.D.; Gallinger, S. Recurrence after liver resection for hepatocellular carcinoma: Risk factors, treatment, and outcomes. Surgery 2007, 141, 330–339. [Google Scholar] [CrossRef]
- Sherman, M. Recurrence of hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 2045–2047. [Google Scholar] [CrossRef]
- Portolani, N.; Coniglio, A.; Ghidoni, S.; Giovanelli, M.; Benetti, A.; Tiberio, G.A.; Giulini, S.M. Early and late recurrence after liver resection for hepatocellular carcinoma: Prognostic and therapeutic implications. Ann. Surg. 2006, 2006, 229–235. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to buld a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Batts, K.P.; Ludwig, J. Chronic hepatitis. An update on terminology and reporting. Am. J. Surg. Pathol. 1995, 19, 1409–1417. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467. [Google Scholar] [CrossRef]
- Uno, H.; Cai, T.; Pencina, M.; D’Agostino, R.; Wei, L. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat. Med. 2011, 30, 1105–1117. [Google Scholar] [CrossRef]
- Pencina, M.; D’Agostino, R., Sr.; D’Agostino, R., Jr.; Vasan, R. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Pencina, M.; D’Agostino, R.; Pencina, K.; Janssens, A.C.; Greenland, P. Interpreting incremental value of markers added to risk prediction models. Am. J. Epidemiol. 2012, 176, 473–481. [Google Scholar] [CrossRef]
- Pencina, M.; D’Agostino, R., Sr.; Demler, O.V. Novel metrics for evaluating improvement in discrimination: Net reclassification and integrated discrimination improvement for normal variables and nested models. Stat. Med. 2012, 31, 101–113. [Google Scholar] [CrossRef]
- Steyerberg, E.; Vickers, A.; Cook, N.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.; Kattan, M. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef]
- Uno, H.; Tian, L.; Cai, T.; Kohane, I.; Wei, L. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat. Med. 2013, 32, 2430–2442. [Google Scholar] [CrossRef]
- Mehta, N.; Heimbach, J.; Harnois, D.M.; Sapisochin, G.; Dodge, J.L.; Lee, D.; Burns, J.M.; Sanchez, W.; Greig, P.D.; Grant, D.R.; et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol. 2017, 3, 493–500. [Google Scholar] [CrossRef]
- Mehta, N.; Dodge, J.L.; Roberts, J.P.; Yao, F.Y. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am. J. Transplant. 2018, 18, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The tumor burden score: A new ‘metro-ticket’ prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Moris, D.; Hyer, J.M.; Bagante, F.; Sahara, K.; Moro, A.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; et al. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br. J. Surg. 2020, 7, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.H.; Zhong, J.; Berhane, S.; Toyoda, H.; Cucchetti, A.; Shi, K.; Tada, T.; Chong, C.C.; Xiang, B.-D.; Li, L.-Q.; et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018, 69, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, T.; Lu, Q.; Li, M.; Guo, J.Y.; Shen, Y.; Wu, Z.; Nan, K.-J.; Lv, Y.; Zhang, X.-F. Surgical resection improves long-term survival of patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages. Cancer Manag. Res. 2018, 10, 361–369. [Google Scholar] [CrossRef]
- Cho, Y.; Sinn, D.H.; Yu, S.J.; Gwak, G.Y.; Kim, J.H.; Yoo, Y.J.; Jun, D.W.; Kim, T.Y.; Lee, H.Y.; Cho, E.J.; et al. Survival analysis of single large (>5 cm) hepatocellular carcinoma patients: BCLC A versus, B. PLoS ONE 2016, 11, e0165722. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Delman, K.A.; Vauthey, J.N.; Nagorney, D.M.; Ng, I.O.L.; Ikai, I.; Yamaoka, Y.; Belghiti, J.; Lauwers, G.Y.; Poon, R.T.; et al. Tumor size predicts vascular invasion and histologic grade: Implciations for selection of surgical treatment fo hepatocellular carcinoma. Liver Transplant. 2005, 11, 1086–1092. [Google Scholar] [CrossRef]
- Hoffman, D.; Mehta, N. Recurrence of hepatocellular carcinoma following liver transplantation. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 91–102. [Google Scholar] [CrossRef]
- Ang, S.F.; Ng, E.S.; Li, H.; Ong, Y.H.; Choo, S.P.; Ngeow, J.; Toh, H.C.; Lim, K.H.; Yap, H.Y.; Tan, C.K.; et al. The Singapore Liver Cancer Recurrence (SLICER) Score for relapse prediction in patients with surgically resected hepatocellular carcinoma. PLoS ONE 2015, 10, e0118658. [Google Scholar]
- Xu, X.F.; Xing, H.; Han, J.; Li, Z.L.; Lau, W.Y.; Zhou, Y.H.; Gu, W.M.; Wang, H.; Chen, T.H.; Zeng, Y.Y.; et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019, 154, 209–217. [Google Scholar] [CrossRef]
- Lee, D.; Sapisochin, G.; Mehta, N.; Gorgen, A.; Musto, K.R.; Hajda, H.; Yao, F.Y.; Hodge, D.O.; Carter, R.E.; Harnois, D.M. Surveillance for HCC After Liver Transplantation: Increased Monitoring May Yield Aggressive Treatment Options and Improved Postrecurrence Survival. Transplantation 2020, 104, 2105–2112. [Google Scholar] [CrossRef]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.-Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.-H.; Tak, W.Y.; et al. Adjuvant Sorafenib for Hepatocellular Carcinoma After Resection or Ablation (STORM): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef]
- Chung, A.Y.; Ooi, L.L.; Machin, D.; Tan, S.B.; Goh, B.K.P.; Wong, J.S.; Chen, Y.M.; Li, P.C.N.; Gandhi, M.; Thng, C.H.; et al. Adjuvant hepatic intra-arterial iodine-131-lipiodol following curative resection of hepatocellular carcinoma: A prospective randomized trial. World J. Surg. 2013, 37, 1356–1361. [Google Scholar] [CrossRef]
- Renzulli, M.; Brocchi, S.; Cucchetti, A.; Mazzotti, F.; Mosconi, C.; Sportoetti, C.; Brandi, G.; Pinna, A.D.; Golfieri, R. Can Current Preoperative Imaging Be Used to Detect Microvascular Invasion of Hepatocellular Carcinoma. Radiology 2016, 279, 432–442. [Google Scholar] [CrossRef]
- Roberts, D.E.; Kakar, S.; Mehta, N.; Gill, R.M. A Point-based Histologic Scoring System for Hepatocellular Carcinoma Can Stratify Risk of Posttransplant Tumor Recurrence. Am. J. Surg. Pathol. 2018, 42, 855–865. [Google Scholar] [CrossRef]
- Suh, S.W.; Lee, K.W.; Lee, J.M.; You, T.; Choi, Y.; Kim, H.; Lee, H.W.; Yi, N.-J.; Suh, K.-S. Prediction of aggressiveness in early-stage hepatocellular carcinoma for selection of surgical resection. J. Hepatol. 2014, 60, 1219–1224. [Google Scholar] [CrossRef]
- Poté, N.; Cauchy, F.; Albuquerque, M.; Voitot, H.; Belghiti, J.; Castera, L.; Puy, H.; Bedossa, P.; Paradis, V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J. Hepatol. 2015, 62, 948–954. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, W.; Zhang, Y.; Liu, X.; Li, M.; Wu, Z.; Liu, Z.; Lv, Y.; Wang, B. Prognostic role of pre-treatment serum AFP-L3% in hepatocellular carcinoma: Systematic review and meta-analysis. PLoS ONE 2014, 9, e87011. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, Y.; Kim, H.Y.; Cho, E.J.; Lee, D.H.; Yu, S.J.; Lee, J.W.; Yi, N.-J.; Lee, K.-W.; Kim, S.H.; et al. Serum Tumor Markers Provide Refined Prognostication in Selecting Liver Transplantation Candidate for Hepatocellular Carcinoma Patients Beyond the Milan Criteria. Annals of Surgery. Ann. Surg. 2016, 263, 842–850. [Google Scholar] [CrossRef]
- Chaiteerakij, R.; Zhang, X.; Addissie, B.D.; Mohamed, E.A.; Harmsen, W.S.; Theobald, P.J.; Peters, B.E.; Balsanek, J.G.; Ward, M.M.; Giama, N.H.; et al. Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transplant. 2015, 21, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Kotwani, P.; Chan, W.; Yao, F.; Mehta, N. DCP and AFP-L3 Are Complementary to AFP in Predicting High-Risk Explant Features: Results of a Prospective Study. Clin. Gastroenterol. Hepatol. 2021, 20, 701–703.e2. [Google Scholar] [CrossRef] [PubMed]
| Variables [n, % of Patients Experiencing Outcome] | Overall (n = 179) | No Recurrence (n = 86) | Recurrence (n = 93) |
|---|---|---|---|
| Age, years [median (IQR)] | 63 (57–67) | 61 (55–67) | 63 (57–67) |
| Age at surgery <50 | 22 (12.3%) | 13 (15.1%) | 9 (9.7%) |
| Age at surgery ≥50 | 157 (87.7%) | 73 (84.9%) | 84 (90.3%) |
| Sex | |||
| Female | 50 (27.1%) | 27 (31.4%) | 23 (24.7%) |
| Male | 129 (72.9%) | 59 (68.6%) | 70 (75.3%) |
| Race | |||
| White | 74 (41.8%) | 26 (30.2%) | 48 (51.6%) |
| Asian | 67 (37.9%) | 41 (47.7%) | 26 (28%) |
| Black or AA | 22 (12.4%) | 11 (12.8%) | 11 (11.8%) |
| Other | 14 (7.9%) | 6 (7%) | 8 (8.6%) |
| Underlying Liver Disease | |||
| HBV | 60 (33.5%) | 30 (34.9%) | 30 (32.3%) |
| HCV | 61 (34.1%) | 30 (34.9%) | 31 (33.3%) |
| Cryptogenic | 32 (17.9%) | 12 (14%) | 20 (21.5%) |
| Unknown/Missing | 26 (14.5%) | 14 (16.3%) | 12 (12.9%) |
| AFP, ng/mL [median (IQR)] | 12.3 (3.7, 183.7) | 5.3 (2.6, 59.9) | 41 (5.8, 397.5) |
| ≤20 | 94 (52.5%) | 55 (64%) | 39 (41.9%) |
| 21–99 | 27 (15.1%) | 12 (14%) | 15 (16%) |
| 100–999 | 29 (16.2%) | 9 (10.5%) | 20 (21.5%) |
| 1000+ | 29 (16.2%) | 10 (11.6%) | 19 (20.4%) |
| Bilirubin, mg/dL [median (IQR)] | 0.80 (0.60, 1.10) | 0.80 (0.60, 1.20) | 0.80 (0.60, 1) |
| Albumin, g/dL [median (IQR)] | 3.90 (3.30, 4.15) | 4.00 (3.42, 4.20) | 3.80 (3.10, 4.10) |
| ALBI Score | −2.57 (−2.82, −2.05) | −2.66 (−2.86, −2.12) | −2.48 (−2.74, −2.02) |
| ALBI Grade 1 | 86 (48%) | 48 (56%) | 38 (41%) |
| ALBI Grade 2 | 85 (47%) | 33 (38%) | 52 (56%) |
| ALBI Grade 3 | 8 (4.5%) | 5 (5.8%) | 3 (3.2%) |
| Pre-Operative LRT | 35 (19.6%) | 15 (17.4%) | 20 (21.5%) |
| Pre-Operative TACE | 26 (14.5%) | 10 (11.6%) | 16 (17.2%) |
| Pre-Operative Y-90 | 5 (2.8%) | 3 (3.5%) | 2 (2.2%) |
| Pre-Operative RFA | 2 (1.1%) | 1 (1.2%) | 1 (1.1%) |
| Pre-Operative Bland Embolization | 2 (1.1%) | 1 (1.2%) | 1 (1.1%) |
| Radiologic Findings | |||
| Number of Nodules | |||
| 1 | 147 (82.1%) | 74 (86.09%) | 73 (78.5%) |
| 2 | 19 (10.6%) | 6 (7%) | 13 (14%) |
| 3 | 8 (4.5%) | 4 (4.7%) | 4 (4.3%) |
| 4+ | 4 (2.2%) | 1 (1.2%) | 3 (3.2%) |
| 2+ | 31 (17.3%) | 11 (12.8%) | 20 (21.5%) |
| Largest Nodule Size (cm) (mean, 95% CI) | 6.07 (5.41, 6.73) | 5.35 (4.36, 6.34) | 6.74 (5.85, 7.62) |
| Aggregate Nodule Size (cm) (mean, 95% CI) | 6.44 (5.73, 7.14) | 5.54 (4.49, 6.58) | 7.25 (6.32, 8.18) |
| Liver Pathology | |||
| Cirrhosis | 65 (36.3%) | 28 (32.6%) | 37 (39.8%) |
| Fibrosis (missing = 0) | |||
| 0 | 47 (26.3%) | 18 (20.9%) | 29 (31.2%) |
| 1 | 17 (9.5%) | 14 (16.3%) | 3 (3.2%) |
| 2 | 19 (10.6%) | 9 (10.5%) | 10 (10.8%) |
| 3 | 31 (17.3%) | 17 (19.8%) | 14 (15.1%) |
| 4 | 65 (36.3%) | 28 (32.6%) | 37 (39.8%) |
| Steatosis (missing = 15) | |||
| 0 | 104 (58.1%) | 52 (60.5%) | 52 (55.9%) |
| 1 | 49 (27.4%) | 25 (29.1%) | 24 (25.8%) |
| 2/3 | 11 (6.1%) | 8 (9.3%) | 3 (3.2%) |
| Inflammation (missing = 12) | |||
| 0 | 54 (30.2%) | 27 (31.4%) | 27 (29%) |
| 1 | 60 (33.5%) | 31 (36%) | 29 (31.2%) |
| 2/3 | 53 (29.6%) | 27 (31.4%) | 26 (28%) |
| Tumor Pathology | |||
| Differentiation | |||
| Well | 26 (14.7%) | 15 (17.4%) | 11 (11.8%) |
| Well-Moderate | 19 (10.7%) | 10 (11.6%) | 9 (9.7%) |
| Moderate | 86 (48.6%) | 37 (43%) | 49 (52.7%) |
| Moderate-Poor | 29 (16.4%) | 15 (17.4%) | 14 (15.1%) |
| Poor | 17 (9.6%) | 7 (8.1%) | 10 (10.8%) |
| Number of Nodules | |||
| 1 | 147 (82.1%) | 82 (95.3%) | 65 (69.9%) |
| 2 | 21 (11.8%) | 4 (4.7%) | 17 (18.3%) |
| 3 | 5 (2.8%) | 0 (0%) | 5 (5.4%) |
| 4+ | 6 (3.4%) | 0 (0%) | 6 (6.5%) |
| 2+ | 32 (18%) | 4 (4.7%) | 28 (30.1%) |
| Largest Nodule Size (cm) (mean, 95% CI) | 6.26 (5.54–6.98) | 5.38 (4.27–6.49) | 7.07 (6.15–8) |
| Aggregate Nodule Size (cm) (mean, 95% CI) | 6.74 (5.96, 7.51) | 5.43 (4.33, 6.54) | 7.93 (6.89, 8.98) |
| Vascular Invasion | |||
| No | 128 (71.9%) | 77 (89.5%) | 51 (54.8%) |
| Yes | 50 (28.1%) | 8 (9.3%) | 42 (45.2%) |
| Capsular Involvement | 67 (38.5%) | 21 (24.4%) | 46 (49.5%) |
| Margin Status | |||
| R0 | 163 (91%) | 79 (91.9%) | 84 (90.3%) |
| ≥R1 | 16 (9%) | 7 (8.1%) | 9 (9.7%) |
| Number of Nodes Examined (mean, 95% CI) | 0.33 (0.15–0.51) | 0.36 (0.04, 0.67) | 0.31 (0.12, 0.5) |
| Variables [n, % of Patients Meeting Criteria] | Overall (n = 179) | No Recurrence (n = 86) | Recurrence (n = 93) |
|---|---|---|---|
| Radiologic Criteria | |||
| Within Milan | 92 | 55 (59.8%) | 37 (40.2%) |
| Outside Milan but within UCSF | 26 | 7 (26.9%) | 19 (73.1%) |
| Outside UCSF | 61 | 24 (39.3%) | 37 (60.7%) |
| Outside Milan | 87 | 41 (47.1%) | 56 (52.9%) |
| Pathologic Criteria | |||
| Within Milan | 87 | 60 (69%) | 27 (31%) |
| Outside Milan but within UCSF | 23 | 8 (34.8%) | 15 (65.2%) |
| Outside UCSF | 69 | 18 (26.1%) | 51 (73.9%) |
| Outside Milan | 92 | 26 (28.3%) | 66 (71.7%) |
| Variable | Comparison | Univariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value |
|---|---|---|---|---|---|
| Patient Characteristic | |||||
| Asian Race | Vs. White Race | 0.46 (0.28–0.74) | <0.01 | 0.86 (0.49, 1.51) | 0.60 |
| AFP | |||||
| 100–999 | Vs. ≤20 | 2.37 (1.38–4.08) | <0.01 | 2.01 (1.06, 3.80) | 0.03 |
| ≥1000 | Vs. ≤20 | 2.47 (1.42–4.28) | <0.01 | 2.02 (1.07, 3.82) | 0.03 |
| Radiology | |||||
| Aggregate Nodule Size (cm) | Per cm diameter | 1.05 (1.01-1.09) | 0.02 | 0.97 (0.85, 1.13) | 0.81 |
| Beyond Milan but within UCSF | Vs. within Milan | 2.43 (1.39–4.24) | <0.01 | 1.82 (0.93, 3.53) | 0.08 |
| Beyond UCSF | Vs. within Milan | 1.72 (1.09–2.71) | 0.02 | 1.36 (0.50. 3.69) | 0.54 |
| Pathology | |||||
| Fibrosis (Batts-Ludwig Criteria) | |||||
| 1 | vs. 0 | 0.19 (0.06–0.63) | 0.01 | 0.35 (0.09, 1.31) | 0.12 |
| Tumor | |||||
| Nodule # | |||||
| 2+ | vs. 1 | 3.11 (1.98–4.88) | <0.01 | 2.67 (1.623, 4.391) | <0.01 |
| Largest Nodule Size (cm) | Per cm diameter | 1.04 (1.00–1.08) | 0.03 | 0.82 (0.67, 1.01) | 0.06 |
| Aggregate Nodule Size (cm) | Per cm aggregate diameter | 1.05 (1.02–1.09) | <0.01 | 1.22 (1.02, 1.47) | 0.03 |
| Vascular Invasion | Vs. None | 3.57 (2.35–5.40) | <0.01 | 2.25 (1.30, 3.89) | <0.01 |
| Capsular Involvement | Vs. None | 1.81 (1.20–2.73) | <0.01 | 1.12 (0.67, 1.88) | 0.66 |
| Transplant Criteria: Beyond Milan but within UCSF | vs. within Milan | 3.20 (1.68–6.08) | <0.01 | ** | |
| Transplant Criteria: Beyond UCSF | vs. within Milan | 3.44 (2.14–5.53) | <0.01 | ** |
| Variable | Hazard Ratio | p-Value | RESTORE Points |
|---|---|---|---|
| Pre-Op AFP | |||
| ≤20 | Ref | 0 | |
| 21–99 | 1.37 (0.79–2.26) | 0.35 | 1 |
| ≥100 | 1.78 (1.08–2.92\3) | 0.02 | 2 |
| Vascular Invasion | |||
| No | Ref | 0 | |
| Yes | 2.77 (1.72–4.44) | <0.01 | 3 |
| Lesion No. | |||
| 1 lesion w/in Milan | Ref | 0 | |
| 1 lesion outside Milan | 1.33 (0.79–2.26) | 0.29 | 1 |
| 2+ lesions | 3.39 (1.93–5.97) | <0.01 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, D.; Shui, A.; Gill, R.; Syed, S.; Mehta, N. Resected Tumor Outcome and Recurrence (RESTORE) Index for Hepatocellular Carcinoma Recurrence after Resection. Cancers 2023, 15, 2433. https://doi.org/10.3390/cancers15092433
Hoffman D, Shui A, Gill R, Syed S, Mehta N. Resected Tumor Outcome and Recurrence (RESTORE) Index for Hepatocellular Carcinoma Recurrence after Resection. Cancers. 2023; 15(9):2433. https://doi.org/10.3390/cancers15092433
Chicago/Turabian StyleHoffman, Daniel, Amy Shui, Ryan Gill, Shareef Syed, and Neil Mehta. 2023. "Resected Tumor Outcome and Recurrence (RESTORE) Index for Hepatocellular Carcinoma Recurrence after Resection" Cancers 15, no. 9: 2433. https://doi.org/10.3390/cancers15092433





