Reshaping the Pancreatic Cancer Microenvironment at Different Stages with Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
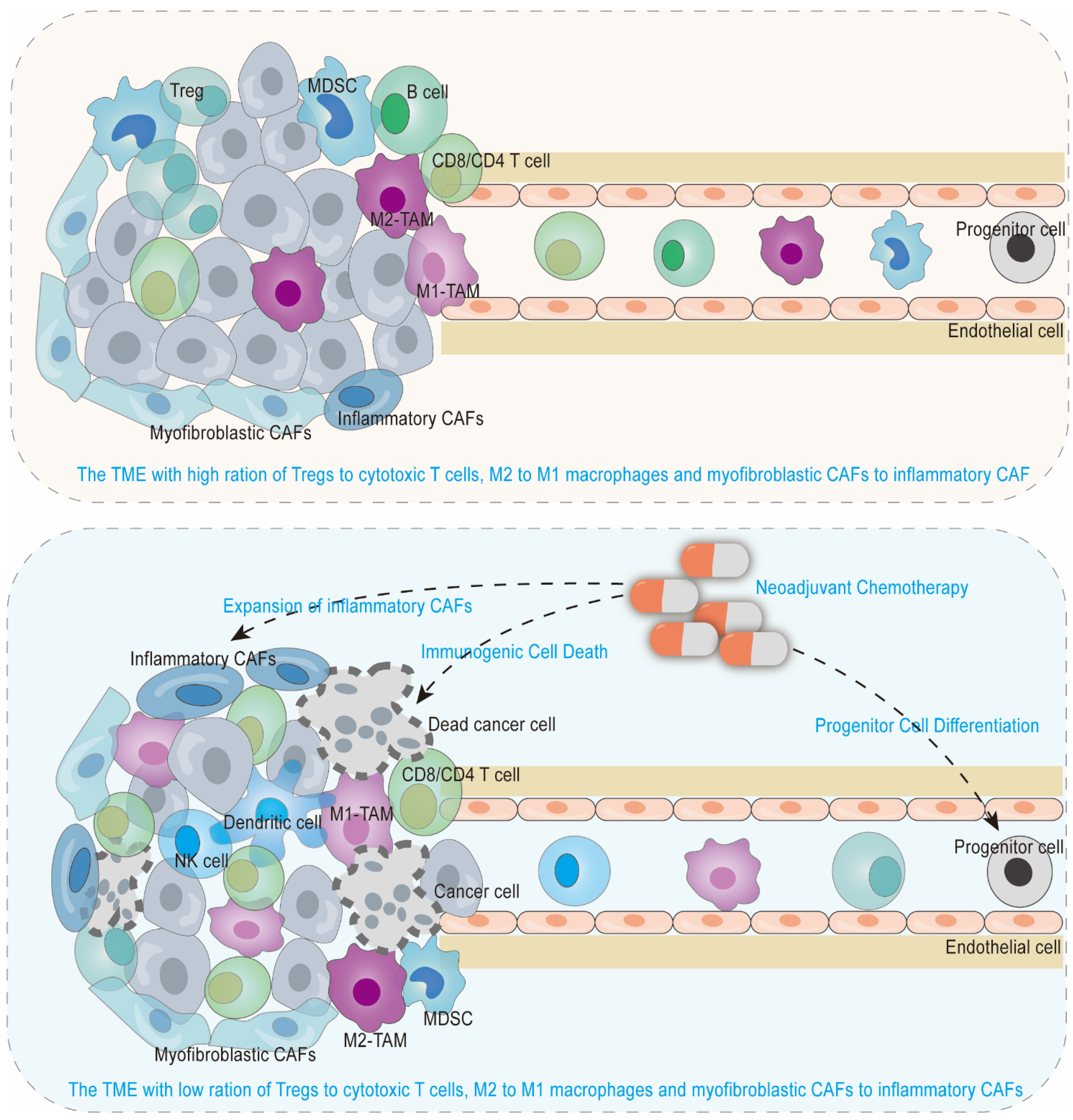
2. An Anti-Tumorigenic Immune Microenvironment by Neoadjuvant Chemotherapy in Borderline Resectable and Locally Advanced Pancreatic Cancer
2.1. Anti-Tumor Immune Microenvironment Induced by Immunogenic Cell Death
2.2. Recruited Anti-Tumor Immune Cells from Progenitor Cell Differentiation
2.3. Reshaped Tumor Immune Cells in Quantity, Space and Function
2.4. Responsive Biomarkers in Reshaped Tumor Immune Microenvironment
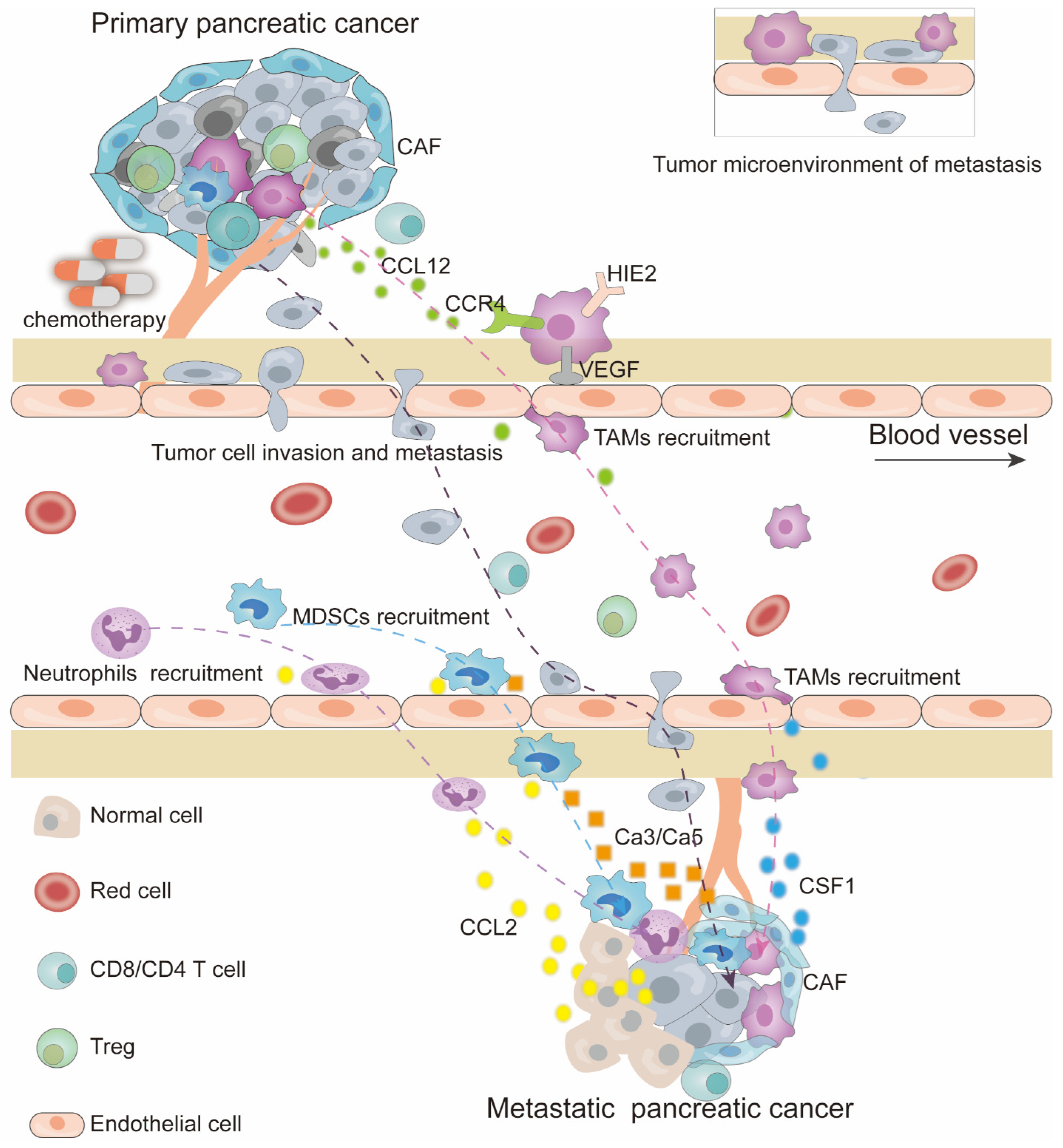
3. Immunosuppressive Myeloid Cell Recruitment and Metastasis by Chemotherapy
3.1. Primary Tumor Immune Microenvironment Reshaped by Chemotherapy
3.2. Micro-Metastatic Niche Induced by Chemotherapy
3.3. Impact of Chemotherapeutic Course and Dose
3.4. Strategies to Prevent Chemotherapy-Induced Tumor Recurrence/Metastasis
4. Metabolic Substances and Exosomes Participate in the TME Remodeling
4.1. Metabolic Remodeling in the TME Due to Chemotherapy
4.2. Exosome-Mediated Reshaping of the TME by Chemotherapy
5. Malignant Cells with Cancer Stem Cell and Epithelial-to-Mesenchymal Transition Characteristics after Chemotherapy
5.1. Malignant Cells Reshaped by Chemotherapy
5.2. The Commonality of Cancer Stem Cells and Partial Epithelial-to-Mesenchymal Transition Cells under Chemotherapy
5.3. Cancer Stem Cell
5.4. Partial-Epithelial-to-Mesenchymal Transition Cells
6. Adaptive Cancer-Associated Fibroblasts under Chemotherapy to Support Tumor Cell Survival and Metastasis
6.1. Cancer-Associated Fibroblasts Support Pancreatic Cancer Cells Survival
6.2. Cancer-Associated Fibroblasts Induce Micro-Metastatic Niche
7. Targeted Therapy to Synergize with Chemotherapy
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hank, T.; Büchler, M.W.; Neoptolemos, J.P. Neoadjuvant Chemotherapy in Pancreatic Cancer. JAMA Surg. 2021, 156, 397. [Google Scholar] [CrossRef] [PubMed]
- Perri, G.; Prakash, L.; Qiao, W.; Varadhachary, G.R.; Wolff, R.; Fogelman, D.; Overman, M.; Pant, S.; Javle, M.; Koay, E.J.; et al. Response and Survival Associated with First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2020, 155, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Gillen, S.; Schuster, T.; Meyer Zum Büschenfelde, C.; Friess, H.; Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef] [PubMed]
- Gourgou-Bourgade, S.; Bascoul-Mollevi, C.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Boige, V.; et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized trial. J. Clin. Oncol. 2013, 31, 23–29. [Google Scholar] [CrossRef]
- Tabernero, J.; Chiorean, E.G.; Infante, J.R.; Hingorani, S.R.; Ganju, V.; Weekes, C.; Scheithauer, W.; Ramanathan, R.K.; Goldstein, D.; Penenberg, D.N.; et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist 2015, 20, 143–150. [Google Scholar] [CrossRef]
- Dhir, M.; Zenati, M.S.; Hamad, A.; Singhi, A.D.; Bahary, N.; Hogg, M.E.; Zeh, H.J.; Zureikat, A.H. FOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Neoadjuvant Treatment of Resectable and Borderline Resectable Pancreatic Head Adenocarcinoma. Ann. Surg. Oncol. 2018, 25, 1896–1903. [Google Scholar] [CrossRef]
- Klein-Brill, A.; Amar-Farkash, S.; Lawrence, G.; Collisson, E.A.; Aran, D. Comparison of FOLFIRINOX vs Gemcitabine Plus Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Ductal Adenocarcinoma. JAMA Netw. Open. 2022, 5, e2216199. [Google Scholar] [CrossRef]
- Randazzo, O.; Papini, F.; Mantini, G.; Gregori, A.; Parrino, B.; Liu, D.S.; Cascioferro, S.; Carbone, D.; Peters, G.J.; Frampton, A.E. “Open Sesame?”: Biomarker status of the human equilibrative nucleoside transporter-1 and molecular mechanisms influencing its expression and activity in the uptake and cytotoxicity of gemcitabine in pancreatic cancer. Cancers 2020, 12, 3206. [Google Scholar] [CrossRef]
- Öman, M.; Wettergren, Y.; Odin, E.; Westermark, S.; Naredi, P.; Hemmingsson, O.; Taflin, H. Pharmacokinetics of preoperative intraperitoneal 5-FU in patients with pancreatic ductal adenocarcinoma. Cancer Chemother. Pharmacol. 2021, 88, 619–631. [Google Scholar] [CrossRef]
- Heiduk, M.; Plesca, I.; Glück, J.; Müller, L.; Digomann, D.; Reiche, C.; von Renesse, J.; Decker, R.; Kahlert, C.; Sommer, U.; et al. Neoadjuvant chemotherapy drives intratumoral T cells toward a proinflammatory profile in pancreatic cancer. JCI Insight 2022, 7, e152761. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Q.; Li, M.; Guo, H.; Liu, W.; Wang, F.; Tian, X.; Yang, Y. Single-cell RNA-seq reveals dynamic change in tumor microenvironment during pancreatic ductal adenocarcinoma malignant progression. EBioMedicine 2021, 66, 103315. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Wang, J.; Lou, Y.; Hong, Z.; Wei, S.; Sun, K.; Wang, J.; Chen, Y.; Sheng, J.; et al. Dynamic profiling of immune microenvironment during pancreatic cancer development suggests early intervention and combination strategy of immunotherapy. EBioMedicine 2022, 78, 103958. [Google Scholar] [CrossRef] [PubMed]
- Bernard, V.; Semaan, A.; Huang, J.; San Lucas, F.A.; Mulu, F.C.; Stephens, B.M.; Guerrero, P.A.; Huang, Y.; Zhao, J.; Kamyabi, N. Single-Cell Transcriptomics of Pancreatic Cancer Precursors Demonstrates Epithelial and Microenvironmental Heterogeneity as an Early Event in Neoplastic ProgressionSingle-Cell RNA Sequencing of Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, B.T.; Devisme, A.; Andrieux, G.; Vyas, F.; Aliar, K.; McCloskey, C.W.; Macklin, A.; Jang, G.H.; Denroche, R.; Romero, J.M.; et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 2021, 184, 5577–5592.e5518. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, J.; Wang, G.; Xu, D. Targeting Proliferating Tumor-Infiltrating Macrophages Facilitates Spatial Redistribution of CD8(+) T Cells in Pancreatic Cancer. Cancers 2022, 14, 1474. [Google Scholar] [CrossRef]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, B.; Li, H. The Roles of Frequently Mutated Genes of Pancreatic Cancer in Regulation of Tumor Microenvironment. Technol. Cancer Res. Treat. 2020, 19, 1533033820920969. [Google Scholar] [CrossRef]
- Samstein, R.M.; Krishna, C.; Ma, X.; Pei, X.; Lee, K.-W.; Makarov, V.; Kuo, F.; Chung, J.; Srivastava, R.M.; Purohit, T.A.; et al. Mutations in BRCA1 and BRCA2 differentially affect the tumor microenvironment and response to checkpoint blockade immunotherapy. Nat. Cancer 2020, 1, 1188–1203. [Google Scholar] [CrossRef]
- Galeano Niño, J.L.; Wu, H.; LaCourse, K.D.; Kempchinsky, A.G.; Baryiames, A.; Barber, B.; Futran, N.; Houlton, J.; Sather, C.; Sicinska, E.; et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 2022, 611, 810–817. [Google Scholar] [CrossRef]
- Hwang, W.L.; Jagadeesh, K.A.; Guo, J.A.; Hoffman, H.I.; Yadollahpour, P.; Reeves, J.W.; Mohan, R.; Drokhlyansky, E.; Van Wittenberghe, N.; Ashenberg, O.; et al. Single-nucleus and spatial transcriptome profiling of pancreatic cancer identifies multicellular dynamics associated with neoadjuvant treatment. Nat. Genet. 2022, 54, 1178–1191. [Google Scholar] [CrossRef]
- Farren, M.R.; Sayegh, L.; Ware, M.B.; Chen, H.R.; Gong, J.; Liang, Y.; Krasinskas, A.; Maithel, S.K.; Zaidi, M.; Sarmiento, J.M.; et al. Immunologic alterations in the pancreatic cancer microenvironment of patients treated with neoadjuvant chemotherapy and radiotherapy. JCI Insight 2020, 5, e130362. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, X.; Peng, X.; May Chen, M.J.; Li, Z.; Wei, B.; Wen, X.; Wei, B.; Dong, Y.; Bu, Z.; et al. Multi-omics characterization of molecular features of gastric cancer correlated with response to neoadjuvant chemotherapy. Sci. Adv. 2020, 6, eaay4211. [Google Scholar] [CrossRef] [PubMed]
- Woolston, A.; Barber, L.J.; Griffiths, B.; Pich, O.; Lopez-Bigas, N.; Matthews, N.; Rao, S.; Watkins, D.; Chau, I.; Starling, N.; et al. Mutational signatures impact the evolution of anti-EGFR antibody resistance in colorectal cancer. Nat. Ecol. Evol. 2021, 5, 1024–1032. [Google Scholar] [CrossRef]
- Kim, R.; An, M.; Lee, H.; Mehta, A.; Heo, Y.J.; Kim, K.M.; Lee, S.Y.; Moon, J.; Kim, S.T.; Min, B.H.; et al. Early Tumor-Immune Microenvironmental Remodeling and Response to First-Line Fluoropyrimidine and Platinum Chemotherapy in Advanced Gastric Cancer. Cancer Discov. 2022, 12, 984–1001. [Google Scholar] [CrossRef] [PubMed]
- Mota Reyes, C.; Teller, S.; Muckenhuber, A.; Konukiewitz, B.; Safak, O.; Weichert, W.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Neoadjuvant Therapy Remodels the Pancreatic Cancer Microenvironment via Depletion of Protumorigenic Immune Cells. Clin. Cancer Res. 2020, 26, 220–231. [Google Scholar] [CrossRef]
- Vorontsova, A.; Cooper, T.J.; Haj-Shomaly, J.; Benguigui, M.; Levin, S.; Manobla, B.; Menachem, R.; Timaner, M.; Raviv, Z.; Shaked, Y. Chemotherapy-induced tumor immunogenicity is mediated in part by megakaryocyte-erythroid progenitors. Oncogene 2023, 42, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; James, C.A.; Cullinan, D.R.; Hogg, G.D.; Mudd, J.L.; Zuo, C.; Takchi, R.; Caldwell, K.E.; Liu, J.; DeNardo, D.G.; et al. Neoadjuvant FOLFIRINOX Therapy Is Associated with Increased Effector T Cells and Reduced Suppressor Cells in Patients with Pancreatic Cancer. Clin. Cancer Res. 2021, 27, 6761–6771. [Google Scholar] [CrossRef]
- Dias Costa, A.; Väyrynen, S.A.; Chawla, A.; Zhang, J.; Väyrynen, J.P.; Lau, M.C.; Williams, H.L.; Yuan, C.; Morales-Oyarvide, V.; Elganainy, D.; et al. Neoadjuvant Chemotherapy Is Associated with Altered Immune Cell Infiltration and an Anti-Tumorigenic Microenvironment in Resected Pancreatic Cancer. Clin. Cancer Res. 2022, 28, 5167–5179. [Google Scholar] [CrossRef]
- Michelakos, T.; Cai, L.; Villani, V.; Sabbatino, F.; Kontos, F.; Fernández-Del Castillo, C.; Yamada, T.; Neyaz, A.; Taylor, M.S.; Deshpande, V.; et al. Tumor Microenvironment Immune Response in Pancreatic Ductal Adenocarcinoma Patients Treated with Neoadjuvant Therapy. J. Natl. Cancer Inst. 2021, 113, 182–191. [Google Scholar] [CrossRef]
- Carstens, J.L.; Correa de Sampaio, P.; Yang, D.; Barua, S.; Wang, H.; Rao, A.; Allison, J.P.; LeBleu, V.S.; Kalluri, R. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 2017, 8, 15095. [Google Scholar] [CrossRef]
- Okubo, S.; Suzuki, T.; Hioki, M.; Shimizu, Y.; Toyama, H.; Morinaga, S.; Gotohda, N.; Uesaka, K.; Ishii, G.; Takahashi, S.; et al. The immunological impact of preoperative chemoradiotherapy on the tumor microenvironment of pancreatic cancer. Cancer Sci. 2021, 112, 2895–2904. [Google Scholar] [CrossRef]
- Werba, G.; Weissinger, D.; Kawaler, E.A.; Zhao, E.; Kalfakakou, D.; Dhara, S.; Wang, L.; Lim, H.B.; Oh, G.; Jing, X.; et al. Single-cell RNA sequencing reveals the effects of chemotherapy on human pancreatic adenocarcinoma and its tumor microenvironment. Nat. Commun. 2023, 14, 797. [Google Scholar] [CrossRef]
- Graeser, M.; Feuerhake, F.; Gluz, O.; Volk, V.; Hauptmann, M.; Jozwiak, K.; Christgen, M.; Kuemmel, S.; Grischke, E.M.; Forstbauer, H.; et al. Immune cell composition and functional marker dynamics from multiplexed immunohistochemistry to predict response to neoadjuvant chemotherapy in the WSG-ADAPT-TN trial. J. Immunother. Cancer 2021, 9, e002198. [Google Scholar] [CrossRef] [PubMed]
- De Castro Silva, I.; Bianchi, A.; Deshpande, N.U.; Sharma, P.; Mehra, S.; Garrido, V.T.; Saigh, S.J.; England, J.; Hosein, P.J.; Kwon, D.; et al. Neutrophil-mediated fibroblast-tumor cell il-6/stat-3 signaling underlies the association between neutrophil-to-lymphocyte ratio dynamics and chemotherapy response in localized pancreatic cancer: A hybrid clinical-preclinical study. Elife 2022, 11, e78921. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Liu, C.; Zhang, W.; He, S.; Wang, F.; Wang, J.; Li, Q.; Zhou, F. Serum levels of IL-6 and CRP can predict the efficacy of mFOLFIRINOX in patients with advanced pancreatic cancer. Front. Oncol. 2022, 12, 964115. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Pastoriza, J.M.; Wang, Y.; Harney, A.S.; Entenberg, D.; Pignatelli, J.; Sharma, V.P.; Xue, E.A.; Cheng, E.; D’Alfonso, T.M.; et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci. Transl. Med. 2017, 9, eaan0026. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Jalgaonkar, S.P.; Middleton, J.D.; Hai, T. Stress-inducible gene Atf3 in the noncancer host cells contributes to chemotherapy-exacerbated breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, E7159–E7168. [Google Scholar] [CrossRef]
- Hughes, R.; Qian, B.Z.; Rowan, C.; Muthana, M.; Keklikoglou, I.; Olson, O.C.; Tazzyman, S.; Danson, S.; Addison, C.; Clemons, M.; et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 2015, 75, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Baghdadi, M.; Tsuchikawa, T.; Wada, H.; Nakamura, T.; Abe, H.; Nakanishi, S.; Usui, Y.; Higuchi, K.; Takahashi, M.; et al. Chemotherapy-Derived Inflammatory Responses Accelerate the Formation of Immunosuppressive Myeloid Cells in the Tissue Microenvironment of Human Pancreatic Cancer. Cancer Res. 2015, 75, 2629–2640. [Google Scholar] [CrossRef]
- Mitchem, J.B.; Brennan, D.J.; Knolhoff, B.L.; Belt, B.A.; Zhu, Y.; Sanford, D.E.; Belaygorod, L.; Carpenter, D.; Collins, L.; Piwnica-Worms, D.; et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013, 73, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Samain, R.; Brunel, A.; Douché, T.; Fanjul, M.; Cassant-Sourdy, S.; Rochotte, J.; Cros, J.; Neuzillet, C.; Raffenne, J.; Duluc, C.; et al. Pharmacologic Normalization of Pancreatic Cancer-Associated Fibroblast Secretome Impairs Prometastatic Cross-Talk with Macrophages. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1405–1436. [Google Scholar] [CrossRef] [PubMed]
- Monteran, L.; Ershaid, N.; Doron, H.; Zait, Y.; Scharff, Y.; Ben-Yosef, S.; Avivi, C.; Barshack, I.; Sonnenblick, A.; Erez, N. Chemotherapy-induced complement signaling modulates immunosuppression and metastatic relapse in breast cancer. Nat. Commun. 2022, 13, 5797. [Google Scholar] [CrossRef]
- Bellomo, G.; Rainer, C.; Quaranta, V.; Astuti, Y.; Raymant, M.; Boyd, E.; Stafferton, R.; Campbell, F.; Ghaneh, P.; Halloran, C.M.; et al. Chemotherapy-induced infiltration of neutrophils promotes pancreatic cancer metastasis via Gas6/AXL signalling axis. Gut 2022, 71, 2284–2299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chandra, V.; Riquelme Sanchez, E.; Dutta, P.; Quesada, P.R.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020, 217, e20190354. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.P.; Qin, W.T.; Yang, Q.; Chen, D.Y.; Li, Q.; Zhou, K.X.; Huang, P.Q.; Xu, C.J.; Li, J.; et al. Identification of a subset of immunosuppressive P2RX1-negative neutrophils in pancreatic cancer liver metastasis. Nat. Commun. 2021, 12, 174. [Google Scholar] [CrossRef]
- Gingis-Velitski, S.; Loven, D.; Benayoun, L.; Munster, M.; Bril, R.; Voloshin, T.; Alishekevitz, D.; Bertolini, F.; Shaked, Y. Host response to short-term, single-agent chemotherapy induces matrix metalloproteinase-9 expression and accelerates metastasis in mice. Cancer Res. 2011, 71, 6986–6996. [Google Scholar] [CrossRef]
- Toste, P.A.; Nguyen, A.H.; Kadera, B.E.; Duong, M.; Wu, N.; Gawlas, I.; Tran, L.M.; Bikhchandani, M.; Li, L.; Patel, S.G.; et al. Chemotherapy-Induced Inflammatory Gene Signature and Protumorigenic Phenotype in Pancreatic CAFs via Stress-Associated MAPK. Mol. Cancer Res. 2016, 14, 437–447. [Google Scholar] [CrossRef]
- Steger, G.G.; Kubista, E.; Hausmaninger, H.; Gnant, M.; Tausch, C.; Lang, A.; Samonigg, H.; Galid, A.; Jakesz, R. 6 vs. 3 cycles of epirubicin/docetaxel + G-CSF in operable breast cancer: Results of ABCSG-14. J. Clin. Oncol. 2004, 22, 553. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhou, Q.C.; Iasonos, A.; Chi, D.S.; Zivanovic, O.; Sonoda, Y.; Gardner, G.; Broach, V.; O’Cearbhaill, R.; Konner, J.A.; et al. Pre-operative neoadjuvant chemotherapy cycles and survival in newly diagnosed ovarian cancer: What is the optimal number? A Memorial Sloan Kettering Cancer Center Team Ovary study. Int. J. Gynecol. Cancer 2020, 30, 1915–1921. [Google Scholar] [CrossRef]
- Hasnis, E.; Alishekevitz, D.; Gingis-Veltski, S.; Bril, R.; Fremder, E.; Voloshin, T.; Raviv, Z.; Karban, A.; Shaked, Y. Anti-Bv8 antibody and metronomic gemcitabine improve pancreatic adenocarcinoma treatment outcome following weekly gemcitabine therapy. Neoplasia 2014, 16, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Cham, K.; Baker, J.; Takhar, K.; Flexman, J.; Wong, M.; Owen, D.; Yung, A.; Kozlowski, P.; Reinsberg, S.; Chu, E. Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma. Br. J. Cancer 2010, 103, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hessmann, E.; Patzak, M.; Klein, L.; Chen, N.; Kari, V.; Ramu, I.; Bapiro, T.; Frese, K.K.; Gopinathan, A.; Richards, F. Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer. Gut 2018, 67, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Habib, J.R.; Kinny-Köster, B.; Bou-Samra, P.; Alsaad, R.; Sereni, E.; Javed, A.A.; Ding, D.; Cameron, J.L.; Lafaro, K.J.; Burns, W.R.; et al. Surgical Decision-Making in Pancreatic Ductal Adenocarcinoma: Modeling Prognosis Following Pancreatectomy in the Era of Induction and Neoadjuvant Chemotherapy. Ann. Surg. 2023, 277, 151–158. [Google Scholar] [CrossRef]
- Wu, V.S.; Elshami, M.; Stitzel, H.J.; Lee, J.J.; Hue, J.J.; Kyasaram, R.K.; Hardacre, J.M.; Ammori, J.B.; Winter, J.M.; Selfridge, J.E.; et al. Why the Treatment Sequence Matters: Interplay Between Chemotherapy Cycles received, Cumulative dose Intensity, and Survival in Resected Early-Stage Pancreas Cancer. Ann. Surg. 2023; ahead of print. [Google Scholar] [CrossRef]
- Truty, M.J.; Kendrick, M.L.; Nagorney, D.M.; Smoot, R.L.; Cleary, S.P.; Graham, R.P.; Goenka, A.H.; Hallemeier, C.L.; Haddock, M.G.; Harmsen, W.S. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann. Surg. 2021, 273, 341–349. [Google Scholar] [CrossRef]
- Pratt, H.G.; Steinberger, K.J.; Mihalik, N.E.; Ott, S.; Whalley, T.; Szomolay, B.; Boone, B.A.; Eubank, T.D. Macrophage and Neutrophil Interactions in the Pancreatic Tumor Microenvironment Drive the Pathogenesis of Pancreatic Cancer. Cancers 2021, 14, 194. [Google Scholar] [CrossRef]
- Nywening, T.M.; Belt, B.A.; Cullinan, D.R.; Panni, R.Z.; Han, B.J.; Sanford, D.E.; Jacobs, R.C.; Ye, J.; Patel, A.A.; Gillanders, W.E.; et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2018, 67, 1112–1123. [Google Scholar] [CrossRef]
- Zhu, Y.; Knolhoff, B.L.; Meyer, M.A.; Nywening, T.M.; West, B.L.; Luo, J.; Wang-Gillam, A.; Goedegebuure, S.P.; Linehan, D.C.; DeNardo, D.G. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014, 74, 5057–5069. [Google Scholar] [CrossRef]
- Nielsen, S.R.; Strøbech, J.E.; Horton, E.R.; Jackstadt, R.; Laitala, A.; Bravo, M.C.; Maltese, G.; Jensen, A.R.D.; Reuten, R.; Rafaeva, M.; et al. Suppression of tumor-associated neutrophils by lorlatinib attenuates pancreatic cancer growth and improves treatment with immune checkpoint blockade. Nat. Commun. 2021, 12, 3414. [Google Scholar] [CrossRef]
- Tuerhong, A.; Xu, J.; Shi, S.; Tan, Z.; Meng, Q.; Hua, J.; Liu, J.; Zhang, B.; Wang, W.; Yu, X.; et al. Overcoming chemoresistance by targeting reprogrammed metabolism: The Achilles’ heel of pancreatic ductal adenocarcinoma. Cell. Mol. Life Sci. 2021, 78, 5505–5526. [Google Scholar] [CrossRef]
- Guillermet-Guibert, J.; Davenne, L.; Pchejetski, D.; Saint-Laurent, N.; Brizuela, L.; Guilbeau-Frugier, C.; Delisle, M.B.; Cuvillier, O.; Susini, C.; Bousquet, C. Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol. Cancer 2009, 8, 809–820. [Google Scholar] [CrossRef]
- Gu, Z.; Du, Y.; Zhao, X.; Wang, C. Tumor microenvironment and metabolic remodeling in gemcitabine-based chemoresistance of pancreatic cancer. Cancer Lett. 2021, 521, 98–108. [Google Scholar] [CrossRef]
- Van Nyen, T.; Planque, M.; van Wagensveld, L.; Duarte, J.A.G.; Zaal, E.A.; Talebi, A.; Rossi, M.; Körner, P.R.; Rizzotto, L.; Moens, S.; et al. Serine metabolism remodeling after platinum-based chemotherapy identifies vulnerabilities in a subgroup of resistant ovarian cancers. Nat. Commun. 2022, 13, 4578. [Google Scholar] [CrossRef] [PubMed]
- Pranzini, E.; Pardella, E.; Muccillo, L.; Leo, A.; Nesi, I.; Santi, A.; Parri, M.; Zhang, T.; Uribe, A.H.; Lottini, T.; et al. SHMT2-mediated mitochondrial serine metabolism drives 5-FU resistance by fueling nucleotide biosynthesis. Cell. Rep. 2022, 40, 111233. [Google Scholar] [CrossRef] [PubMed]
- Amrutkar, M.; Verbeke, C.S.; Finstadsveen, A.V.; Dorg, L.; Labori, K.J.; Gladhaug, I.P. Neoadjuvant chemotherapy is associated with an altered metabolic profile and increased cancer stemness in patients with pancreatic ductal adenocarcinoma. Mol. Oncol. 2023, 17, 59–81. [Google Scholar] [CrossRef]
- Lee, W.; Oh, M.; Kim, J.S.; Park, Y.; Kwon, J.W.; Jun, E.; Song, K.B.; Lee, J.H.; Hwang, D.W.; Yoo, C.; et al. Metabolic activity by FDG-PET/CT after neoadjuvant chemotherapy in borderline resectable and locally advanced pancreatic cancer and association with survival. Br. J. Surg. 2021, 109, 61–70. [Google Scholar] [CrossRef]
- Sun, W.; Ren, Y.; Lu, Z.; Zhao, X. The potential roles of exosomes in pancreatic cancer initiation and metastasis. Mol. Cancer 2020, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, S.; Yang, F.; Au, J.L.; Wientjes, M.G. Nontoxic doses of suramin enhance activity of doxorubicin in prostate tumors. J. Pharm. Exp. 2001, 299, 426–433. [Google Scholar]
- Andrade, L.N.S.; Otake, A.H.; Cardim, S.G.B.; da Silva, F.I.; Ikoma Sakamoto, M.M.; Furuya, T.K.; Uno, M.; Pasini, F.S.; Chammas, R. Extracellular Vesicles Shedding Promotes Melanoma Growth in Response to Chemotherapy. Sci. Rep. 2019, 9, 14482. [Google Scholar] [CrossRef]
- Fanini, F.; Fabbri, M. Cancer-derived exosomic microRNAs shape the immune system within the tumor microenvironment: State of the art. Semin. Cell. Dev. Biol. 2017, 67, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tang, H.; Li, Q.; Chen, G.; Li, D. Exosomes and their roles in the chemoresistance of pancreatic cancer. Cancer Med. 2022, 11, 4979–4988. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.; Richards, K.E.; Hill, R. Gemcitabine-Induced Exosome Hypersecretion Increases the Chemoresistance and Migration of Pancreatic Cancer Cells. FASEB J. 2017, 31, 775.20. [Google Scholar]
- Bhatta, B.; Cooks, T. Reshaping the tumor microenvironment: Extracellular vesicles as messengers of cancer cells. Carcinogenesis 2020, 41, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Zhuang, X.; Samykutty, A.; Mu, J.; Zhang, L.; Cao, P.; Yan, J.; Miller, D. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene 2017, 36, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Srivastava, S.K.; Bhardwaj, A.; Owen, L.B.; Singh, A.P. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: A novel target for therapy. Br. J. Cancer 2010, 103, 1671–1679. [Google Scholar] [CrossRef]
- Arora, S.; Bhardwaj, A.; Singh, S.; Srivastava, S.K.; McClellan, S.; Nirodi, C.S.; Piazza, G.A.; Grizzle, W.E.; Owen, L.B.; Singh, A.P. An undesired effect of chemotherapy: Gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor κB- and hypoxia-inducible factor 1α-mediated up-regulation of CXCR4. J. Biol. Chem. 2013, 288, 21197–21207. [Google Scholar] [CrossRef]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef]
- Bockorny, B.; Macarulla, T.; Semenisty, V.; Borazanci, E.; Feliu, J.; Ponz-Sarvise, M.; Abad, D.G.; Oberstein, P.; Alistar, A.; Muñoz, A.; et al. Motixafortide and Pembrolizumab Combined to Nanoliposomal Irinotecan, Fluorouracil, and Folinic Acid in Metastatic Pancreatic Cancer: The COMBAT/KEYNOTE-202 Trial. Clin. Cancer Res. 2021, 27, 5020–5027. [Google Scholar] [CrossRef]
- Lambert, A.W.; Weinberg, R.A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 2021, 21, 325–338. [Google Scholar] [CrossRef]
- Tsubakihara, Y.; Ohata, Y.; Okita, Y.; Younis, S.; Eriksson, J.; Sellin, M.E.; Ren, J.; Ten Dijke, P.; Miyazono, K.; Hikita, A.; et al. TGFβ selects for pro-stemness over pro-invasive phenotypes during cancer cell epithelial-mesenchymal transition. Mol. Oncol. 2022, 16, 2330–2354. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, C.F.; Bastos, N.; Adem, B.; Batista, I.; Duraes, C.; Melo, C.A.; Castaldo, S.A.; Campos-Laborie, F.; Moutinho-Ribeiro, P.; Morão, B.; et al. Extracellular Vesicles from Pancreatic Cancer Stem Cells Lead an Intratumor Communication Network (EVNet) to fuel tumour progression. Gut 2022, 71, 2043–2068. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Yang, J.; Zhan, H.; Zhou, Z.; Jiang, Y.; Shi, X.; Fan, X.; Zhang, J.; Luo, W.; et al. Zinc-Dependent Regulation of ZEB1 and YAP1 Coactivation Promotes Epithelial-Mesenchymal Transition Plasticity and Metastasis in Pancreatic Cancer. Gastroenterology 2021, 160, 1771–1783.e1771. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.-C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Ding, Q.; Miyazaki, Y.; Kuwahata, T.; Tsukasa, K.; Takao, S. mTOR plays critical roles in pancreatic cancer stem cells through specific and stemness-related functions. Sci. Rep. 2013, 3, 3230. [Google Scholar] [CrossRef]
- Mueller, M.T.; Hermann, P.C.; Witthauer, J.; Rubio-Viqueira, B.; Leicht, S.F.; Huber, S.; Ellwart, J.W.; Mustafa, M.; Bartenstein, P.; D’Haese, J.G.; et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology 2009, 137, 1102–1113. [Google Scholar] [CrossRef]
- Suzuki, S.; Okada, M.; Shibuya, K.; Seino, M.; Sato, A.; Takeda, H.; Seino, S.; Yoshioka, T.; Kitanaka, C. JNK suppression of chemotherapeutic agents-induced ROS confers chemoresistance on pancreatic cancer stem cells. Oncotarget 2015, 6, 458–470. [Google Scholar] [CrossRef]
- Sher, G.; Masoodi, T.; Patil, K.; Akhtar, S.; Kuttikrishnan, S.; Ahmad, A.; Uddin, S. Dysregulated FOXM1 signaling in the regulation of cancer stem cells. Semin. Cancer Biol. 2022, 86, 107–121. [Google Scholar] [CrossRef]
- Nilsson, M.B.; Sun, H.; Robichaux, J.; Pfeifer, M.; McDermott, U.; Travers, J.; Diao, L.; Xi, Y.; Tong, P.; Shen, L.; et al. A YAP/FOXM1 axis mediates EMT-associated EGFR inhibitor resistance and increased expression of spindle assembly checkpoint components. Sci. Transl. Med. 2020, 12, eaaz4589. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Z.; Gao, Y.; Xie, D.; Wei, D.; Cui, J.; Mishra, L.; Huang, S.; Zhang, Y.; Xie, K. Activation of vitamin D receptor signaling downregulates the expression of nuclear FOXM1 protein and suppresses pancreatic cancer cell stemness. Clin. Cancer Res. 2015, 21, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.S.; Moccia, T.; Iannelli, F.; Testa, C.; Vitagliano, C.; Minopoli, M.; Camerlingo, R.; De Riso, G.; De Cecio, R.; Bruzzese, F.; et al. HDAC class I inhibitor domatinostat sensitizes pancreatic cancer to chemotherapy by targeting cancer stem cell compartment via FOXM1 modulation. J. Exp. Clin. Cancer Res. 2022, 41, 83. [Google Scholar] [CrossRef] [PubMed]
- Timaner, M.; Letko-Khait, N.; Kotsofruk, R.; Benguigui, M.; Beyar-Katz, O.; Rachman-Tzemah, C.; Raviv, Z.; Bronshtein, T.; Machluf, M.; Shaked, Y. Therapy-Educated Mesenchymal Stem Cells Enrich for Tumor-Initiating Cells. Cancer Res. 2018, 78, 1253–1265. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Yang, J.; Cui, X.; Zhou, Z.; Zhan, H.; Ding, K.; Tian, X.; Yang, Z.; Fung, K.A.; et al. ZIP4 Increases Expression of Transcription Factor ZEB1 to Promote Integrin α3β1 Signaling and Inhibit Expression of the Gemcitabine Transporter ENT1 in Pancreatic Cancer Cells. Gastroenterology 2020, 158, 679–692.e671. [Google Scholar] [CrossRef]
- van Staalduinen, J.; Baker, D.; Ten Dijke, P.; van Dam, H. Epithelial-mesenchymal-transition-inducing transcription factors: New targets for tackling chemoresistance in cancer? Oncogene 2018, 37, 6195–6211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Kong, D.; Banerjee, S.; Ahmad, A.; Azmi, A.S.; Ali, S.; Abbruzzese, J.L.; Gallick, G.E.; Sarkar, F.H. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009, 69, 2400–2407. [Google Scholar] [CrossRef]
- Lund, K.; Dembinski, J.L.; Solberg, N.; Urbanucci, A.; Mills, I.G.; Krauss, S. Slug-dependent upregulation of L1CAM is responsible for the increased invasion potential of pancreatic cancer cells following long-term 5-FU treatment. PLoS ONE 2015, 10, e0123684. [Google Scholar] [CrossRef]
- Carstens, J.L.; Yang, S.; Correa de Sampaio, P.; Zheng, X.; Barua, S.; McAndrews, K.M.; Rao, A.; Burks, J.K.; Rhim, A.D.; Kalluri, R. Stabilized epithelial phenotype of cancer cells in primary tumors leads to increased colonization of liver metastasis in pancreatic cancer. Cell. Rep. 2021, 35, 108990. [Google Scholar] [CrossRef]
- Semaan, A.; Bernard, V.; Kim, D.U.; Lee, J.J.; Huang, J.; Kamyabi, N.; Stephens, B.M.; Qiao, W.; Varadhachary, G.R.; Katz, M.H.; et al. Characterisation of circulating tumour cell phenotypes identifies a partial-EMT sub-population for clinical stratification of pancreatic cancer. Br. J. Cancer 2021, 124, 1970–1977. [Google Scholar] [CrossRef]
- Richards, K.; Zeleniak, A.; Fishel, M.; Wu, J.; Littlepage, L.; Hill, R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2016, 36, 1770–1778. [Google Scholar] [CrossRef]
- Wei, L.; Ye, H.; Li, G.; Lu, Y.; Zhou, Q.; Zheng, S.; Lin, Q.; Liu, Y.; Li, Z.; Chen, R. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell. Death Dis. 2018, 9, 1065. [Google Scholar] [CrossRef]
- Liu, H.; Shi, Y.; Qian, F. Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Adv. Drug. Deliv. Rev. 2021, 172, 37–51. [Google Scholar] [CrossRef]
- Lotti, F.; Jarrar, A.M.; Pai, R.K.; Hitomi, M.; Lathia, J.; Mace, A.; Gantt, G.A., Jr.; Sukhdeo, K.; DeVecchio, J.; Vasanji, A.; et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J. Exp. Med. 2013, 210, 2851–2872. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Jeong, J.; Lee, D.S.; Hyeon, D.Y.; Park, G.W.; Jeon, S.; Lee, K.B.; Jang, J.Y.; Hwang, D.; Kim, H.M.; et al. PD-L1-directed PlGF/VEGF blockade synergizes with chemotherapy by targeting CD141(+) cancer-associated fibroblasts in pancreatic cancer. Nat. Commun. 2022, 13, 6292. [Google Scholar] [CrossRef]
- Kawahara, K.; Takano, S.; Furukawa, K.; Takayashiki, T.; Kuboki, S.; Ohtsuka, M. The effect of the low stromal ratio induced by neoadjuvant chemotherapy on recurrence patterns in borderline resectable pancreatic ductal adenocarcinoma. Clin. Exp. Metastasis 2022, 39, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Che, L.-H.; Liu, J.-W.; Huo, J.-P.; Luo, R.; Xu, R.-M.; He, C.; Li, Y.-Q.; Zhou, A.-J.; Huang, P.; Chen, Y.-Y.; et al. A single-cell atlas of liver metastases of colorectal cancer reveals reprogramming of the tumor microenvironment in response to preoperative chemotherapy. Cell. Discov. 2021, 7, 80. [Google Scholar] [CrossRef]
- Huanwen, W.; Zhiyong, L.; Xiaohua, S.; Xinyu, R.; Kai, W.; Tonghua, L. Intrinsic chemoresistance to gemcitabine is associated with constitutive and laminin-induced phosphorylation of FAK in pancreatic cancer cell lines. Mol. Cancer 2009, 8, 1–16. [Google Scholar] [CrossRef]
- Takeda, H.; Okada, M.; Suzuki, S.; Kuramoto, K.; Sakaki, H.; Watarai, H.; Sanomachi, T.; Seino, S.; Yoshioka, T.; Kitanaka, C. Rho-associated protein kinase (ROCK) inhibitors inhibit survivin expression and sensitize pancreatic cancer stem cells to gemcitabine. Anticancer Res. 2016, 36, 6311–6318. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Xia, Q.; Paradiso, G.; Ling, J.; Moccia, T.; Carbone, C.; Budillon, A.; Abbruzzese, J.L.; Chiao, P.J. Modulation of pancreatic cancer chemoresistance by inhibition of TAK1. J. Natl. Cancer Inst. 2011, 103, 1190–1204. [Google Scholar] [CrossRef]
- Sun, J.; Russell, C.C.; Scarlett, C.J.; McCluskey, A. Small molecule inhibitors in pancreatic cancer. RSC Med. Chem. 2020, 11, 164–183. [Google Scholar] [CrossRef]
- Murphy, K.J.; Reed, D.A.; Vennin, C.; Conway, J.R.; Nobis, M.; Yin, J.X.; Chambers, C.R.; Pereira, B.A.; Lee, V.; Filipe, E.C. Intravital imaging technology guides FAK-mediated priming in pancreatic cancer precision medicine according to Merlin status. Sci. Adv. 2021, 7, eabh0363. [Google Scholar] [CrossRef]
- Murphy, K.J.; Zhu, J.; Trpceski, M.; Pereira, B.A.; Timpson, P.; Herrmann, D. Focal adhesion kinase priming in pancreatic cancer, altering biomechanics to improve chemotherapy. Biochem. Soc. Trans. 2022, 50, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Tan, Y.; Su, C.; Wang, T.; Gao, Z.; Song, D.; Zhao, J.; Liao, Y.; Liu, X.; Jiang, Y. Inhibition of protein FAK enhances 5-FU chemosensitivity to gastric carcinoma via p53 signaling pathways. Comput. Struct. Biotechnol. J. 2020, 18, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hegde, S.; Knolhoff, B.L.; Zhu, Y.; Herndon, J.M.; Meyer, M.A.; Nywening, T.M.; Hawkins, W.G.; Shapiro, I.M.; Weaver, D.T.; et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016, 22, 851–860. [Google Scholar] [CrossRef]
- Pecoraro, C.; Parrino, B.; Cascioferro, S.; Puerta, A.; Avan, A.; Peters, G.J.; Diana, P.; Giovannetti, E.; Carbone, D. A New Oxadiazole-Based Topsentin Derivative Modulates Cyclin-Dependent Kinase 1 Expression and Exerts Cytotoxic Effects on Pancreatic Cancer Cells. Molecules 2021, 27, 19. [Google Scholar] [CrossRef]
- Wijnen, R.; Pecoraro, C.; Carbone, D.; Fiuji, H.; Avan, A.; Peters, G.J.; Giovannetti, E.; Diana, P. Cyclin dependent kinase-1 (CDK-1) inhibition as a novel therapeutic strategy against pancreatic ductal adenocarcinoma (PDAC). Cancers 2021, 13, 4389. [Google Scholar] [CrossRef]
- Peng, J.; Sun, B.-F.; Chen, C.-Y.; Zhou, J.-Y.; Chen, Y.-S.; Chen, H.; Liu, L.; Huang, D.; Jiang, J.; Cui, G.-S.; et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell. Res. 2019, 29, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Babiker, H.M.; Karass, M.; Recio-Boiles, A.; Chandana, S.R.; McBride, A.; Mahadevan, D. Everolimus for the treatment of advanced pancreatic ductal adenocarcinoma (PDAC). Expert. Opin. Investig. Drugs 2019, 28, 583–592. [Google Scholar] [CrossRef]
- Peng, T.; Dou, Q.P. Everolimus Inhibits Growth of Gemcitabine-Resistant Pancreatic Cancer Cells via Induction of Caspase-Dependent Apoptosis and G2/M Arrest. J. Cell. Biochem. 2017, 118, 2722–2730. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef]
- Zhou, D.C.; Jayasinghe, R.G.; Herndon, J.M.; Storrs, E.; Mo, C.-K.; Wu, Y.; Fulton, R.S.; Wyczalkowski, M.A.; Fronick, C.C.; Fulton, L.A. Spatial drivers and pre-cancer populations collaborate with the microenvironment in untreated and chemo-resistant pancreatic cancer. Nat. Genet. 2021, 54, 1390–1405. [Google Scholar] [CrossRef] [PubMed]

| Combined Treatment Strategy | Target | Target Inhibitor | Chemotherapy | Other Regimens | Phase | (Estimated) Number Enrolled | NCT Number PMID Number |
|---|---|---|---|---|---|---|---|
| Immunotherapy | PD-1 | Camrelizumab | NabPaclitaxel/Gemcitabine | NA | Phase 2 | 117 | NCT04498689 |
| Immunotherapy | PD-1 | Camrelizumab | NabPaclitaxel/Gemcitabine | NA | Phase 3 | 401 | NCT04674956 |
| Immunotherapy | PD-1 | Pembrolizumab | Gemcitabine | Defactinib | Phase 1 | 43 | NCT02546531 |
| Immunotherapy | PD-L1 | Durvalumab | Nab-Paclitaxel/Gemcitabine | Oleclumab | Phase 2 | 30 | NCT04940286 |
| Immunotherapy | CTLA-4 | Ipilimumab | Gemcitabine | NA | Phase 1b | 21 | NCT01473940 |
| Immunotherapy | CTLA-4 | Ipilimumab | FOLFIRINOX | Vaccine | Phase 2 | 83 | NCT01896869 |
| Immunotherapy | CTLA-4 | Zalifrelimab | Nab-Paclitaxel/Gemcitabine | NLM-001 | Phase 1/2 | 24 | NCT04827953 |
| Immunotherapy | LAG3 | IMP321 | Gemcitabine | NA | Phase 1 | 18 | NCT00732082 |
| Targeted therapy | CXCR4 | Motixafortide | Irinotecan/Fluorouracil /Folinic Acid | pembrolizumab | phase 2a | 80 | NCT02826486 |
| Targeted therapy | CXCR4 | Motixafortide | Onivyde/Leucovorin/5FU | pembrolizumab | Phase 2a | 137 | NCT02826486 |
| Targeted therapy | CCR2 | PF-04136309 | FOLFIRINOX | NA | phase 1b | 44 | NCT01413022. |
| Targeted therapy | CCR2 | PF-04136309 | Nab-Paclitaxel/Gemcitabine | NA | phase 1b | NCT02732938 | |
| Targeted therapy | PARP | Veliparib | 5fluorouracil/oxaliplatin | NA | Phase 1/2 | 64 | NCT01489865 |
| Targeted therapy | EGFR | Erlotinib | Gemcitabine | NA | Phase 3 | 569 | PMID:17452677 |
| Targeted therapy | EGFR | Nimotuzumab | Gemcitabine | NA | Phase 2b | 192 | PMID:28961832 |
| Targeted therapy | EGFR | Cetuximab | Gemcitabine | NA | Phase 2 | 41 | PMID:15226328 |
| Targeted therapy | EGFR | Matuzumab | Gemcitabine | NA | Phase 1 | 17 | PMID:16622465 |
| Targeted therapy | VEGF | Bevacizumab | Gemcitabine/capecitabine | erlotinib | Phase 1/2 | 44 | PMID:24613126 |
| Targeted therapy | VEGF | Bevacizumab | Gemcitabine | NA | Phase 2 | 52 | PMID:16258101 |
| Characteristics of Various Sub-Microenvironment | ||
|---|---|---|
| Sub-Microenvironment | Intermediated/Reactive Submicroenvironment | Deserted Sub-Microenvironment |
| CAFs characteristics | CAFs dedifferentiated | CAFs activated and pro-inflammatory |
| Stroma | ECM-rich stroma | ECM-rare stroma |
| Overall immune features | Immune-cold | Immune-hot |
| Immune cell distribution | Small/Scattered immune clusters | Uninterrupted clusters with all immune cells |
| Main immune cell type | CD20 B cells | CD3/CD8 T cells; CD68 macrophages |
| Tumor cells feature | Poorly differentiated tumor cells | Well-differentiated tumor cells |
| Response to chemotherapy | Sensitive to chemotherapy | Resistant to chemotherapy |
| Cellar stress response | Rare | Heat shock; Hypoxia; Metabolic stress |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, M.; Ying, Y.; Zhang, Z.; Liu, L.; Wang, W. Reshaping the Pancreatic Cancer Microenvironment at Different Stages with Chemotherapy. Cancers 2023, 15, 2448. https://doi.org/10.3390/cancers15092448
Peng M, Ying Y, Zhang Z, Liu L, Wang W. Reshaping the Pancreatic Cancer Microenvironment at Different Stages with Chemotherapy. Cancers. 2023; 15(9):2448. https://doi.org/10.3390/cancers15092448
Chicago/Turabian StylePeng, Maozhen, Ying Ying, Zheng Zhang, Liang Liu, and Wenquan Wang. 2023. "Reshaping the Pancreatic Cancer Microenvironment at Different Stages with Chemotherapy" Cancers 15, no. 9: 2448. https://doi.org/10.3390/cancers15092448
APA StylePeng, M., Ying, Y., Zhang, Z., Liu, L., & Wang, W. (2023). Reshaping the Pancreatic Cancer Microenvironment at Different Stages with Chemotherapy. Cancers, 15(9), 2448. https://doi.org/10.3390/cancers15092448





