Decision Tree Analyses for Prediction of QoL over a One-Year Period in Breast Cancer Patients: An Added Value of Patient-Reported Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Group and Treatment
2.2. Study Procedure
2.3. Measures
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics of the Sample
| Demographic Characteristics | N (%) | Medical Characteristics | N (%) |
|---|---|---|---|
| Mean age (SD, range) | 57.4 (9.0, 28–79) | AJCC stage | |
| Marital status | Stage 0 | 15(8.1) | |
| Living alone | 41 (22.2) | Stage I | 95 (51.4) |
| Married/cohabiting | 143 (77.3) | Stage IIA | 43 (23.2) |
| Missing | 1 (0.5) | Stage IIB | 16 (8.6) |
| Education | Stage III | 16 (8.6) | |
| Primary school (7–10 grade) | 48 (25.9) | Comorbidity (Yes) | 48 (25.9) |
| Vocational (1–2 grades) | 59 (31.9) | Cardiovascular | 26 (14.0) |
| High school (2–4 grades) | 24 (13.0) | Pulmonary | 7 (3.8) |
| University, 3 years | 23 (12.4) | Other | 15 (8.1) |
| University >3 years | 29 (15.7) | Breast surgery | |
| Missing | 2 (1.1) | Conservative | 134 (72.4) |
| Employment | Radical | 51 (27.6) | |
| No * | 129 (69.7) | Radiotherapy | |
| Yes | 52 (28.1) | Local | 122 (65.9) |
| Missing | 4 (2.2) | Locoregional | 63 (34.1) |
| Annual family income in NOK | Chemotherapy (Yes) | 77 (41.6) | |
| <300,000 | 31 (16.8) | Endocrine therapy (Yes) | 102 (55.1) |
| 300,000–499,000 | 53 (28.6) | Trastuzumab (Yes) | 24 (13.0) |
| >500,000 | 84 (45.4) | ||
| Missing | 17 (9.2) | ||
| Function | Mean (SD, range) | Symptoms | Mean (SD, range) |
| Physical Function | 87.0 (15.7, 26.7–100) | Fatigue | 25.6 (23.4, 0–100) |
| Role Function | 79.1 (26.8, 0–100) | Pain | 14.9 (23.2, 0–100) |
| Emotional Function | 81.6 (18.9, 25–100) | Insomnia | 24.6 (28.6, 0–100) |
| Cognitive Function | 86.4 (18.4, 0–100) | ||
| Social Function | 79.0 (23.6, 0–100) | Global QoL | 75.7 (19.8, 16.7–100) |
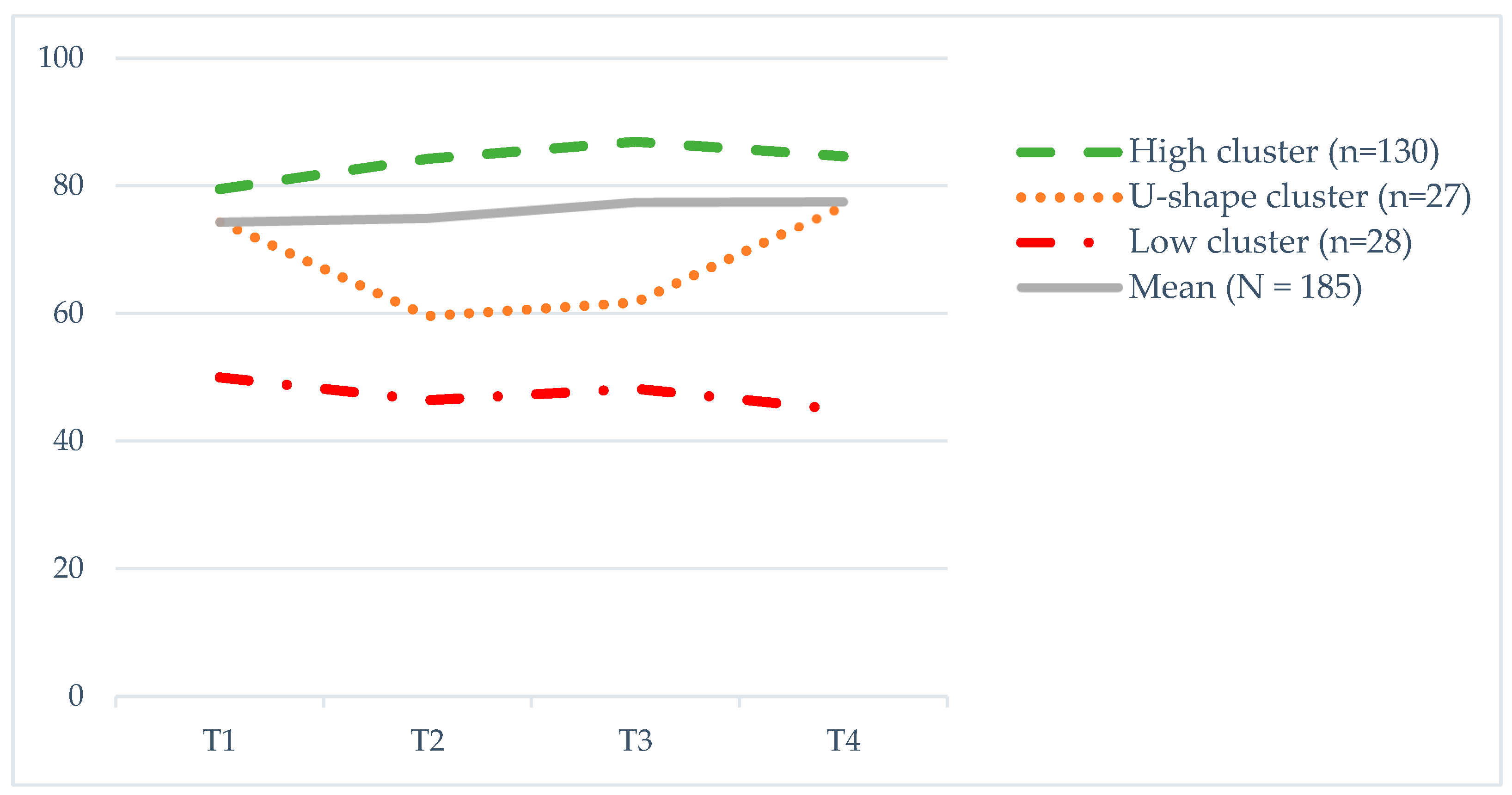
3.2. Clusters of Global QoL Trajectories
3.3. Baseline Characteristics and Differences between Clusters of Global QoL Trajectories
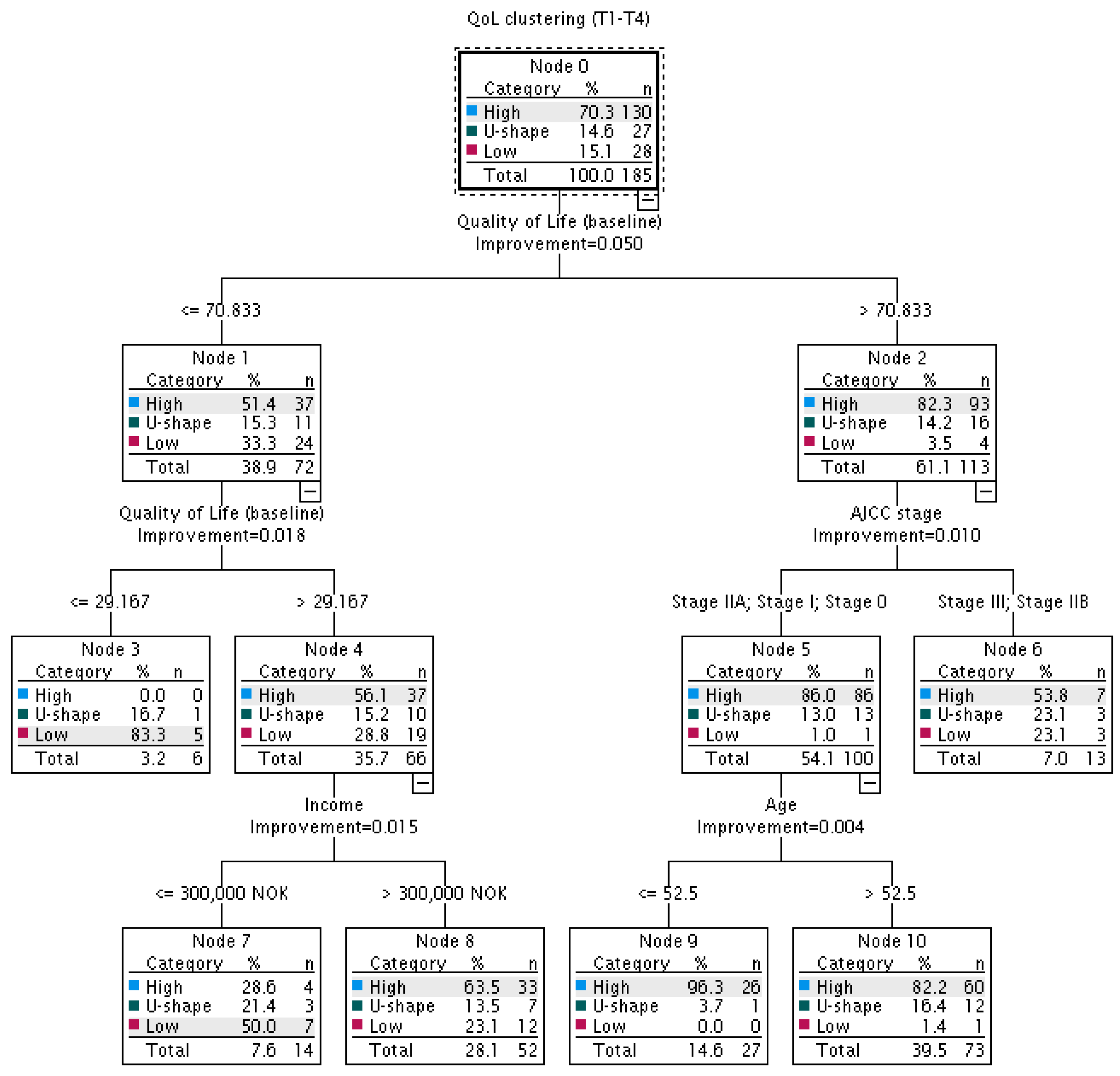
3.4. ‘Basic’ Model—Decision Tree Analysis
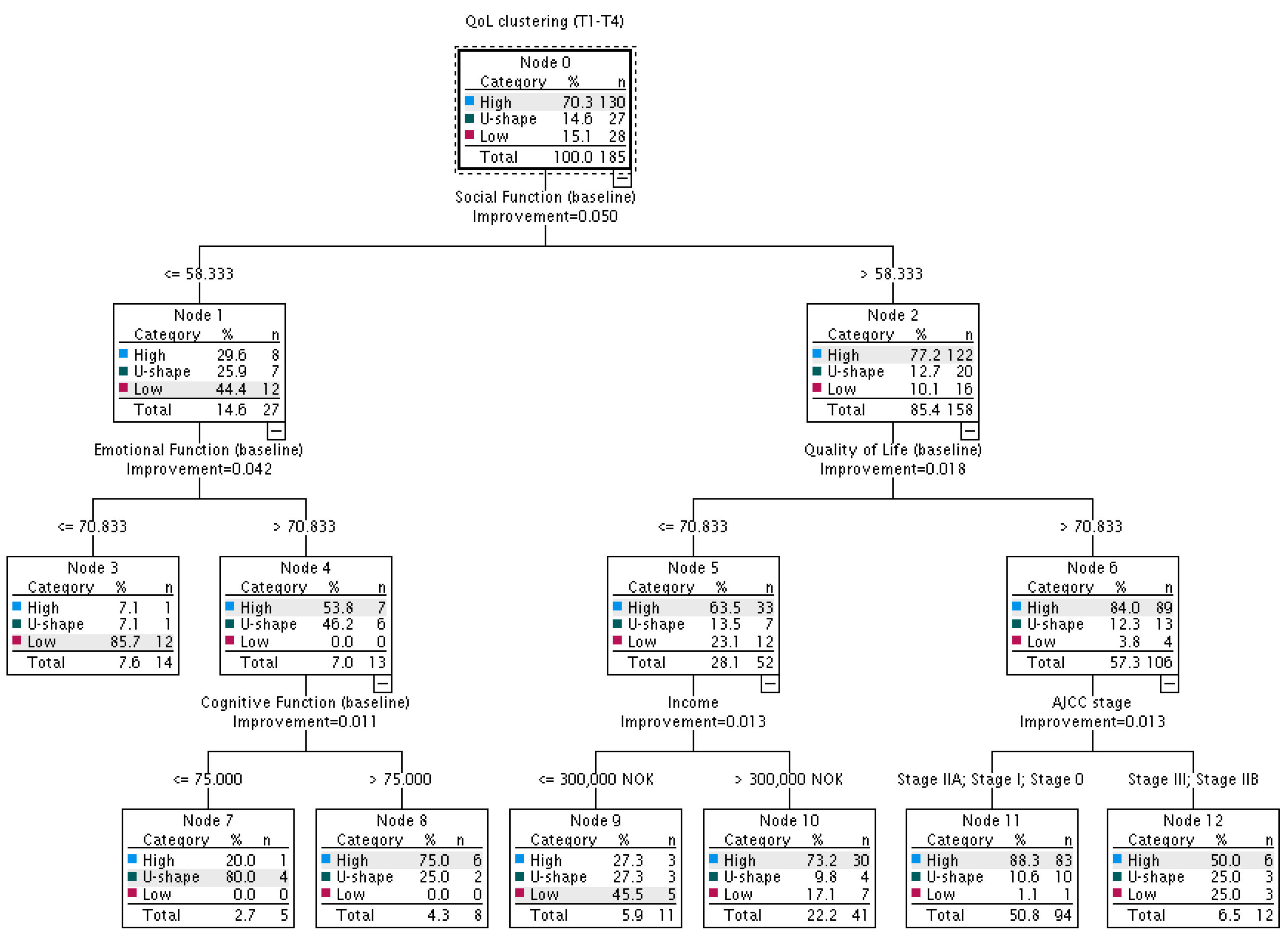
3.5. ‘Enriched’ Model—Decision Tree Analysis
3.6. Comparison of the ‘Basic’ and ‘Enriched’ Decision Trees
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sebri, V.; Durosini, I.; Mazzoni, D.; Pravettoni, G. The Body after Cancer: A Qualitative Study on Breast Cancer Survivors’ Body Representation. Int. J. Environ. Res. Public Health 2022, 19, 12515. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Sansoni, J.; Morris, D.; Grootemaat, P.; Thompson, C. Patient-Reported Outcome Measures: Literature Review. In: Australian Commission of Safety and Quality in Health Care. 2016. Available online: https://www.safetyandquality.gov.au/publications-and-resources/resource-library/patient-reported-outcome-measures-literature-review (accessed on 17 January 2023).
- Wilson, I.B.; Cleary, P.D. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995, 273, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Diener, E. Subjective well-being. Psychol. Bull. 1995, 95, 542–575. [Google Scholar] [CrossRef]
- Benyamini, Y. Self-ratings of health and longevity: A health psychologist’s viewpoint on epidemiological findings. Eur. Health Psychol. 2008, 10, 10–11. [Google Scholar]
- Kotronoulas, G.; Kearney, N.; Maguire, R.; Harrow, A.; Di Domenico, D.; Croy, S.; MacGillivray, S. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J. Clin. Oncol. 2014, 32, 1480–1501. [Google Scholar] [CrossRef]
- Benyamini, Y. Why does self-rated health predict mortality? An update on current knowledge and a research agenda for psychologists. Psychol. Health 2011, 26, 1407–1413. [Google Scholar] [CrossRef]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- Browall, M.; Östlund, U.; Henoch, I.; Wengström, Y. The course of Health Related Quality of Life in postmenopausal women with breast cancer from breast surgery and up to five years post-treatment. Breast 2013, 22, 952–957. [Google Scholar] [CrossRef]
- Hsu, T.; Ennis, M.; Hood, N. Quality of life in long-term breast cancer survivors. J. Clin. Oncol. 2013, 31, 3540–3548. [Google Scholar] [CrossRef]
- Koch, L.; Jansen, L.; Herrmann, A.; Stegmaier, C.; Holleczek, B.; Singer, S.; Brenner, H.; Arndt, V. Quality of life in long-term breast cancer survivors—A 10-year longitudinal population-based study. Acta Oncol. 2013, 52, 1119–1128. [Google Scholar] [CrossRef]
- Lazarewicz, M.A.; Włodarczyk, D.; Lundgren, S.; Reidunsdatter, R.J. Diversity in changes of HRQoL over a 1-year period after radiotherapy in Norwegian breast cancer patients: Results of cluster analyses. Qual. Life Res. 2019, 28, 1521–1530. [Google Scholar] [CrossRef]
- Neuner, J.M.; Zokoe, N.; McGinley, E.L.; Pezzin, L.E.; Yen, T.W.F.; Schapira, M.M.; Nattinger, A.B. Quality of life among a population-based cohort of older patients with breast cancer. Breast 2014, 23, 609–616. [Google Scholar] [CrossRef]
- Abrahams, H.J.G.; Gielissen, M.F.M.; Schmits, I.C.; Verhagen, C.A.H.H.V.M.; Rovers, M.M.; Knoop, H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12,327 breast cancer survivors. Ann. Oncol. 2016, 27, 965–974. [Google Scholar] [CrossRef]
- Fallbjörk, U.; Rasmussen, B.H.; Karlsson, S.; Slander, P. Aspects of body image after mastectomy due to breast cancer—A two-year follow-up study. Eur. J. Oncol. Nurs. 2013, 17, 340–345. [Google Scholar] [CrossRef]
- Liu, L.; Fiorentino, L.; Rissling, M.; Natarajan, L.; Parker, B.A.; Dimsdale, J.E.; Mills, P.J.; Sadler, G.R.; Ancoli-Israel, S. Decreased health-related quality of life in women with breast cancer is associated with poor sleep. Behav. Sleep Med. 2013, 11, 189–206. [Google Scholar] [CrossRef]
- Winters, Z.E.; Afzal, M.; Balta, V.; Freeman, J.; Llewellyn-Bennett, R.; Rayter, Z.; Cook, J.; Greenwood, R.; King, M.T.; Hallam, S.; et al. Patient-reported outcomes and their predictors at 2- and 3-year follow-up after immediate latissimus dorsi breast reconstruction and adjuvant treatment. Br. J. Surg. 2016, 103, 524–536. [Google Scholar] [CrossRef]
- Durá-Ferrandis, E.; Mandelblatt, J.S.; Clapp, J.; Luta, G.; Faul, L.; Kimmick, G.; Cohen, H.J.; Yung, R.L.; Hurria, A. Personality, coping, and social support as predictors of long-term quality-of-life trajectories in older breast cancer survivors: CALGB protocol 369901 (Alliance). Psycho-Oncology 2017, 26, 1914–1921. [Google Scholar] [CrossRef]
- Lopes-Conceição, L.; Brandão, M.; Araújo, N.; Severo, M.; Dias, T.; Peleteiro, B.; Fontes, F.; Pereira, S.; Lunet, N. Quality of life trajectories during the first three years after diagnosis of breast cancer: The NEON-BC study. J. Public Health 2019, 43, 521–531. [Google Scholar] [CrossRef]
- Hamer, J.; McDonald, R.; Zhang, L.; Verma, S.; Leahey, A.; Ecclestone, C.; Bedard, G.; Pulenzas, N.; Bhatia, A.; Chow, R.; et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support. Care Cancer 2017, 25, 409–419. [Google Scholar] [CrossRef]
- Ou, H.T.; Chung, W.P.; Su, P.F.; Lin, T.H.; Lin, J.Y.; Wen, Y.C.; Fang, W. Health-related quality of life associated with different cancer treatments in Chinese breast cancer survivors in Taiwan. Eur. J. Cancer Care 2019, 28, e13069. [Google Scholar] [CrossRef]
- Reidunsdatter, R.J.; Albrektsen, G.; Hjermstad, M.J.; Rannestad, T.; Oldervoll, L.M.; Lundgren, S. One-year course of fatigue after post-operative radiotherapy in Norwegian breast cancer patients—Comparison to general population. Acta Oncol. 2013, 52, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A.; on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual, 3rd ed; European Organization for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- Yim, O.; Ramdeen, K.T. Hierarchical cluster analysis: Comparison of three linkage measures and application to psychological data. Quant. Methods Psychol. 2015, 11, 8–21. [Google Scholar] [CrossRef]
- Leech, N.L.; Barrett, K.C.; Morgan, G.A. IBM SPSS for Intermediate Statistics: Use and Interpretation, 4th ed.; Taylor and Francis Group: New York, NY, USA, 2011. [Google Scholar]
- Podgorelec, V.; Kokol, P.; Stiglic, B.; Rozman, I. Decision trees: An overview and their use in medicine. J. Med. Syst. 2002, 26, 445–463. [Google Scholar] [CrossRef] [PubMed]
- Åsberg, R.E.; Nilsen, M.; Hjermstad, M.J.; Reinertsen, K.V.; Karlsen, J.; Giskeødegård, G.F.; Reidunsdatter, R.J. Norwegian general population normative data for the EORTC questionnaires; the core QLQ-C30, the sexual health questionnaire QLQ-SHQ22, and sexual domains of the QLQ-BR23/BR45. medRxiv 2023. [Google Scholar] [CrossRef]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Lopes-Conceição, L.; Brandão, M.; Araújo, N.; Severo, M.; Dias, T.; Peleteiro, B.; Fontes, F.; Pereira, S.; Lunet, N. Quality of life trajectories in breast cancer patients: An updated analysis 5 years after diagnosis. J. Public Health 2021, 43, e133–e134. [Google Scholar] [CrossRef]
- Sehl, M.; Lu, X.; Silliman, R.; Ganz, P.A. Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J. Cancer Surviv. 2013, 7, 20–31. [Google Scholar] [CrossRef]
- Goyal, N.G.; Levine, B.J.; Van Zee, K.J.; Naftalis, E.; Avis, N.E. Trajectories of quality of life following breast cancer diagnosis. Breast Cancer Res. Treat. 2018, 169, 163–173. [Google Scholar] [CrossRef]
- Boothby, E.J.; Clark, M.S.; Bargh, J.A. Shared experiences are amplified. Psychol. Sci. 2014, 25, 2209–2216. [Google Scholar] [CrossRef]
- Myers, D.G. The funds, friends, and faith of happy people. Am. Psychol. 2000, 55, 56–67. [Google Scholar] [CrossRef]
- Joly, F.; Lange, M.; Dos Santos, M.; Vaz-Luis, I.; Di Meglio, A. Long-term fatigue and cognitive disorders in breast cancer survivors. Cancers 2019, 11, 1896. [Google Scholar] [CrossRef]
- Aizpurua-Perez, I.; Perez-Tejada, J. Resilience in women with breast cancer: A systematic review. Eur. J. Oncol. Nurs. 2020, 49, 101854. [Google Scholar] [CrossRef]
- Savioni, L.; Triberti, S.; Durosini, I.; Sebri, V.; Pravettoni, G. Cancer patients’ participation and commitment to psychological interventions: A scoping review. Psychol. Health 2022, 37, 1022–1055. [Google Scholar] [CrossRef]
| T1 | T2 | T3 | T4 | F(p) | Differences * | |
|---|---|---|---|---|---|---|
| QoL cluster | M (SD) | M (SD) | M (SD) | M (SD) | ||
| High (n = 130) | 79.5 (17.6) | 84.2 (13.3) | 86.9 (11.5) | 84.6 (14.8) | 11.0 (<0.001) a, eta = 0.079 | T1 < T2,T3,T4 |
| U-shape (n = 27) | 74.4 (11.1) | 59.6 (16.6) | 61.7 (12.9) | 76.9 (14.7) | 10.8 (<0.001), eta = 0.293 | T1,T4 > T2,T3 |
| Low (n = 28) | 50.0 (14.2) | 46.4 (13.1) | 48.2 (13.9) | 44.9 (15.8) | 0.797 (0.499) | no sig. dif. |
| N(%) | |||
|---|---|---|---|
| High Cluster (n = 130) | U-Shape Cluster (n = 27) | Low Cluster (n = 28) | |
| Demographic Characteristics | |||
| Mean age (SD, range) | 57.2 (8.99, 28–89) | 55.2 (8.81, 36–71) | 60.3 (8.84, 41–79) |
| Marital status | |||
| Living alone | 25 (19.2) | 9 (33.3) | 7 (25.0) |
| Married/cohabiting | 104 (80.0) | 18 (66.7) | 21 (75.0) |
| Missing | 1 (0.8) | ||
| Education | |||
| Primary school (7–10 grade) | 31 (23.8) | 8 (29.6) | 9 (32.1) |
| Vocational (1–2 grades) | 46 (35.4) | 5 (18.5) | 8 (28.6) |
| High school (2–4 grades) | 15 (11.5) | 4 (14.8) | 5 (17.9) |
| University | 36 (27.7) | 10 (37.0) | 6 (21.4) |
| Missing | 2 (1.5) | ||
| Employment | |||
| No * | 86 (66.2) | 19 (70.4) | 24 (85.7) |
| Yes | 42 (2.3) | 6 (22.2) | 4 (14.3) |
| Missing | 2 (1.5) | 2 (7.4) | |
| Annual family income in NOK | |||
| <300,000 | 16 (12.3) | 5 (18.5) | 10 (35.7) |
| 300,000–499,000 | 40 (30.8) | 6 (22.2) | 7 (25.0) |
| >500,000 | 65 (50.0) | 8 (29.6) | 11 (39.3) |
| Missing | 9 (6.9) | 8 (29.6) | |
| Medical characteristics | |||
| AJCC stage | |||
| Stage 0 | 13 (10.0) | — | 2 (7.1) |
| Stage I | 67 (51.5) | 14 (51.9) | 14 (50.0) |
| Stage IIA | 32 (24.6) | 6 (22.2) | 5 (17.9) |
| Stage IIB | 9 (6.9) | 4 (14.8) | 3 (10.7) |
| Stage III | 9 (6.9) | 3 (11.1) | 4 (14.3) |
| Comorbidity (Yes) | 29 (22.3) | 7 (25.9) | 12 (42.9) |
| Breast surgery | |||
| Conservative | 98 (75.4) | 14 (51.9) | 22 (78.6) |
| Radical | 32 (24.6) | 13 (48.1) | 6 (21.4) |
| Radiotherapy | |||
| Local | 89 (68.5) | 13 (48.1) | 20 (71.4) |
| Locoregional | 41 (31.5) | 14 (51.9) | 8 (28.6) |
| Chemotherapy (Yes) | 55 (42.3) | 15 (55.6) | 7 (25.0) |
| Endocrine therapy (Yes) | 65 (50.0) | 18 (66.7) | 19 (67.9) |
| Trastuzumab (Yes) | 22 (16.9) | 2 (7.4) | — |
| Mean (SD) | Differences * | |||
|---|---|---|---|---|
| High (n = 130) | U-Shape (n = 27) | Low (n = 28) | ||
| Global QoL | 80.8 (16.0) | 72.6 (20.6) | 53.7 (20.3) | H&U > L |
| Physical Function | 89.9 (13.0) | 87.5 (12.4) | 72.8 (21.6) | H&U > L |
| Role Function | 83.7 (24.0) | 70.0 (28.1) | 66.0 (32.5) | H > U&L |
| Emotional Function | 84.9 (16.2) | 85.4 (14.8) | 62.3 (22.9) | H&U > L |
| Cognitive Function | 89.3 (15.9) | 86.1 (16.1) | 72.8 (25.0) | H&U > L |
| Social Function | 84.4 (17.6) | 75.0 (24.6) | 56.8 (32.8) | H&U > L |
| Fatigue | 20.5 (20.6) | 26.2 (23.3) | 49.4 (22.5) | H&U < L |
| Pain | 10.7 (17.5) | 14.0 (21.3) | 35.8 (25.4) | H&U < L |
| Insomnia | 22.3 (27.6) | 18.7 (21.7) | 40.7 (33.8) | H&U < L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarewicz, M.A.; Wlodarczyk, D.; Johansen Reidunsdatter, R. Decision Tree Analyses for Prediction of QoL over a One-Year Period in Breast Cancer Patients: An Added Value of Patient-Reported Outcomes. Cancers 2023, 15, 2474. https://doi.org/10.3390/cancers15092474
Lazarewicz MA, Wlodarczyk D, Johansen Reidunsdatter R. Decision Tree Analyses for Prediction of QoL over a One-Year Period in Breast Cancer Patients: An Added Value of Patient-Reported Outcomes. Cancers. 2023; 15(9):2474. https://doi.org/10.3390/cancers15092474
Chicago/Turabian StyleLazarewicz, Magdalena Anna, Dorota Wlodarczyk, and Randi Johansen Reidunsdatter. 2023. "Decision Tree Analyses for Prediction of QoL over a One-Year Period in Breast Cancer Patients: An Added Value of Patient-Reported Outcomes" Cancers 15, no. 9: 2474. https://doi.org/10.3390/cancers15092474
APA StyleLazarewicz, M. A., Wlodarczyk, D., & Johansen Reidunsdatter, R. (2023). Decision Tree Analyses for Prediction of QoL over a One-Year Period in Breast Cancer Patients: An Added Value of Patient-Reported Outcomes. Cancers, 15(9), 2474. https://doi.org/10.3390/cancers15092474








