Early Adverse Event Derived Biomarkers in Predicting Clinical Outcomes in Patients with Advanced Non-Small Cell Lung Cancer Treated with Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Background of Adverse Event (AE)
1.2. Potential Clinical Utility
1.3. Challenges in AE Analysis
1.3.1. Complexity of AE Data
1.3.2. Limitations of Existing Strategies
1.3.3. Potential Biased Analysis with Less Predictive Value
2. Methods
2.1. Derivation of AE Biomarkers
2.1.1. Treatment Relatedness
2.1.2. Grade
2.1.3. Measurement
2.2. AE Data Type
2.2.1. Overall AE Level
2.2.2. Toxicity Category Level
2.2.3. Individual AE level
2.3. Early AE
2.4. Clinical Outcomes and Statistical Analysis
2.5. Global Discovery Analysis
2.6. Study Cohorts
3. Results
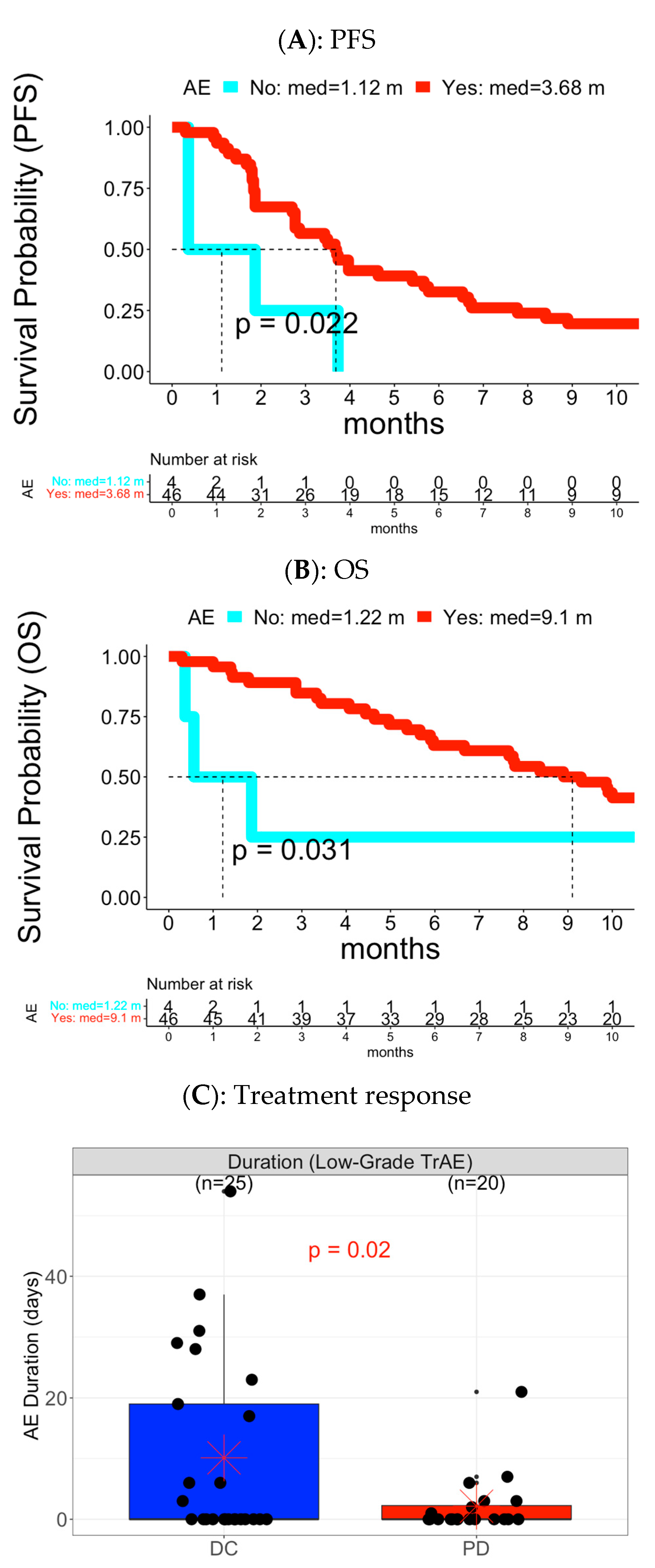
3.1. Cohort A
3.1.1. Analysis of Overall AE Level


3.1.2. Analysis of Toxicity Category Level and Individual AE Level
3.2. Cohort B
3.2.1. Analysis of Overall AE level
3.2.2. Analysis of Toxicity Category Level and Individual AE Level
4. Discussion
4.1. Informative Predictive Biomarkers
4.2. Unique AE Metrics and Full Spectrum of AE Data Analysis
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hommes, J.W.; Verheijden, R.J.; Suijkerbuijk, K.P.M.; Hamann, D. Biomarkers of Checkpoint Inhibitor Induced Immune-Related Adverse Events-A Comprehensive Review. Front. Oncol. 2020, 10, 585311. [Google Scholar] [CrossRef] [PubMed]
- Judd, J.; Zibelman, M.; Handorf, E.; O’Neill, J.; Ramamurthy, C.; Bentota, S.; Doyle, J.; Uzzo, R.G.; Bauman, J.; Borghaei, H.; et al. Immune-Related Adverse Events as a Biomarker in Non-Melanoma Patients Treated with Programmed Cell Death 1 Inhibitors. Oncol. 2017, 22, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Lisberg, A.; Tucker, D.A.; Goldman, J.W.; Wolf, B.; Carroll, J.; Hardy, A.; Morris, K.; Linares, P.; Adame, C.; Spiegel, M.L.; et al. Treatment-Related Adverse Events Predict Improved Clinical Outcome in NSCLC Patients on KEYNOTE-001 at a Single Center. Cancer Immunol. Res. 2018, 6, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Reguart, N.; Auclin, E.; Besse, B. Immune-Related Adverse Events and Outcomes in Patients with Advanced Non-Small Cell Lung Cancer: A Predictive Marker of Efficacy? J. Thorac. Oncol. 2019, 14, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Bai, C.; Xu, J.; Shen, L.; Li, J.; Zhou, Z.; Li, Z.; Chi, Y.; Yu, X.; et al. Treatment-related adverse events as predictive biomarkers of efficacy in patients with advanced neuroendocrine tumors treated with surufatinib: Results from two phase III studies. ESMO Open 2022, 7, 100453. [Google Scholar] [CrossRef]
- Cho, Y.-T.; Lin, Y.-T.; Yang, C.-W.; Chu, C.-Y. Cutaneous immune-related adverse events among Taiwanese cancer patients receiving immune checkpoint inhibitors link to a survival benefit. Sci. Rep. 2022, 12, 7021. [Google Scholar] [CrossRef]
- Dahlberg, S.E.; Sandler, A.B.; Brahmer, J.R.; Schiller, J.H.; Johnson, D.H. Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J. Clin. Oncol. 2010, 28, 949–954. [Google Scholar] [CrossRef]
- Izzedine, H.; Derosa, L.; Le Teuff, G.; Albiges, L.; Escudier, B. Hypertension and angiotensin system inhibitors: Impact on outcome in sunitinib-treated patients for metastatic renal cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2015, 26, 1128–1133. [Google Scholar] [CrossRef]
- Lauko, A.; Thapa, B.; Sharma, M.; Muhsen, B.; Barnett, A.; Rauf, Y.; Borghei-Razavi, H.; Tatineni, V.; Patil, P.; Mohammadi, A.; et al. Neutrophil to lymphocyte ratio influences impact of steroids on efficacy of immune checkpoint inhibitors in lung cancer brain metastases. Sci. Rep. 2021, 11, 7490. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Lalani, A.-K.A.; Xie, W.; Martini, D.J.; Steinharter, J.A.; Norton, C.K.; Krajewski, K.M.; Duquette, A.; Bossé, D.; Bellmunt, J.; Van Allen, E.M.; et al. Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J. ImmunoTherapy Cancer 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Y.; Zhang, B.; Wang, X.; Mo, H.; Jiao, Y.; Xu, J.; Huang, J. Prognostic and predictive impact of neutrophil-to-lymphocyte ratio and HLA-I genotyping in advanced esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitor monotherapy. Thorac. Cancer 2022, 13, 1631–1641. [Google Scholar] [CrossRef]
- Teraoka, S.; Fujimoto, D.; Morimoto, T.; Kawachi, H.; Ito, M.; Sato, Y.; Nagata, K.; Nakagawa, A.; Otsuka, K.; Uehara, K.; et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non–Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J. Thorac. Oncol. 2017, 12, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- George, G.C.; Barata, P.C.; Campbell, A.; Chen, A.; Cortes, J.E.; Hyman, D.M.; Jones, L.; Karagiannis, T.; Klaar, S.; Le-Rademacher, J.G.; et al. Improving attribution of adverse events in oncology clinical trials. Cancer Treat. Rev. 2019, 76, 33–40. [Google Scholar] [CrossRef]
- Luo, H.; Schumacher, O.; Galvao, D.A.; Newton, R.U.; Taaffe, D.R. Adverse Events Reporting of Clinical Trials in Exercise Oncology Research (ADVANCE): Protocol for a Scoping Review. Front. Oncol. 2022, 12, 841266. [Google Scholar] [CrossRef]
- Maillet, D.; Blay, J.Y.; You, B.; Rachdi, A.; Gan, H.K.; Peron, J. The reporting of adverse events in oncology phase III trials: A comparison of the current status versus the expectations of the EORTC members. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2016, 27, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Le-Rademacher, J.; Hillman, S.L.; Meyers, J.; Loprinzi, C.L.; Limburg, P.J.; Mandrekar, S.J. Statistical controversies in clinical research: Value of adverse events relatedness to study treatment: Analyses of data from randomized double-blind placebo-controlled clinical trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2017, 28, 1183–1190. [Google Scholar] [CrossRef]
- Sartor, O. Adverse Event Reporting in Clinical Trials: Time to Include Duration as Well as Severity. Oncologist 2018, 23, 1. [Google Scholar] [CrossRef]
- Carbini, M.; Suarez-Farinas, M.; Maki, R.G. A Method to Summarize Toxicity in Cancer Randomized Clinical Trials. Clin. Cancer Res. 2018, 24, 4968–4975. [Google Scholar] [CrossRef]
- Thanarajasingam, G.; Atherton, P.J.; Novotny, P.J.; Loprinzi, C.L.; Sloan, J.A.; Grothey, A. Longitudinal adverse event assessment in oncology clinical trials: The Toxicity over Time (ToxT) analysis of Alliance trials NCCTG N9741 and 979254. Lancet Oncol. 2016, 17, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Gelber, R.D.; Simes, R.J.; Glasziou, P.; Coates, A.S. Costs and benefits of adjuvant therapy in breast cancer: A quality-adjusted survival analysis. J. Clin. Oncol. 1989, 7, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Razaee, Z.S.; Amini, A.A.; Diniz, M.A.; Tighiouart, M.; Yothers, G.; Rogatko, A. On the properties of the toxicity index and its statistical efficiency. Stat. Med. 2021, 40, 1535–1552. [Google Scholar] [CrossRef]
- Gong, Q.; Tong, B.; Strasak, A.; Fang, L. Analysis of safety data in clinical trials using a recurrent event approach. Pharm. Stat. 2014, 13, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hengelbrock, J.; Gillhaus, J.; Kloss, S.; Leverkus, F. Safety data from randomized controlled trials: Applying models for recurrent events. Pharm. Stat. 2016, 15, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Cabarrou, B.; Gomez-Roca, C.; Viala, M.; Rabeau, A.; Paulon, R.; Loirat, D.; Munsch, N.; Delord, J.P.; Filleron, T. Modernizing adverse events analysis in oncology clinical trials using alternative approaches: Rationale and design of the MOTIVATE trial. Investig. New Drugs 2020, 38, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.C.; Choueiri, T.K.; Feuilly, M.; Meng, J.; Lister, J.; Marteau, F.; Falchook, A.D.; Morris, M.J.; George, D.J.; Feldman, D.R. Quality-adjusted survival with first-line cabozantinib or sunitinib for advanced renal cell carcinoma in the CABOSUN randomized clinical trial (Alliance). Cancer 2020, 126, 5311–5318. [Google Scholar] [CrossRef]
- Lee, C.H.; Wan, Y.; Smith, A.; Xie, R.; Motzer, R.J. Quality-adjusted Time Without Symptoms or Toxicity (Q-TWiST) for Lenvatinib plus Everolimus Versus Everolimus Monotherapy in Patients with Advanced Renal Cell Carcinoma. Eur. Urol. Open Sci. 2021, 31, 1–9. [Google Scholar] [CrossRef]
- Cocks, K.; Contente, M.; Simpson, S.; DeRosa, M.; Taylor, F.C.; Shaw, J.W. A Q-TWiST Analysis Comparing Nivolumab and Therapy of Investigator’s Choice in Patients with Recurrent/Metastatic Platinum-Refractory Squamous Cell Carcinoma of the Head and Neck. Pharmacoeconomics 2019, 37, 1041–1047. [Google Scholar] [CrossRef]
- Huang, M.; Pietanza, M.C.; Samkari, A.; Pellissier, J.; Burke, T.; Chandwani, S.; Kong, F.; Pickard, A.S. Q-TWiST Analysis to Assess Benefit-Risk of Pembrolizumab in Patients with PD-L1-Positive Advanced or Metastatic Non-small Cell Lung Cancer. Pharmacoeconomics 2019, 37, 105–116. [Google Scholar] [CrossRef]
- McDermott, D.F.; Shah, R.; Gupte-Singh, K.; Sabater, J.; Luo, L.; Botteman, M.; Rao, S.; Regan, M.M.; Atkins, M. Quality-adjusted survival of nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone among treatment-naive patients with advanced melanoma: A quality-adjusted time without symptoms or toxicity (Q-TWiST) analysis. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2019, 28, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Giobbie-Hurder, A.; Gelber, R.D.; Regan, M.M. Challenges of guarantee-time bias. J. Clin. Oncol. 2013, 31, 2963–2969. [Google Scholar] [CrossRef] [PubMed]
- Ang, F.L.I.; Rowland, A.; Modi, N.D.; McKinnon, R.A.; Sorich, M.J.; Hopkins, A.M. Early Adverse Events predict Survival Outcomes in HER2-positive Advanced Breast Cancer Patients treated with Lapatinib plus Capecitabine. J. Cancer 2020, 11, 3327–3333. [Google Scholar] [CrossRef]
- Russano, M.; Cortellini, A.; Giusti, R.; Russo, A.; Zoratto, F.; Rastelli, F.; Gelibter, A.; Chiari, R.; Nigro, O.; De Tursi, M.; et al. Clinical outcomes of NSCLC patients experiencing early immune-related adverse events to PD-1/PD-L1 checkpoint inhibitors leading to treatment discontinuation. Cancer Immunol. Immunother. CII 2022, 71, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nature Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef]
- Thanarajasingam, G.; Hubbard, J.M.; Sloan, J.A.; Grothey, A. The Imperative for a New Approach to Toxicity Analysis in Oncology Clinical Trials. J. Natl. Cancer Inst. 2015, 107, djv216. [Google Scholar] [CrossRef]
- Gray, J.E.; Saltos, A.N.; Tanvetyanon, T.; Haura, E.B.; Creelan, B.C.; Antonia, S.J.; Shafique, M.; Zheng, H.; Dai, W.; Saller, J.J.; et al. Phase 1/1b study of pembrolizumab plus vorinostat in advanced/metastatic non-small cell lung cancer. Clin. Cancer Res. 2019, 25, 6623–6632. [Google Scholar] [CrossRef]
- Chiappori, A.A.; Creelan, B.; Tanvetyanon, T.; Gray, J.E.; Haura, E.B.; Thapa, R.; Barlow, M.L.; Chen, Z.; Chen, D.T.; Beg, A.A.; et al. Phase I Study of Taminadenant (PBF509/NIR178), an Adenosine 2A Receptor Antagonist, with or without Spartalizumab (PDR001), in Patients with Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2022, 28, 2313–2320. [Google Scholar] [CrossRef]
- Schmidinger, M.; Vogl, U.M.; Bojic, M.; Lamm, W.; Heinzl, H.; Haitel, A.; Clodi, M.; Kramer, G.; Zielinski, C.C. Hypothyroidism in patients with renal cell carcinoma: Blessing or curse? Cancer 2011, 117, 534–544. [Google Scholar] [CrossRef]
- Buda-Nowak, A.; Kucharz, J.; Dumnicka, P.; Kuzniewski, M.; Herman, R.M.; Zygulska, A.L.; Kusnierz-Cabala, B. Sunitinib-induced hypothyroidism predicts progression-free survival in metastatic renal cell carcinoma patients. Med. Oncol. 2017, 34, 68. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2017, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Garfield, D. Hypothyroidism promotes survival. Lancet Oncol. 2002, 3, 328. [Google Scholar] [CrossRef] [PubMed]
- Assi, H.A.; Asch, A.S.; Machiorlatti, M.; Vesely, S.K.; Ibrahimi, S. Development of thrombocytopenia is associated with improved survival in patients treated with immunotherapy. Future Sci. OA 2020, 6, FSO581. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Liver enzymes, metabolomics and genome-wide association studies: From systems biology to the personalized medicine. World J. Gastroenterol. 2015, 21, 711–725. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Ahmed, H.; ElHalawani, H. Risk of elevated transaminases in non-small cell lung cancer (NSCLC) patients treated with erlotinib, gefitinib and afatinib: A meta-analysis. Expert Rev. Respir. Med. 2016, 10, 223–234. [Google Scholar] [CrossRef]





| Measurement Type | |||||
|---|---|---|---|---|---|
| Occurrence | Sum of All Unique AEs | Sum of All AEs | Sum of All AE Duration | ||
| Grade/ Treatment relatedness | Any-grade | x | x | x | x |
| Treatment related any-grade | x | x | x | x | |
| Low-grade (1 or 2) | x | x | x | x | |
| Treatment related low-grade | x | x | x | x | |
| High-grade (3 or higher) | x | x | x | x | |
| Treatment related high-grade | x | x | x | x | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.-T.; Saltos, A.N.; Rose, T.; Thompson, Z.J.; Thapa, R.; Chiappori, A.; Gray, J.E. Early Adverse Event Derived Biomarkers in Predicting Clinical Outcomes in Patients with Advanced Non-Small Cell Lung Cancer Treated with Immunotherapy. Cancers 2023, 15, 2521. https://doi.org/10.3390/cancers15092521
Chen D-T, Saltos AN, Rose T, Thompson ZJ, Thapa R, Chiappori A, Gray JE. Early Adverse Event Derived Biomarkers in Predicting Clinical Outcomes in Patients with Advanced Non-Small Cell Lung Cancer Treated with Immunotherapy. Cancers. 2023; 15(9):2521. https://doi.org/10.3390/cancers15092521
Chicago/Turabian StyleChen, Dung-Tsa, Andreas N. Saltos, Trevor Rose, Zachary J. Thompson, Ram Thapa, Alberto Chiappori, and Jhanelle E. Gray. 2023. "Early Adverse Event Derived Biomarkers in Predicting Clinical Outcomes in Patients with Advanced Non-Small Cell Lung Cancer Treated with Immunotherapy" Cancers 15, no. 9: 2521. https://doi.org/10.3390/cancers15092521





