Effects of Reoperation Timing on Survival among Recurrent Glioblastoma Patients: A Retrospective Multicentric Descriptive Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- The tumor volume increased by >20–30% or a tumor was rediscovered after radiological disappearance.
- The patient’s clinical status was satisfactory (KS ≥ 70% and PS WHO ≤ gr. 2).
- The tumor was localized, without multifocality.
- The minimum expected tumor volume reduction was above 80%.
2.2. Investigation of IDH Mutation and MGMT Promotor Methylation
2.3. Statistical Methods
3. Results
3.1. Effect of Progression Time on PSS
3.2. Adjusted Model of PPS
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, B.M.; Cloughesy, T.F. Adult glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Van Den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase iii study: 5-year analysis of the eortc-ncic trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Lakomy, R.; Kazda, T.; Selingerova, I.; Poprach, A.; Pospisil, P.; Belanova, R.; Fadrus, P.; Vybihal, V.; Smrcka, M.; Jancalek, R.; et al. Real-World Evidence in Glioblastoma: Stupp’s Regimen after a Decade. Front. Oncol. 2020, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Berger, M.S. Surgical oncology for gliomas: The state of the art. Nat. Rev. Clin. Oncol. 2018, 15, 112–125. [Google Scholar] [CrossRef]
- Darefsky, A.S.; King, J.T., Jr.; Dubrow, R. Adult glioblastoma multiforme survival in the temozolomide era: A population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer 2012, 118, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Neth, B.J.; Carabenciov, I.D.; Ruff, M.W.; Johnson, D.R. Neurologist. Temporal Trends in Glioblastoma Survival: Progress then Plateau. Neurologist 2022, 27, 119–124. [Google Scholar] [CrossRef]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro Oncol. 2013, 15, 4–27. [Google Scholar] [CrossRef]
- Lu, G.; Rao, M.; Zhu, P.; Liang, B.; El-Nazer, R.T.; Fonkem, E.; Bhattacharjee, M.B.; Zhu, J.J. Triple-drug Therapy With Bevacizumab, Irinotecan, and Temozolomide Plus Tumor Treating Fields for Recurrent Glioblastoma: A Retrospective Study. Front. Neurol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Kesari, S.; Ram, Z. EF-14 Trial Investigators. Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: A post hoc analysis of the EF-14 trial. CNS Oncol. 2017, 6, 185–193. [Google Scholar] [CrossRef]
- Ansstas, G.; Tran, D.D. Treatment with Tumor-Treating Fields Therapy and Pulse Dose Bevacizumab in Patients with Bevacizumab-Refractory Recurrent Glioblastoma: A Case Series. Case Rep. Neurol. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Marko, N.F.; Weil, R.J.; Schroeder, J.L.; Lang, F.F.; Suki, D.; Sawaya, R.E. Extent of resection of glioblastoma revisited: Personal ized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J. Clin. Oncol. 2014, 32, 774–782. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neurooncol. 2016, 130, 269–282. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–764; discussion 264–266. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Sporikova, Z.; Slavkovsky, R.; Tuckova, L.; Kalita, O.; Megova Houdova, M.; Ehrmann, J.; Hajduch, M.; Hrabalek, L.; Vaverka, M. IDH1/2 Mutations in Patients With Diffuse Gliomas: A Single Centre Retrospective Massively Parallel Sequencing Analysis. Appl. Immunohistochem. Mol. Morphol. 2022, 30, 178–183. [Google Scholar] [CrossRef]
- Urbanovska, I.; Megova, M.H.; Dwight, Z.; Kalita, O.; Uvirova, M.; Simova, J.; Tuckova, L.; Buzrla, P.; Palecek, T.; Hajduch, M.; et al. IDH Mutation Analysis in Glioma Patients by CADMA Compared with SNaPshot Assay and two Immunohistochemical Methods. Pathol. Oncol. Res. 2019, 25, 971–978. [Google Scholar] [CrossRef]
- Kalita, O.; Sporikova, Z.; Hajduch, M.; Megova Houdova, M.; Slavkovsky, R.; Hrabalek, L.; Halaj, M.; Klementova, Y.; Dolezel, M.; Drabek, J.; et al. The Influence of Gene Aberrations on Survival in Resected IDH Wildtype Glioblastoma Patients: A Single-Institution Study. Curr. Oncol. 2021, 28, 20122. [Google Scholar] [CrossRef]
- Perrini, P.; Gambacciani, C.; Weiss, A.; Pasqualetti, F.; Delishaj, D.; Paiar, F.; Morganti, R.; Vannozzi, R.; Lutzemberger, L. Survival outcomes following repeat surgery for recurrent glioblastoma: A single-center retrospective analysis. J. Neurooncol. 2017, 131, 585–591. [Google Scholar] [CrossRef]
- Yong, R.L.; Wu, T.; Mihatov, N.; Shen, M.J.; Brown, M.A.; Zaghloul, K.A.; Park, G.E.; Park, J.K. Residual tumor volume and patient survival following reoperation for recurrent glioblastoma. J. Neurosurg. 2014, 121, 802–809. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Sheean, T.; Bonney, P.A.; Maurer, A.J.; Teo, C. Aggressive repeat surgery for focally recurrent primary glioblastoma: Outcomes and theoretical framework. Neurosurg. Focus 2015, 38, E11. [Google Scholar] [CrossRef]
- Suchorska, B.; Weller, M.; Tabatabai, G.; Senft, C.; Hau, P.; Sabel, M.C.; Herrlinger, U.; Ketter, R.; Schlegel, U.; Marosi, C.; et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the director trial. Neuro Oncol. 2016, 18, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Hodges, T.; Arko, L.; Shen, M.; Dello Iacono, D.; McNabb, A.; Olsen Bailey, N.; Kreisl, T.N.; Iwamoto, F.M.; Sul, J.; et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J. Clin. Oncol. 2010, 28, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Kim, J.H.; Nam, D.H.; Kim, C.Y.; Chung, S.B.; Kim, Y.H.; Seol, H.J.; Kim, T.M.; Choi, S.H.; Lee, S.H.; et al. A practical scoring system to determine whether to proceed with surgical resection in recurrent glioblastoma. Neuro Oncol. 2013, 15, 1096–1101. [Google Scholar] [CrossRef]
- Cote, D.J.; Balak, N.; Brennum, J.; Holsgrove, D.T.; Kitchen, N.; Kolenda, H.; Moojen, W.A.; Schaller, K.; Robe, P.A.; Mathiesen, T.; et al. Ethical difficulties in the innovative surgical treatment of patients with recurrent glioblastoma multi forme. J. Neurosurg. 2017, 126, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576; discussion 564–576. [Google Scholar] [CrossRef]
- Stummer, W.; Meinel, T.; Ewelt, C.; Martus, P.; Jakobs, O.; Felsberg, J.; Reifenberger, G. Prospective cohort study of radio therapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J. Neurooncol. 2012, 108, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Tonn, J.C.; Mehdorn, H.M.; Nestler, U.; Franz, K.; Goetz, C.; Bink, A.; Pichlmeier, U.; ALA-Glioma Study Group. Counterbalancing risks and gains from extended resections in malignant glioma surgery: A supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J. Neurosurg. 2011, 114, 613–623. [Google Scholar] [CrossRef]
- Roh, T.H.; Kang, S.G.; Moon, J.H.; Sung, K.S.; Park, H.H.; Kim, S.H.; Kim, E.H.; Hong, C.K.; Suh, C.O.; Chang, J.H. Survival benefit of lobectomy over gross-total resection without lobectomy in cases of glioblastoma in the noneloquent area: A retrospective study. J. Neurosurg. 2019, 132, 895–901. [Google Scholar] [CrossRef]
- Rubin, M.C.; Sagberg, L.M.; Jakola, A.S.; Solheim, O. Primary versus recurrent surgery for glioblastoma-a prospective cohort study. Acta Neurochir. 2022, 164, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Wirtz, C.R.; König, R.W. Impact of extent of resection and recurrent surgery on clinical outcome and overall survival in a consecutive series of 170 patients for glioblastoma in intraoperative high field magnetic resonance imaging. J. Neurosurg. Sci. 2017, 61, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Colamaria, A.; Fochi, N.P.; Sacco, M.; Landriscina, M.; Parbonetti, G.; de Notaris, M.; Coppola, G.; De Santis, E.; Giordano, G.; et al. Recurrent Glioblastoma Treatment: State of the Art and Future Perspectives in the Precision Medicine Era. Biomedicines 2022, 10, 1927. [Google Scholar] [CrossRef]
- Yoo, J.; Yoon, S.J.; Kim, K.H.; Jung, I.H.; Lim, S.H.; Kim, W.; Yoon, H.I.; Kim, S.H.; Sung, K.S.; Roh, T.H.; et al. Patterns of recurrence according to the extent of resection in patients with IDH-wild-type glioblastoma: A retrospective study. J. Neurosurg. 2021, 137, 533–543. [Google Scholar] [CrossRef]
- Tatari, N.; Khan, S.; Livingstone, J.; Zhai, K.; Mckenna, D.; Ignatchenko, V.; Chokshi, C.; Gwynne, W.D.; Singh, M.; Revill, S.; et al. The proteomic landscape of glioblastoma recurrence reveals novel and targetable immunoregulatory drivers. Acta Neuropathol. 2022, 144, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.T.; Timmons, J.; Callahan, A.; O’Loughlin, L.; Giarusso, B.; Alsop, D.C. Phase I study of low-dose metronomic temozolomide for recurrent malignant gliomas. BMC Cancer 2016, 16, 914. [Google Scholar] [CrossRef] [PubMed]
- Van Vugt, V.A.; Piccioni, D.E.; Brown, B.D.; Brown, T.; Saria, M.G.; Juarez, T.; Kesari, S. Retrospective analysis of safety and feasibility of a 3 days on/11 days off temozolomide dosing regimen in recurrent adult malignant gliomas. CNS Oncol. 2014, 3, 257–265. [Google Scholar] [CrossRef]
- Han, S.J.; Rolston, J.D.; Molinaro, A.M.; Clarke, J.L.; Prados, M.D.; Chang, S.M.; Berger, M.S.; DeSilva, A.; Butowski, N.A. Phase II trialof 7 days on/7 days off temozolmide for recurrent high-grade glioma. Neuro Oncol. 2014, 16, 1255–1262. [Google Scholar] [CrossRef]
- Omuro, A.; Chan, T.A.; Abrey, L.E.; Khasraw, M.; Reiner, A.S.; Kaley, T.J.; Deangelis, L.M.; Lassman, A.B.; Nolan, C.P.; Gavrilovic, I.T.; et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol. 2013, 15, 242–250. [Google Scholar] [CrossRef]
- Archavlis, E.; Tselis, N.; Birn, G.; Ulrich, P.; Baltas, D.; Zamboglou, N. Survival analysis of HDR brachytherapy versus reoperation versus temozolomide alone: A retrospective cohort analysis of recurrent glioblastoma multiforme. BMJ Open 2013, 3, e002262. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Lesser, G.J.; Drappatz, J.; Ligon, K.L.; Hammond, S.N.; Lee, E.Q.; Reardon, D.R.; Fadul, C.E.; Plotkin, S.R.; Batchelor, T.T.; et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol. 2013, 15, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Arakawa, Y.; Ueba, T.; Oda, M.; Nishida, N.; Akiyama, Y.; Tsukahara, T.; Iwasaki, K.; Mikuni, N.; Miyamoto, S. Phase I/II Study of Temozolomide Plus Nimustine Chemotherapy for Recurrent Malignant Gliomas: Kyoto Neuro-oncology Group. Neurol. Med.-Chir. 2017, 57, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, L.; Yang, T.; Qiao, Y.; Xia, Y.; Liu, L.; Li, C.; Lu, P.; Jiang, X. A pilot clinical study of apatinib plus irinotecan in patients with recurrent high-grade glioma: Clinical Trial/Experimental Study. Medicine 2017, 96, e9053. [Google Scholar] [CrossRef] [PubMed]
- Reynés, G.; Martínez-Sales, V.; Vila, V.; Balañá, C.; Pérez-Segura, P.; Vaz, M.A.; Benavides, M.; Gallego, O.; Palomero, I.; Gil-Gil, M.; et al. Phase II trial of irinotecan and metronomic temozolomide in patients with recurrent glioblastoma. Anticancer Drugs 2016, 27, 133–137. [Google Scholar] [CrossRef]
- Di Cristofori, A.; Zarino, B.; Fanizzi, C.; Fornara, G.A.; Bertani, G.; Rampini, P.; Carrabba, G.; Caroli, M. Analysis of factors influencing the access to concomitant chemo-radiotherapy in elderly patients with high grade gliomas: Role of MMSE, age and tumor volume. J. Neurooncol. 2017, 134, 377–385. [Google Scholar] [CrossRef]
- Franceschi, E.; Stupp, R.; van den Bent, M.J.; van Herpen, C.; Laigle Donadey, F.; Gorlia, T.; Hegi, M.; Lhermitte, B.; Strauss, L.C.; Allgeier, A.; et al. EORTC 26083 phase I/II trial of dasatinib in combination with CCNU in patients with recurrent glioblastoma. Neuro Oncol. 2012, 14, 1503–1510. [Google Scholar] [CrossRef]
- Klein, J.; Juratli, T.A.; Radev, Y.; Daubner, D.; Soucek, S.; Schackert, G.; Krex, D. Safety and Effectiveness of Bis-Chloroethylnitrosourea Wafer Chemotherapy in Elderly Patients with Recurrent Glioblastoma. Oncology 2017, 93, 43–50. [Google Scholar] [CrossRef]
- Minniti, G.; Niyazi, M.; Alongi, F.; Navarria, P.; Belka, C. Current status and recent advances in reirradiation of glioblastoma. Radiat. Oncol. 2021, 16, 36. [Google Scholar] [CrossRef]
- Tsien, C.I.; Pugh, S.L.; Dicker, A.P.; Raizer, J.J.; Matuszak, M.M.; Lallana, E.C.; Huang, J.; Algan, O.; Deb, N.; Portelance, L.; et al. NRG Oncology/RTOG1205: A Randomized Phase II Trial of Concurrent Bevacizumab and Reirradiation Versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma. J. Clin. Oncol. 2022, 41, JCO2200164. [Google Scholar] [CrossRef]
- Ciammella, P.; Podgornii, A.; Galeandro, M.; D’Abbiero, N.; Pisanello, A.; Botti, A.; Cagni, E.; Iori, M.; Iotti, C. Hypofractionated stereotactic radiation therapy for recurrent glioblastoma: Single institutional experience. Radiat. Oncol. 2013, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Straube, C.; Antoni, S.; Gempt, J.; Zimmer, C.; Meyer, B.; Schlegel, J.; Schmidt-Graf, F.; Combs, S.E. Re-irradiation in elderly patients with glioblastoma: A single institution experience. J. Neurooncol. 2019, 142, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, E.; Lampl, C.; Track, C.; Nieder, C.; Pichler, J.; Hammer, J.; Geinitz, H. Re-irradiation of recurrent glioblastoma as part of a sequential multimodality treatment concept. Clin. Transl. Oncol. 2019, 21, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, K.; Paulsen, F.; Schittenhelm, J.; Borchers, C.; Skardelly, M.; Zips, D.; Eckert, F. Prognostic parameters and outcome after re-irradiation for progressive glioblastoma. Acta Neurol. Scand. 2017, 136, 239–245. [Google Scholar] [CrossRef]
- Hasan, S.; Chen, E.; Lanciano, R.; Yang, J.; Hanlon, A.; Lamond, J.; Arrigo, S.; Ding, W.; Mikhail, M.; Ghaneie, A.; et al. Salvage Fractionated Stereotactic Radiotherapy with or without Chemotherapy and Immunotherapy for Recurrent Glioblastoma Multiforme: A Single Institution Experience. Front. Oncol. 2015, 5, 106. [Google Scholar] [CrossRef]
- Pinzi, V.; Orsi, C.; Marchetti, M.; Milanesi, I.M.; Bianchi, L.C.; DiMeco, F.; Cuccarini, V.; Farinotti, M.; Ferroli, P.; Finocchiaro, G.; et al. Radiosurgery reirradiation for high-grade glioma recurrence: A retrospective analysis. Neurol. Sci. 2015, 36, 1431–1440. [Google Scholar] [CrossRef]
- Greenspoon, J.N.; Sharieff, W.; Hirte, H.; Overholt, A.; Devillers, R.; Gunnarsson, T.; Whitton, A. Fractionated stereotactic radiosurgery with concurrent temozolomide chemotherapy for locally recurrent glioblastoma multiforme: A prospective cohort study. OncoTargets Ther. 2014, 7, 485–490. [Google Scholar] [CrossRef]
- Yazici, G.; Cengiz, M.; Ozyigit, G.; Eren, G.; Yildiz, F.; Akyol, F.; Gurkaynak, M.; Zorlu, F. Hypofractionated stereotactic reirradiation for recurrent glioblastoma. J. Neurooncol. 2014, 120, 117–123. [Google Scholar] [CrossRef]
- Straube, C.; Kessel, K.A.; Zimmer, C.; Schmidt-Graf, F.; Schlegel, J.; Gempt, J.; Meyer, B.; Combs, S.E. A Second Course of Radiotherapy in Patients with Recurrent Malignant Gliomas: Clinical Data on Re-irradiation, Prognostic Factors, and Usefulness of Digital Biomarkers. Curr. Treat. Options Oncol. 2019, 20, 71. [Google Scholar] [CrossRef]
- Li, X.; Jia, Z.; Yan, Y. Efficacy and safety of tumor-treating fields in recurrent glioblastoma: A systematic review and meta-analysis. Acta Neurochir. 2022, 164, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Pirzkall, A.; McGue, C.; Saraswathy, S.; Cha, S.; Liu, R.; Vandenberg, S.; Lamborn, K.R.; Berger, M.S.; Chang, S.M.; Nelson, S.J. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2009, 11, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Meyer, J.E.; Han, S.J.; Cha, S.; Butowski, N.A. Early tumor growth between initial resection and radiotherapy of glioblastoma: Incidence and impact on clinical outcomes. J. Neurooncol. 2017, 134, 213–219. [Google Scholar] [CrossRef]
- Palmer, J.D.; Bhamidipati, D.; Shukla, G.; Sharma, D.; Glass, J.; Kim, L.; Evans, J.J.; Judy, K.; Farrell, C.; Andrews, D.W.; et al. Rapid Early Tumor Progression is Prognostic in Glioblastoma Patients. Am. J. Clin. Oncol. 2019, 42, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Lakomy, R.; Kazda, T.; Selingerova, I.; Poprach, A.; Pospisil, P.; Belanova, R.; Fadrus, P.; Smrcka, M.; Vybihal, V.; Jancalek, R.; et al. Pre-Radiotherapy Progression after Surgery of Newly Diagnosed Glioblastoma: Corroboration of New Prognostic Variable. Diagnostics 2020, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- De Barros, A.; Attal, J.; Roques, M.; Nicolau, J.; Sol, J.C.; Cohen-Jonathan-Moyal, E.; Roux, F.E. Impact on survival of early tumor growth between surgery and radiotherapy in patients with de novo glioblastoma. J. Neurooncol. 2019, 142, 489–497. [Google Scholar] [CrossRef]
- Wee, C.W.; Kim, E.; Kim, T.M.; Park, C.K.; Kim, J.W.; Choi, S.H.; Yoo, R.E.; Lee, S.T.; Kim, I.H. Impact of interim progression during the surgery-to-radiotherapy interval and its predictors in glioblastoma treated with temozolomide-based radiochemotherapy. J. Neurooncol. 2017, 134, 169–175. [Google Scholar] [CrossRef]
- Merkel, A.; Soeldner, D.; Wendl, C.; Urkan, D.; Kuramatsu, J.B.; Seliger, C.; Proescholdt, M.; Eyupoglu, I.Y.; Hau, P.; Uhl, M. Early postoperative tumor progression predicts clinical outcome in glioblastoma-implication for clinical trials. J. Neurooncol. 2017, 132, 249–254. [Google Scholar] [CrossRef]
- Majós, C.; Cos, M.; Castañer, S.; Pons, A.; Gil, M.; Fernández-Coello, A.; Macià, M.; Bruna, J.; Aguilera, C. Preradiotherapy MR. Imaging: A Prospective Pilot Study of the Usefulness of Performing an MR Examination Shortly before Radiation Therapy in Patients with Glioblastoma. AJNR Am. J. Neuroradiol. 2016, 37, 2224–2230. [Google Scholar] [CrossRef]
- Farace, P.; Amelio, D.; Ricciardi, G.K.; Zoccatelli, G.; Magon, S.; Pizzini, F.; Alessandrini, F.; Sbarbati, A.; Amichetti, M.; Beltramello, A. Early MRI changes in glioblastoma in the period between surgery and adjuvant therapy. J. Neurooncol. 2013, 111, 177–185. [Google Scholar] [CrossRef]
- Waqar, M.; Roncaroli, F.; Lehrer, E.J.; Palmer, J.D.; Villanueva-Meyer, J.; Braunstein, S.; Hall, E.; Aznar, M.; De Witt Hamer, P.C.; D’Urso, P.I.; et al. Rapid early progression (REP) of glioblastoma is an independent negative prognostic factor: Results from a systematic review and meta-analysis. Neurooncol. Adv. 2022, 4, vdac075. [Google Scholar] [CrossRef]
- Boiardi, A.; Silvani, A.; Eoli, M.; Lamperti, E.; Salmaggi, A.; Gaviani, P.; Fiumani, A.; Botturi, A.; Falcone, C.; Solari, A.; et al. Treatment of recurrent glioblastoma: Can local delivery of mitoxantrone improve survival? J. Neurooncol. 2008, 88, 105–113. [Google Scholar] [CrossRef]
- Chen, Y.R.; Sole, J.; Ugiliweneza, B.; Johnson, E.; Burton, E.; Woo, S.Y.; Koutourousiou, M.; Williams, B.; Boakye, M.; Skirboll, S. National trends for reoperation in older patients with glioblastoma. World Neurosurg. 2018, 113, e179–e189. [Google Scholar] [CrossRef]
- Delgado-Fernandez, J.; Garcia-Pallero, M.Á.; Blasco, G.; Penanes, J.R.; Gil-Simoes, R.; Pulido, P.; Sola, R.G. Usefulness of reintervention in recurrent glioblastoma: An indispensable weapon for increasing survival. World Neurosurg. 2017, 108, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Skeie, B.S.; Enger, P.Ø.; Brøgger, J.; Ganz, J.C.; Thorsen, F.; Heggdal, J.I.; Pedersen, P.H. Gamma knife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg. 2012, 78, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Wann, A.; Tully, P.A.; Barnes, E.H.; Lwin, Z.; Jeffree, R.; Drummond, K.J.; Gan, H.; Khasraw, M. Outcomes after second surgery for recurrent glioblastoma: A retrospective case-control study. J. Neurooncol. 2018, 137, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Zanello, M.; Roux, A.; Ursu, R.; Peeters, S.; Bauchet, L.; Noel, G.; Guyotat, J.; Le Reste, P.J.; Faillot, T.; Litre, F.; et al. Recurrent glioblastomas in the elderly after maximal first-line treatment: Does preserved overall condition warrant a maximal second-line treatment? J. Neurooncol. 2017, 135, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Zadnik, P.; Weingart, J.D.; Olivi, A.; Gallia, G.L.; Blakeley, J.; Lim, M.; Brem, H.; Quiñones-Hinojosa, A. Multiple resections for patients with glioblastoma: Prolonging survival. J. Neurosurg. 2013, 118, 812–820. [Google Scholar] [CrossRef]
- Stark, A.M.; Hedderich, J.; Held-Feindt, J.; Mehdorn, H.M. Glioblastoma–the consequences of advanced patient age on treatment and survival. Neurosurg. Rev. 2007, 30, 56–61; discussion 61–62. [Google Scholar] [CrossRef]
- Azoulay, M.; Santos, F.; Shenouda, G.; Petrecca, K.; Oweida, A.; Guiot, M.C.; Owen, S.; Panet-Raymond, V.; Souhami, L.; Abdulkarim, B.S. Benefit of re-operation and salvage therapies for recurrent glioblastoma multiforme: Results froma single institution. J. Neurooncol. 2017, 132, 419–426. [Google Scholar] [CrossRef]
- Clarke, J.L.; Ennis, M.M.; Yung, W.K.; Chang, S.M.; Wen, P.Y.; Cloughesy, T.F.; Deangelis, L.M.; Robins, H.I.; Lieberman, F.S.; Fine, H.A.; et al. Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol. 2011, 13, 1118–1124. [Google Scholar] [CrossRef]
- Ortega, A.; Sarmiento, J.M.; Ly, D.; Nuno, M.; Mukherjee, D.; Black, K.L.; Patil, C.G. Multiple resections and survival of recurrent glioblastoma patients in the temozolomide era. J. Clin. Neurosci. 2016, 24, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tugcu, B.; Postalci, L.S.; Gunaldi, O.; Tanriverdi, O.; Akdemir, H. Efficacy of clinical prognostic factors on survival in patients with glioblastoma. Turk. Neurosurg. 2010, 20, 117–125. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quiñones-Hinojosa, A.R. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef]
- Lu, V.M.; Jue, T.R.; McDonald, K.L.; Rovin, R.A. The survival effect of repeat surgery at glioblastoma recurrence and its trend: A systematic review and metaanalysis. World Neurosurg. 2018, 115, 453–459.e3. [Google Scholar] [CrossRef]
- Nava, F.; Tramacere, I.; Fittipaldo, A.; Bruzzone, M.G.; DiMeco, F.; Fariselli, L.; Finocchiaro, G.; Pollo, B.; Salmaggi, A.; Silvani, A.; et al. Survival effect of first- and second-line treatments for patients with primary glioblastoma: A cohort study from a prospective registry, 1997–2010. Neuro Oncol. 2014, 16, 719–727. [Google Scholar] [CrossRef]
- Voisin, M.R.; Zuccato, J.A.; Wang, J.Z.; Zadeh, G. Surgery for Recurrent Glioblastoma Multiforme: A Retrospective Case Control Study. World Neurosurg. 2022, 166, e624–e631. [Google Scholar] [CrossRef]
- Goldman, D.A.; Hovinga, K.; Reiner, A.S.; Esquenazi, Y.; Tabar, V.; Panageas, K.S. The relationship between repeat resection and overall survival in patients with glioblastoma: A time-dependent analysis. J. Neurosurg. 2018, 129, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, J.; Gastmeier, P.; Wolkewitz, M.; Schumacher, M. An easy mathematical proof showed that time-dependent bias inevitably leads to biased effect estimation. J. Clin. Epidemiol. 2008, 61, 1216–1221. [Google Scholar] [CrossRef]
- Anderson, J.R.; Cain, K.C.; Gelber, R.D. Analysis of survival by tumor response. J. Clin. Oncol. 1983, 1, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Wang, Z.F.; Pan, Z.Y.; Péus, D.; Delgado-Fernandez, J.; Pallud, J.; Li, Z.Q. A Meta-Analysis of Survival Outcomes. Following Reoperation in Recurrent Glioblastoma: Time to Consider the Timing of Reoperation. Front. Neurol. 2019, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Clavreul, A.; Autier, L.; Lemée, J.M.; Augereau, P.; Soulard, G.; Bauchet, L.; Figarella-Branger, D.; Menei, P.; Network, F.G. Management of Recurrent Glioblastomas: What Can We Learn from the French Glioblastoma Biobank? Cancers 2022, 14, 5510. [Google Scholar] [CrossRef] [PubMed]
| Variables | Median (Min–Max); Number (%) |
|---|---|
| Age | |
| Years | 55 (24–79) |
| Follow-Up | |
| Months | 20.3 (4.1–158.6) |
| Time to Progression (TTP) | |
| Months | 10.1 (0.1–75.5) |
| Overall Survival (OS) | |
| Events (%) | 88 (83%) |
| Median (95% CI) | 22.5 (19.4, 29.9) |
| 1y-surv. ± SE[%] | 85 ± 3.5 |
| 2y-surv. ± SE[%] | 48 ± 5.1 |
| Post-Progression Surgery Survival (PSS) | |
| Events (%) | 88 (83%) |
| Median (95% CI) | 9.8 (8.5, 12.5) |
| 1y-surv. ± SE[%] | 40 ± 5 |
| 2y-surv. ± SE[%] | 33 ± 6 |
| Sex | |
| Women | 48 (45.3%) |
| Men | 58 (54.7%) |
| IDH Methylation Status | |
| wt | 67 (88.2%) |
| mt | 9 (11.8%) |
| Unknown | 30 patients |
| MGMT Methylation | |
| No | 30 (58.8%) |
| Yes | 21 (41.2%) |
| Unknown | 55 patients |
| Radicality of Initial Surgery | |
| GTR | 60 (60.6.%) |
| STR or Partial Resection | 39 (39.4%) |
| Unknown | 7 patients |
| Stupp Protocol After Initial Surgery | |
| No | 12 (11.8%) |
| Yes | 90 (88.2%) |
| Unknown | 4 patients |
| Oncotherapy After Initial Surgery | |
| No therapy | 5 (4.9%) |
| RT Only | 7 (6.9%) |
| ChemoRT + Adj. CHT | 71 (69.6%) |
| ChemoRT Only | 14 (13.7%) |
| RT Only and Then CHT Only | 5 (4.9%) |
| Unknown | 4 patients |
| Karnofsky Score (Before Surgery-1stPD) | |
| <80 | 19 (20.4%) |
| 80–100 | 74 (79.6%) |
| Unknown | 13 patients |
| Radicality of Surgery-1stPD | |
| GTR | 40 (56.3%) |
| STR or Partial | 31 (43.7%) |
| Unknown | 35 patients |
| Oncotherapy After Surgery-1stPD | |
| No Therapy | 8 (13.1%) |
| RT | 4 (6.6%) |
| CHT | 40 (65.6%) |
| ChemoRT and Adj. CHT | 4 (6.6%) |
| ChemoRT | 2 (3.3%) |
| RT Only and Then CHT Only | 2 (3.3%) |
| Other | 1 (1.6%) |
| Unknown | 45 patients |
| Characteristic | N | TTP | PSS | |||
|---|---|---|---|---|---|---|
| (Total 109) | Median (Min–Max) | p-Value * | Log-Rank, p-Value | Cox Regression, HR (95% CI) | Cox Regression, p-Value | |
| Sex | ||||||
| Female | 48 | 10.5 (0.1–32) | 0.399 | 0.132 | ||
| Male | 58 | 9.8 (1.5–75.5) | 1.4 (0.9, 2.14) | 0.133 | ||
| IDH status | ||||||
| Wild-type | 67 | 9.3 (0.1–55.7) | 0.436 | 0.263 | ||
| Mutated | 9 | 11.2 (3.9–48.1) | 0.6 (0.26, 1.45) | 0.267 | ||
| MGMT Status | ||||||
| Unmetylated | 30 | 8.2 (1.5–28.3) | 0.645 | 0.379 | ||
| Metylated | 21 | 8.2 (1.9–25.4) | 0.7 (0.38, 1.45) | 0.381 | ||
| Radicality of Initial Surgery | - | - | ||||
| GTR | 60 | 10.3 (0.6–73.6) | 0.652 | |||
| STR or Partial Resection | 39 | 9.7 (0.1–75.5) | ||||
| Stupp Protocol After Initial Surgery | - | - | ||||
| No | 12 | 5.8 (0.1–27) | 0.040 | |||
| Yes | 90 | 10.7 (1.2–75.5) | ||||
| Karnofsky Score Before Surgery-1stPD # | ||||||
| <80 | 19 | 9.3 (1.9–25.4) | 0.394 | < 0.001 | ||
| 80–100 | 74 | 10.9 (0.1–75.5) | ||||
| Radicality of Surgery-1stPD # | - | |||||
| GTR | 40 | 0.511 | ||||
| STR or Partial Resection | 31 | |||||
| Stupp Protocol AfterSurgery-1stPD | - | |||||
| No | 53 | 0.968 | ||||
| Yes | 8 | 1 (0.44, 2.21) | 0.968 |
| N | Median (95% CI) | 1y-Surv. ± SE [%] | 2y-Surv. ± SE [%] | HR (95% CI) | pV | Log-Rank, p-Value | ||
|---|---|---|---|---|---|---|---|---|
| 6 | <cutoff | 26 | 12.9 (7.7, NA) | 63.8 ± 10.4 | 34.8 ± 11.1 | 0.131 | ||
| ≥cutoff | 80 | 9.5 (7.9, 11.9) | 91.3 ± 3.2 | 51.7 ± 5.7 | 1.5 (0.88, 2.68) | 0.134 | ||
| 8 | <cutoff | 42 | 9.7 (6.9, 19.5) | 67.9 ± 7.7 | 26.6 ± 7.5 | 0.706 | ||
| ≥cutoff | 64 | 9.9 (8.5, 13.9) | 95.3 ± 2.6 | 60 ± 6.2 | 1.1 (0.7, 1.7) | 0.706 | ||
| 9 | <cutoff | 48 | 9.2 (6.4, 15.9) | 70 ± 7 | 27.5 ± 7 | 0.987 | ||
| ≥cutoff | 58 | 9.9 (9.1, 14.4) | 96.6 ± 2.4 | 62.7 ± 6.5 | 1 (0.65, 1.53) | 0.987 | ||
| 10 | <cutoff | 53 | 7.7 (6.1, 12.9) | 71.1 ± 6.6 | 24.4 ± 6.4 | 0.441 | ||
| ≥cutoff | 53 | 10.8 (9.1, 15.7) | 98.1 ± 1.9 | 68.7 ± 6.5 | 0.8 (0.56, 1.29) | 0.441 | ||
| 12 | <cutoff | 64 | 7.7 (6.7, 11) | 74.7 ± 5.7 | 22.3 ± 5.6 | 0.082 | ||
| ≥cutoff | 42 | 13.2 (9.9, 18.8) | 100 ± 0 | 82.9 ± 5.9 | 0.7 (0.45, 1.05) | 0.083 | ||
| 14 | <cutoff | 72 | 8.5 (6.7, 11) | 77.7 ± 5.1 | 26.4 ± 5.6 | 0.059 | ||
| ≥cutoff | 34 | 13.6 (9.9, 20.5) | 100 ± 0 | 88.2 ± 5.5 | 0.7 (0.42, 1.02) | 0.061 | ||
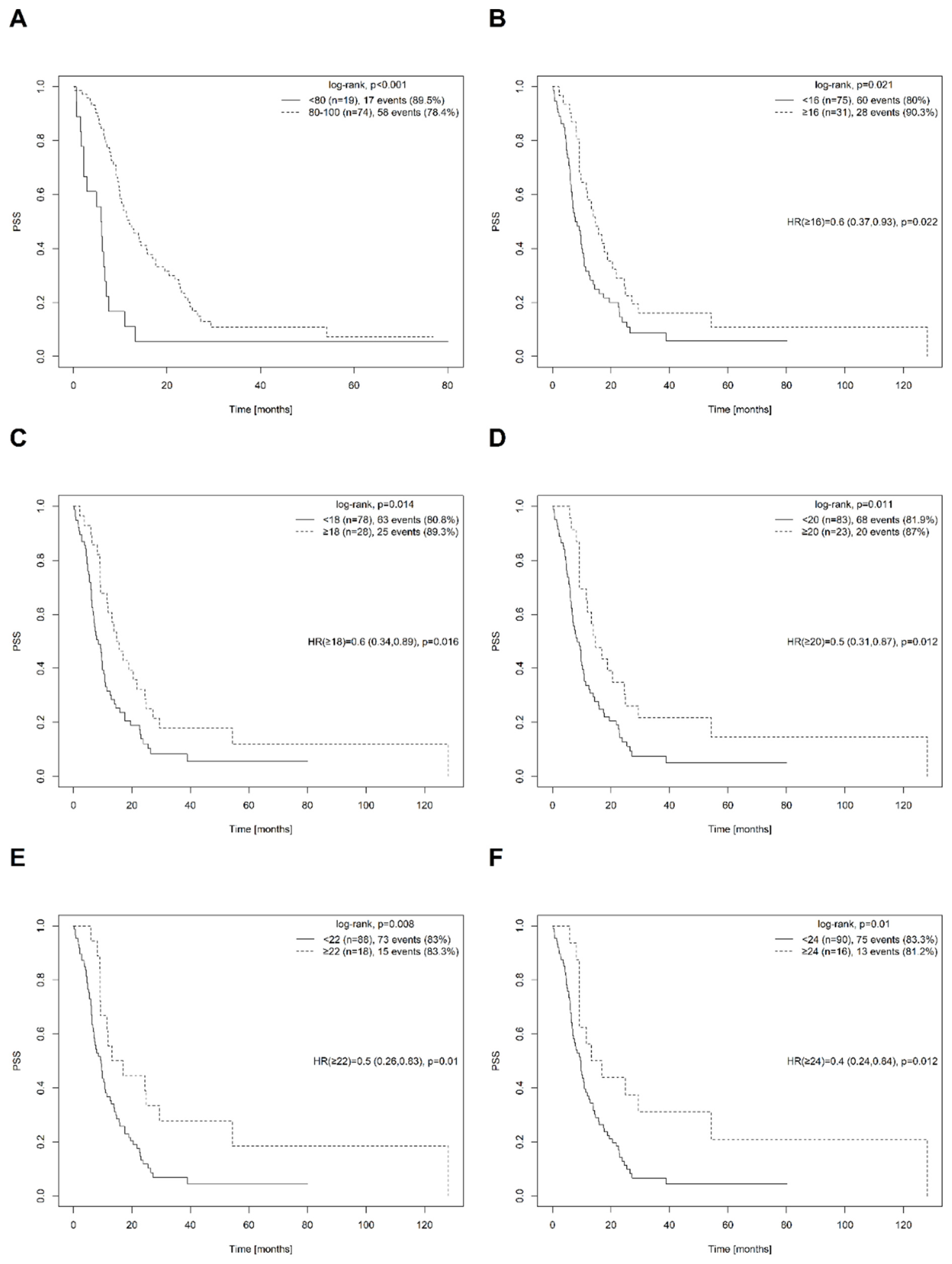
| 16 * | <cutoff | 75 | 8.5 (6.7, 10.8) | 78.7 ± 4.9 | 26.6 ± 5.5 | 0.021 | ||
| ≥cutoff | 31 | 14.7 (9.9, 24.5) | 100 ± 0 | 93.5 ± 4.4 | 0.6 (0.37, 0.93) | 0.022 | ||
| 18 * | <cutoff | 78 | 8.5 (6.8, 10.7) | 79.5 ± 4.7 | 29.9 ± 5.6 | 0.014 | ||
| ≥cutoff | 28 | 15.2 (11.5, 24.9) | 100 ± 0 | 92.9 ± 4.9 | 0.6 (0.34, 0.89) | 0.016 | ||
| 20 * | <cutoff | 83 | 8.5 (6.8, 10.8) | 80.9 ± 4.5 | 31.8 ± 5.5 | 0.011 | ||
| ≥cutoff | 23 | 14.7 (11.5, 29.4) | 100 ± 0 | 100 ± 0 | 0.5 (0.31, 0.87) | 0.012 | ||
| 22 * | <cutoff | 88 | 9.5 (6.9, 11.3) | 82 ± 4.2 | 36.2 ± 5.4 | 0.008 | ||
| ≥cutoff | 18 | 15.1 (9.2, NA) | 100 ± 0 | 100 ± 0 | 0.5 (0.26, 0.83) | 0.010 | ||
| 24 * | <cutoff | 90 | 9.7 (7, 11.9) | 82.4 ± 4.1 | 37.8 ± 5.4 | 0.010 | ||
| ≥cutoff | 16 | 15.1 (9.1, NA) | 100 ± 0 | 100 ± 0 | 0.4 (0.24, 0.84) | 0.012 |
| Factor | HR (95% CI) | p-Value |
|---|---|---|
| Age | 1 (0.99, 1.03) | 0.220 |
| TTP ≥ 6 | 1.4 (0.75, 2.6) | 0.295 |
| Age | 1 (0.99, 1.03) | 0.204 |
| TTP ≥ 8 | 1.1 (0.68, 1.78) | 0.705 |
| Age | 1 (0.99, 1.03) | 0.210 |
| TTP ≥ 9 | 1.1 (0.68, 1.74) | 0.721 |
| Age | 1 (0.99, 1.03) | 0.206 |
| TTP ≥ 10 | 1 (0.62, 1.57) | 0.945 |
| Age | 1 (0.99, 1.03) | 0.208 |
| TTP ≥ 12 | 0.8 (0.48, 1.27) | 0.321 |
| Age | 1 (0.99, 1.03) | 0.201 |
| TTP ≥ 14 | 0.8 (0.47, 1.27) | 0.308 |
| Age | 1 (0.99, 1.03) | 0.213 |
| TTP ≥ 16 | 0.7 (0.41, 1.15) | 0.154 |
| Age | 1 (0.99, 1.03) | 0.212 |
| TTP ≥ 18 | 0.6 (0.37, 1.07) | 0.085 |
| Age | 1 (0.99, 1.03) | 0.275 |
| TTP ≥ 20 | 0.6 (0.33, 1.05) | 0.071 |
| Age | 1 (0.99, 1.03) | 0.275 |
| TTP ≥ 22 * | 0.5 (0.27, 0.96) | 0.036 |
| Age | 1 (0.99, 1.03) | 0.311 |
| TTP ≥ 24 * | 0.5 (0.25, 0.96) | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalita, O.; Kazda, T.; Reguli, S.; Jancalek, R.; Fadrus, P.; Slachta, M.; Pospisil, P.; Krska, L.; Vrbkova, J.; Hrabalek, L.; et al. Effects of Reoperation Timing on Survival among Recurrent Glioblastoma Patients: A Retrospective Multicentric Descriptive Study. Cancers 2023, 15, 2530. https://doi.org/10.3390/cancers15092530
Kalita O, Kazda T, Reguli S, Jancalek R, Fadrus P, Slachta M, Pospisil P, Krska L, Vrbkova J, Hrabalek L, et al. Effects of Reoperation Timing on Survival among Recurrent Glioblastoma Patients: A Retrospective Multicentric Descriptive Study. Cancers. 2023; 15(9):2530. https://doi.org/10.3390/cancers15092530
Chicago/Turabian StyleKalita, Ondrej, Tomas Kazda, Stefan Reguli, Radim Jancalek, Pavel Fadrus, Marek Slachta, Petr Pospisil, Lukas Krska, Jana Vrbkova, Lumir Hrabalek, and et al. 2023. "Effects of Reoperation Timing on Survival among Recurrent Glioblastoma Patients: A Retrospective Multicentric Descriptive Study" Cancers 15, no. 9: 2530. https://doi.org/10.3390/cancers15092530
APA StyleKalita, O., Kazda, T., Reguli, S., Jancalek, R., Fadrus, P., Slachta, M., Pospisil, P., Krska, L., Vrbkova, J., Hrabalek, L., Smrcka, M., & Lipina, R. (2023). Effects of Reoperation Timing on Survival among Recurrent Glioblastoma Patients: A Retrospective Multicentric Descriptive Study. Cancers, 15(9), 2530. https://doi.org/10.3390/cancers15092530







