Rate of Malignant Transformation Differs Based on Diagnostic Criteria for Oral Lichenoid Conditions: A Systematic Review and Meta-Analysis of 24,277 Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Eligibility Criteria
2.3. Data Extraction and Management
2.4. Risk of Bias Assessment
2.5. Evaluation of Quality of Evidence
2.6. Data Analysis
3. Results
3.1. Result of Database Search
3.2. Study Characteristics
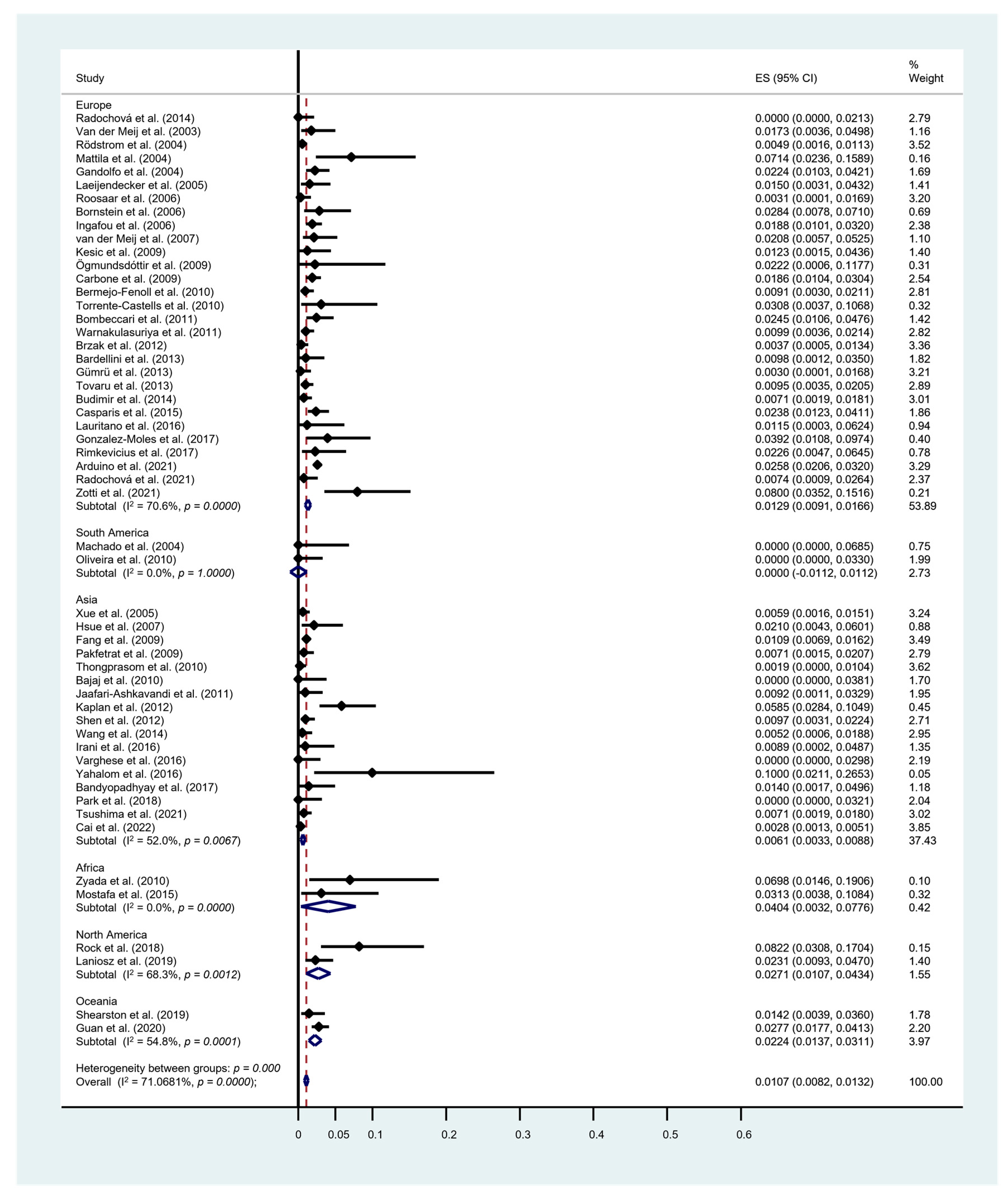
3.3. Rate of Malignant Transformation
3.4. Meta-Analysis for Risk Factor of Malignant Transformation
3.5. Risk of Bias Assessment and Quality of Evidence
4. Discussion
4.1. Strengths
4.2. Limitations
4.3. Recommendations for Future Research and Clinical Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikeda, N.; Handa, Y.; Khim, S.P.; Durward, C.; Axell, T.; Mizuno, T.; Fukano, H.; Kawai, T. Prevalence study of oral mucosal lesions in a selected Cambodian population. Community Dent. Oral Epidemiol. 1995, 23, 49–54. [Google Scholar] [CrossRef]
- Krupaa, R.J.; Sankari, S.L.; Masthan, K.M.; Rajesh, E. Oral lichen planus: An overview. J. Pharm. Bioallied Sci. 2015, 7, S158–S161. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Debta, P.; Dixit, A. Oral Potentially Malignant Disorders: Etiology, Pathogenesis, and Transformation Into Oral Cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar] [CrossRef]
- Krutchkoff, D.J.; Eisenberg, E. Lichenoid dysplasia: A distinct histopathologic entity. Oral Surg. Oral Med. Oral Pathol. 1985, 60, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Rock, L.D.; Laronde, D.M.; Lin, I.; Rosin, M.P.; Chan, B.; Shariati, B.; Zhang, L. Dysplasia Should Not Be Ignored in Lichenoid Mucositis. J. Dent. Res. 2018, 97, 767–772. [Google Scholar] [CrossRef]
- Pimolbutr, K.; Lim, W.T.; Leeson, R.; Hopper, C.; Kalavrezos, N.; Liew, C.; Schilling, C.; Sinha, D.; Jay, A.; Agrawal, R.; et al. Prognosis of oral epithelial dysplasia in individuals with and without oral lichen planus. Oral Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, E.H.; van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Kamath, V.V.; Setlur, K.; Yerlagudda, K. Oral lichenoid lesions—A review and update. Indian J. Dermatol. 2015, 60, 102. [Google Scholar] [CrossRef]
- Muller, S. Oral lichenoid lesions: Distinguishing the benign from the deadly. Mod. Pathol. 2017, 30, S54–S67. [Google Scholar] [CrossRef]
- Farah, C.S.; Fox, S.; Shearston, K.; Newman, L.; Babic, S.; Vacher, M. Lichenoid dysplasia is not a distinct pathological entity. Oral Oncol. 2021, 119, 105362. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; Gonzalez-Moles, M.A.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Hallopeau, H. Sur un cas de lichen de Wilson gingival avec néoplasie voisine dans la région maxillaire. Bull. Soc. Fr. Dermatol. Syphiligr. 1910, 17, 32. [Google Scholar]
- Lodi, G.; Scully, C.; Carrozzo, M.; Griffiths, M.; Sugerman, P.B.; Thongprasom, K. Current controversies in oral lichen planus: Report of an international consensus meeting. Part 1. Viral infections and etiopathogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 40–51. [Google Scholar] [CrossRef]
- Landini, G.; Mylonas, P.; Shah, I.Z.; Hamburger, J. The reported rates of transformation of oral lichen planus. J. Oral Maxillofac. Surg. Med. Pathol. 2014, 26, 213–220. [Google Scholar] [CrossRef]
- Eisenberg, E.; Krutchkoff, D.J. Lichenoid lesions of oral mucosa. Diagnostic criteria and their importance in the alleged relationship to oral cancer. Oral Surg. Oral Med. Oral Pathol. 1992, 73, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J. Am. Acad. Dermatol. 2002, 46, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, E.H.; Mast, H.; van der Waal, I. The possible premalignant character of oral lichen planus and oral lichenoid lesions: A prospective five-year follow-up study of 192 patients. Oral Oncol. 2007, 43, 742–748. [Google Scholar] [CrossRef]
- Van der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009, 45, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Shearston, K.; Fateh, B.; Tai, S.; Hove, D.; Farah, C.S. Oral lichenoid dysplasia and not oral lichen planus undergoes malignant transformation at high rates. J. Oral Pathol. Med. 2019, 48, 538–545. [Google Scholar] [CrossRef]
- Tampa, M.; Caruntu, C.; Mitran, M.; Mitran, C.; Sarbu, I.; Rusu, L.C.; Matei, C.; Constantin, C.; Neagu, M.; Georgescu, S.R. Markers of Oral Lichen Planus Malignant Transformation. Dis. Markers 2018, 2018, 1959506. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.G.; Hirsch, S.A.; Gordon, S.C. The malignant transformation of oral lichen planus and oral lichenoid lesions: A systematic review. J. Am. Dent. Assoc. 2014, 145, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Troiano, G.; Cordaro, M.; Corsalini, M.; Gioco, G.; Lo Muzio, L.; Pignatelli, P.; Lajolo, C. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis. 2019, 25, 693–709. [Google Scholar] [CrossRef]
- Idrees, M.; Kujan, O.; Shearston, K.; Farah, C.S. Oral lichen planus has a very low malignant transformation rate: A systematic review and meta-analysis using strict diagnostic and inclusion criteria. J. Oral Pathol. Med. 2021, 50, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; Group, G.W. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.C.; Sugaya, N.N.; Migliari, D.A.; Matthews, R.W. Oral lichen planus. Clinical aspects and management in fifty-two Brazilian patients. West. Indian Med. J. 2004, 53, 113–117. [Google Scholar]
- Rodstrom, P.O.; Jontell, M.; Mattsson, U.; Holmberg, E. Cancer and oral lichen planus in a Swedish population. Oral Oncol. 2004, 40, 131–138. [Google Scholar] [CrossRef]
- Mattila, R.; Alanen, K.; Syrjanen, S. DNA content as a prognostic marker of oral lichen planus with a risk of cancer development. Anal. Quant. Cytol. Histol. 2004, 26, 278–284. [Google Scholar]
- Gandolfo, S.; Richiardi, L.; Carrozzo, M.; Broccoletti, R.; Carbone, M.; Pagano, M.; Vestita, C.; Rosso, S.; Merletti, F. Risk of oral squamous cell carcinoma in 402 patients with oral lichen planus: A follow-up study in an Italian population. Oral Oncol. 2004, 40, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.L.; Fan, M.W.; Wang, S.Z.; Chen, X.M.; Li, Y.; Wang, L. A clinical study of 674 patients with oral lichen planus in China. J. Oral Pathol. Med. 2005, 34, 467–472. [Google Scholar] [CrossRef]
- Laeijendecker, R.; van Joost, T.; Kuizinga, M.C.; Tank, B.; Neumann, H.A. Premalignant nature of oral lichen planus. Acta Derm. Venereol. 2005, 85, 516–520. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Kalas, L.; Lemp, S.; Altermatt, H.J.; Rees, T.D.; Buser, D. Oral lichen planus and malignant transformation: A retrospective follow-up study of clinical and histopathologic data. Quintessence Int. 2006, 37, 261–271. [Google Scholar] [PubMed]
- Ingafou, M.; Leao, J.C.; Porter, S.R.; Scully, C. Oral lichen planus: A retrospective study of 690 British patients. Oral Dis. 2006, 12, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Hsue, S.S.; Wang, W.C.; Chen, C.H.; Lin, C.C.; Chen, Y.K.; Lin, L.M. Malignant transformation in 1458 patients with potentially malignant oral mucosal disorders: A follow-up study based in a Taiwanese hospital. J. Oral Pathol. Med. 2007, 36, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kesic, L.; Obradovic, R.; Mihailovic, D.; Radicevic, G.; Stankovic, S.; Todorovic, K. Incidence and treatment outcome of oral lichen planus in southeast Serbia in a 10-year period (1997–2007). Vojnosanit. Pregl. 2009, 66, 435–439. [Google Scholar] [CrossRef]
- Fang, M.; Zhang, W.; Chen, Y.; He, Z. Malignant transformation of oral lichen planus: A retrospective study of 23 cases. Quintessence Int. 2009, 40, 235–242. [Google Scholar]
- Pakfetrat, A.; Javadzadeh-Bolouri, A.; Basir-Shabestari, S.; Falaki, F. Oral Lichen Planus: A retrospective study of 420 Iranian patients. Med. Oral Patol. Oral Cir. Bucal 2009, 14, E315–E318. [Google Scholar]
- Ogmundsdottir, H.M.; Bjornsson, J.; Holbrook, W.P. Role of TP53 in the progression of pre-malignant and malignant oral mucosal lesions. A follow-up study of 144 patients. J. Oral Pathol. Med. 2009, 38, 565–571. [Google Scholar] [CrossRef]
- Carbone, M.; Arduino, P.G.; Carrozzo, M.; Gandolfo, S.; Argiolas, M.R.; Bertolusso, G.; Conrotto, D.; Pentenero, M.; Broccoletti, R. Course of oral lichen planus: A retrospective study of 808 northern Italian patients. Oral Dis. 2009, 15, 235–243. [Google Scholar] [CrossRef]
- Oliveira Alves, M.G.; Almeida, J.D.; Balducci, I.; Guimaraes Cabral, L.A. Oral lichen planus: A retrospective study of 110 Brazilian patients. BMC Res. Notes 2010, 3, 157. [Google Scholar] [CrossRef]
- Zyada, M.M.; Fikry, H.E. Immunohistochemical study of syndecan-1 down-regulation and the expression of P35 protein in oral lichen planus: A clinicopathologic correlation with hepatitis C infection in the Egyptian population. Ann. Diagn. Pathol. 2010, 14, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Thongprasom, K.; Youngnak-Piboonratanakit, P.; Pongsiriwet, S.; Laothumthut, T.; Kanjanabud, P.; Rutchakitprakarn, L. A multicenter study of oral lichen planus in Thai patients. J. Investig. Clin. Dent. 2010, 1, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, D.R.; Khoso, N.A.; Devrajani, B.R.; Matlani, B.L.; Lohana, P. Oral lichen planus: A clinical study. J. Coll. Physicians Surg. Pak. 2010, 20, 154–157. [Google Scholar]
- Bermejo-Fenoll, A.; Sanchez-Siles, M.; Lopez-Jornet, P.; Camacho-Alonso, F.; Salazar-Sanchez, N. A retrospective clinicopathological study of 550 patients with oral lichen planus in south-eastern Spain. J. Oral Pathol. Med. 2010, 39, 491–496. [Google Scholar] [CrossRef]
- Torrente-Castells, E.; Figueiredo, R.; Berini-Aytes, L.; Gay-Escoda, C. Clinical features of oral lichen planus. A retrospective study of 65 cases. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e685–e690. [Google Scholar] [CrossRef]
- Jaafari-Ashkavandi, Z.; Mardani, M.; Pardis, S.; Amanpour, S. Oral mucocutaneous diseases: Clinicopathologic analysis and malignant transformation. J. Craniofac Surg. 2011, 22, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kovacevic, T.; Madden, P.; Coupland, V.H.; Sperandio, M.; Odell, E.; Moller, H. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10-year period in South East England. J. Oral Pathol. Med. 2011, 40, 677–683. [Google Scholar] [CrossRef]
- Brzak, B.L.; Mravak-Stipetic, M.; Canjuga, I.; Baricevic, M.; Balicevic, D.; Sikora, M.; Filipovic-Zore, I. The frequency and malignant transformation rate of oral lichen planus and leukoplakia—A retrospective study. Coll. Antropol. 2012, 36, 773–777. [Google Scholar]
- Kaplan, I.; Ventura-Sharabi, Y.; Gal, G.; Calderon, S.; Anavi, Y. The dynamics of oral lichen planus: A retrospective clinicopathological study. Head Neck Pathol. 2012, 6, 178–183. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Liu, W.; Zhu, L.K.; Feng, J.Q.; Tang, G.Y.; Zhou, Z.T. A retrospective clinicopathological study on oral lichen planus and malignant transformation: Analysis of 518 cases. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e943–e947. [Google Scholar] [CrossRef]
- Bardellini, E.; Amadori, F.; Flocchini, P.; Bonadeo, S.; Majorana, A. Clinicopathological features and malignant transformation of oral lichen planus: A 12-years retrospective study. Acta Odontol. Scand. 2013, 71, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Gumru, B. A retrospective study of 370 patients with oral lichen planus in Turkey. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e427–e432. [Google Scholar] [CrossRef] [PubMed]
- Tovaru, S.; Parlatescu, I.; Gheorghe, C.; Tovaru, M.; Costache, M.; Sardella, A. Oral lichen planus: A retrospective study of 633 patients from Bucharest, Romania. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e201–e206. [Google Scholar] [CrossRef] [PubMed]
- Budimir, V.; Richter, I.; Andabak-Rogulj, A.; Vucicevic-Boras, V.; Budimir, J.; Brailo, V. Oral lichen planus—Retrospective study of 563 Croatian patients. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e255–e260. [Google Scholar] [CrossRef]
- Radochova, V.; Drizhal, I.; Slezak, R. A retrospective study of 171 patients with oral lichen planus in the East Bohemia—Czech Republic—Single center experience. J. Clin. Exp. Dent. 2014, 6, e556–e561. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Tail, Y.H.; Wang, W.C.; Chen, C.Y.; Kao, Y.H.; Chen, Y.K.; Chen, C.H. Malignant transformation in 5071 southern Taiwanese patients with potentially malignant oral mucosal disorders. BMC Oral Health 2014, 14, 99. [Google Scholar] [CrossRef]
- Casparis, S.; Borm, J.M.; Tektas, S.; Kamarachev, J.; Locher, M.C.; Damerau, G.; Gratz, K.W.; Stadlinger, B. Oral lichen planus (OLP), oral lichenoid lesions (OLL), oral dysplasia, and oral cancer: Retrospective analysis of clinicopathological data from 2002-2011. Oral Maxillofac. Surg. 2015, 19, 149–156. [Google Scholar] [CrossRef]
- Mostafa, B.; Ahmed, E. Prevalence of oral lichen planus among a sample of the Egyptian population. J. Clin. Exp. Dent. 2015, 7, e7–e12. [Google Scholar] [CrossRef]
- Lauritano, D.; Arrica, M.; Lucchese, A.; Valente, M.; Pannone, G.; Lajolo, C.; Ninivaggi, R.; Petruzzi, M. Oral lichen planus clinical characteristics in Italian patients: A retrospective analysis. Head Face Med. 2016, 12, 18. [Google Scholar] [CrossRef]
- Irani, S.; Esfahani, A.M.; Ghorbani, A. Dysplastic change rate in cases of oral lichen planus: A retrospective study of 112 cases in an Iranian population. J. Oral Maxillofac. Pathol. 2016, 20, 395–399. [Google Scholar] [CrossRef]
- Varghese, S.S.; George, G.B.; Sarojini, S.B.; Vinod, S.; Mathew, P.; Mathew, D.G.; Sebastian, J.; George, A. Epidemiology of Oral Lichen Planus in a Cohort of South Indian Population: A Retrospective Study. J. Cancer Prev. 2016, 21, 55–59. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Behura, S.S.; Nishat, R.; Dash, K.C.; Bhuyan, L.; Ramachandra, S. Clinicopathological Profile and Malignant Transformation in Oral Lichen Planus: A Retrospective Study. J. Int. Soc. Prev. Community Dent. 2017, 7, 116–124. [Google Scholar] [CrossRef]
- Gonzalez-Moles, M.A.; Gil-Montoya, J.A.; Ruiz-Avila, I.; Bravo, M. Is oral cancer incidence among patients with oral lichen planus/oral lichenoid lesions underestimated? J. Oral Pathol. Med. 2017, 46, 148–153. [Google Scholar] [CrossRef]
- Rimkevicius, A.; Aleksejuniene, J.; Puriene, A.; Seinin, D.; Rasteniene, R. Oral lichen planus: A 4-year clinical follow-up study. Turk. J. Med. Sci. 2017, 47, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.J.; Kim, S.H.; Kim, S.B.; Choi, Y.H.; Kim, Y.K.; Yun, P.Y. Factors affecting treatment outcomes in patients with oral lichen planus lesions: A retrospective study of 113 cases. J. Periodontal Implant. Sci. 2018, 48, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Laniosz, V.; Torgerson, R.R.; Ramos-Rodriguez, A.J.; Ma, J.E.; Mara, K.C.; Weaver, A.L.; Bruce, A.J. Incidence of squamous cell carcinoma in oral lichen planus: A 25-year population-based study. Int. J. Dermatol. 2019, 58, 296–301. [Google Scholar] [CrossRef]
- Arduino, P.G.; Magliano, A.; Gambino, A.; Macciotta, A.; Carbone, M.; Conrotto, D.; Karimi, D.; Carrozzo, M.; Broccoletti, R. Risk of Malignant Transformation in 3173 Subjects with Histopathologically Confirmed Oral Lichen Planus: A 33-Year Cohort Study in Northern Italy. Cancers 2021, 13, 5740. [Google Scholar] [CrossRef]
- Radochova, V.; Koberova Ivancakova, R.; Heneberk, O.; Slezak, R. The Characteristics of Patients with Oral Lichen Planus and Malignant Transformation-A Retrospective Study of 271 Patients. Int. J. Environ. Res. Public Health 2021, 18, 6525. [Google Scholar] [CrossRef]
- Tsushima, F.; Sakurai, J.; Uesugi, A.; Oikawa, Y.; Ohsako, T.; Mochizuki, Y.; Hirai, H.; Kayamori, K.; Harada, H. Malignant transformation of oral lichen planus: A retrospective study of 565 Japanese patients. BMC Oral Health 2021, 21, 298. [Google Scholar] [CrossRef]
- Zotti, F.; Nocini, R.; Capocasale, G.; Bertossi, D.; Fior, A.; Peretti, M.; Manfrin, E.; Albanese, M. Oral Lichen Planus: Risk factors of malignant transformation and follow up. Ten years retrospective study. J. Clin. Exp. Dent. 2021, 13, e630–e636. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, J.; Han, Y.; Tang, Q.; Zhang, H.; Li, T. Development and validation of a nomogram prediction model for malignant transformation of oral potentially malignant disorders. Oral Oncol. 2021, 123, 105619. [Google Scholar] [CrossRef]
- Guan, G.; Mei, L.; Polonowita, A.; Hussaini, H.; Seo, B.; Rich, A.M. Malignant transformation in oral lichen planus and lichenoid lesions: A 14-year longitudinal retrospective cohort study of 829 patients in New Zealand. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, E.H.; Schepman, K.P.; van der Waal, I. The possible premalignant character of oral lichen planus and oral lichenoid lesions: A prospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Roosaar, A.; Yin, L.; Sandborgh-Englund, G.; Nyren, O.; Axell, T. On the natural course of oral lichen lesions in a Swedish population-based sample. J. Oral Pathol. Med. 2006, 35, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Bombeccari, G.P.; Guzzi, G.; Tettamanti, M.; Gianni, A.B.; Baj, A.; Pallotti, F.; Spadari, F. Oral lichen planus and malignant transformation: A longitudinal cohort study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 328–334. [Google Scholar] [CrossRef]
- Yahalom, R.; Yarom, N.; Shani, T.; Amariglio, N.; Kaplan, I.; Trakhtenbrot, L.; Hirshberg, A. Oral lichen planus patients exhibit consistent chromosomal numerical aberrations: A follow-up analysis. Head Neck 2016, 38 (Suppl. S1), E741–E746. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.A.; Warnakulasuriya, S.; Gonzalez-Ruiz, I.; Gonzalez-Ruiz, L.; Ayen, A.; Lenouvel, D.; Ruiz-Avila, I.; Ramos-Garcia, P. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.A.; Ramos-Garcia, P.; Warnakulasuriya, S. An appraisal of highest quality studies reporting malignant transformation of oral lichen planus based on a systematic review. Oral Dis. 2021, 27, 1908–1918. [Google Scholar] [CrossRef]
- Gonzalez-Moles, M.A.; Warnakulasuriya, S.; Gonzalez-Ruiz, I.; Ayen, A.; Gonzalez-Ruiz, L.; Ruiz-Avila, I.; Ramos-Garcia, P. Dysplasia in oral lichen planus: Relevance, controversies and challenges. A position paper. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e541–e548. [Google Scholar] [CrossRef]
- Muller, S. The Lichenoid Tissue Reactions of the Oral Mucosa: Oral Lichen Planus and Other Lichenoid Lesions. Surg. Pathol. Clin. 2011, 4, 1005–1026. [Google Scholar] [CrossRef]
- Aghbari, S.M.H.; Abushouk, A.I.; Attia, A.; Elmaraezy, A.; Menshawy, A.; Ahmed, M.S.; Elsaadany, B.A.; Ahmed, E.M. Malignant transformation of oral lichen planus and oral lichenoid lesions: A meta-analysis of 20095 patient data. Oral Oncol. 2017, 68, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, W.; Geng, N.; Tian, K.; Jack Windsor, L. MMPs, TIMP-2, and TGF-beta1 in the cancerization of oral lichen planus. Head Neck 2008, 30, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sayans, M.; Lorenzo-Pouso, A.I.; Chamorro-Petronacci, C.M.; Suarez-Penaranda, J.M.; Padin-Iruegas, E.; Gonzalez-Moles, M.A.; Marichalar-Mendia, X.; Garcia-Garcia, A.; Blanco-Carrion, A. Immunoexpression of Apoptosis and Cell-cycle Arrest Markers in Oral Lichen Planus. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.A.; Ruiz-Avila, I.; Gonzalez-Ruiz, L.; Ayen, A.; Gil-Montoya, J.A.; Ramos-Garcia, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef]
- Reidy, J.T.; McHugh, E.E.; Stassen, L.F. A review of the role of alcohol in the pathogenesis of oral cancer and the link between alcohol-containing mouthrinses and oral cancer. J. Ir. Dent. Assoc. 2011, 57, 200–202. [Google Scholar]
- Mayne, S.T.; Cartmel, B.; Kirsh, V.; Goodwin, W.J., Jr. Alcohol and tobacco use prediagnosis and postdiagnosis, and survival in a cohort of patients with early stage cancers of the oral cavity, pharynx, and larynx. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 3368–3374. [Google Scholar] [CrossRef]
- Marron, M.; Boffetta, P.; Zhang, Z.F.; Zaridze, D.; Wunsch-Filho, V.; Winn, D.M.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; Sturgis, E.M.; et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int. J. Epidemiol. 2010, 39, 182–196. [Google Scholar] [CrossRef]
- Rangel, J.B.; Thuler, L.C.S.; Pinto, J. Prevalence of hepatitis C virus infection and its impact on the prognosis of head and neck cancer patients. Oral Oncol. 2018, 87, 138–143. [Google Scholar] [CrossRef]
- Carrozzo, M.; Scally, K. Oral manifestations of hepatitis C virus infection. World J. Gastroenterol. 2014, 20, 7534–7543. [Google Scholar] [CrossRef]
- Carrozzo, M.; Quadri, R.; Latorre, P.; Pentenero, M.; Paganin, S.; Bertolusso, G.; Gandolfo, S.; Negro, F. Molecular evidence that the hepatitis C virus replicates in the oral mucosa. J. Hepatol. 2002, 37, 364–369. [Google Scholar] [CrossRef]
- Nagao, Y.; Nishida, N.; Toyo-Oka, L.; Kawaguchi, A.; Amoroso, A.; Carrozzo, M.; Sata, M.; Mizokami, M.; Tokunaga, K.; Tanaka, Y. Genome-Wide Association Study Identifies Risk Variants for Lichen Planus in Patients With Hepatitis C Virus Infection. Clin. Gastroenterol. Hepatol. 2017, 15, 937–944.e5. [Google Scholar] [CrossRef] [PubMed]
- Sakamuro, D.; Furukawa, T.; Takegami, T. Hepatitis C virus nonstructural protein NS3 transforms NIH 3T3 cells. J. Virol. 1995, 69, 3893–3896. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.B.; Lagging, L.M.; Meyer, K.; Ray, R. Hepatitis C virus core protein cooperates with ras and transforms primary rat embryo fibroblasts to tumorigenic phenotype. J. Virol. 1996, 70, 4438–4443. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Kim, Y.D.; Park, C.S.; Han, K.D.; Joo, Y.H. Hypertension is associated with oral, laryngeal, and esophageal cancer: A nationwide population-based study. Sci. Rep. 2020, 10, 10291. [Google Scholar] [CrossRef]
- Mouhayar, E.; Salahudeen, A. Hypertension in cancer patients. Tex. Heart Inst. J. 2011, 38, 263–265. [Google Scholar]
- Arai, H.; Saitoh, S.; Matsumoto, T.; Makita, F.; Mitsugi, S.; Yuasa, K.; Takagi, H.; Mori, M. Hypertension as a paraneoplastic syndrome in hepatocellular carcinoma. J. Gastroenterol. 1999, 34, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Al-Maweri, S.A.; Saini, D.; Ismail, N.M.; Ismail, A.R. Oral mucosal lesions in non oral habit diabetic patients and association of diabetes mellitus with oral precancerous lesions. Diabetes Res. Clin. Pract. 2010, 89, 320–326. [Google Scholar] [CrossRef]
- Wu, C.H.; Wu, T.Y.; Li, C.C.; Lui, M.T.; Chang, K.W.; Kao, S.Y. Impact of diabetes mellitus on the prognosis of patients with oral squamous cell carcinoma: A retrospective cohort study. Ann. Surg. Oncol. 2010, 17, 2175–2183. [Google Scholar] [CrossRef]
- Ramos-Garcia, P.; Roca-Rodriguez, M.D.M.; Aguilar-Diosdado, M.; Gonzalez-Moles, M.A. Diabetes mellitus and oral cancer/oral potentially malignant disorders: A systematic review and meta-analysis. Oral Dis. 2021, 27, 404–421. [Google Scholar] [CrossRef]










| Database | Search Terms |
|---|---|
| PubMed | (“Lichen Planus, Oral”[Mesh] or “oral lichen planus”[All Fields] or “oral lichenoid lesion”[All Fields] or “olp”[All Fields] or “oll”[All Fields]) and (“Carcinoma, Squamous Cell”[Mesh] or “squamous cell carcinoma”[All Fields] or “oscc”[All Fields] or “precancer”[All Fields] or “cancer”[All Fields] or “neoplasm”[Mesh] or “neoplasm”[All Fields] or “oral potentially malignant disorder”[All Fields] or “premalignant”[All Fields] or malign * or premalign * or “malignant transformation”[All Fields] or “degeneration”[All Fields] or “carcinogenesis”[Mesh] or “carcinogenesis”[All Fields] or “transformation”[All Fields] or “oncogenesis”[All Fields]) |
| Embase | (‘oral lichen planus’/exp OR ‘oral lichen planus’ OR ‘oral lichenoid lesion’/exp OR ‘oral lichenoid lesion’ OR ‘OLP’ OR ‘OLL’) AND (‘oral squamous cell carcinoma’/exp OR ‘oral squamous cell carcinoma’ OR ‘OSCC’ OR ‘precancer’/exp OR ‘precancer’ OR ‘cancer’ OR ‘malignant neoplasm’/exp OR ‘malignant neoplasm’ OR ‘oral potentially malignant disorder’/exp OR ‘oral potentially malignant disorder’ OR ‘premalignant’ OR ‘malign *’ OR ‘premalign *’ OR ‘malignant transformation’/exp OR ‘malignant transformation’ OR ‘degeneration’/exp OR ‘degeneration’ OR ‘carcinogenesis’/exp OR ‘carcinogenesis’ OR ‘oncogenesis’) |
| Web of Science | (ALL = (oral lichen planus) OR ALL = (oral lichenoid lesion) OR ALL = (OLL) OR ALL = (OLP)) AND (ALL = (oral squamous cell carcinoma) OR ALL = (oscc) OR ALL = (precancer) OR ALL = (cancer) OR ALL = (malignant neoplasm) OR ALL = (oral potentially malignant disorder) OR ALL = (premalignant) OR ALL = (malign *) OR ALL = (premalign *) OR ALL = (malignant transformation) OR ALL = (degeneration) OR ALL = (carcinogenesis) OR ALL = (transformation) OR ALL = (oncogenesis)) |
| Scopus | TITLE-ABS-KEY((“oral lichen planus” OR “oral lichenoid lesion” OR “olp” OR “oll”) AND (“oral squamous cell carcinoma” OR “oscc” OR “precancer” OR “cancer” OR “malignant neoplasm” OR “oral potentially malignant disorder” OR “premalignant” OR “malign *” OR “premalign *” OR “malignant transformation” OR “degeneration” OR “carcinogenesis” OR “transformation” OR “oncogenesis”)) |
| Study/Year | Study Design | Total (n) | OSCC Development | Overall MT Rate (%) | Annual MT Rate (%) | Monthly MT Rate (%) | |
|---|---|---|---|---|---|---|---|
| Van der Meij et al. [73] | 2003 | P | 173 | 3 | 1.73 | 0.65 | 0.054 |
| Machado et al. [26] | 2004 | R | 52 | 0 | 0.00 | 0.00 | 0.000 |
| Rödstrom et al. [27] | 2004 | R | 1028 | 5 | 0.49 | 0.08 | 0.006 |
| Mattila et al. [28] | 2004 | R | 70 | 5 | 7.14 | 2.61 | 0.218 |
| Gandolfo et al. [29] | 2004 | R | 402 | 9 | 2.24 | 0.60 | 0.050 |
| Xue et al. [30] | 2005 | R | 674 | 4 | 0.59 | 0.07 | 0.005 |
| Laeijendecker et al. [31] | 2005 | R | 200 | 3 | 1.50 | 4.19 | 0.349 |
| Roosaar et al. [74] | 2006 | P | 327 | 1 | 0.31 | 0.01 | 0.001 |
| Bornstein et al. [32] | 2006 | R | 141 | 4 | 2.84 | 1.32 | 0.110 |
| Ingafou et al. [33] | 2006 | R | 690 | 13 | 1.88 | - | - |
| Hsue et al. [34] | 2007 | R | 143 | 3 | 2.10 | 1.72 | 0.143 |
| Van der Meij et al. [17] | 2007 | P | 192 | 4 | 2.08 | 0.63 | 0.052 |
| Kesic et al. [35] | 2009 | R | 163 | 2 | 1.23 | - | - |
| Fang et al. [36] | 2009 | R | 2119 | 23 | 1.09 | 0.81 | 0.068 |
| Pakfetrat et al. [37] | 2009 | R | 420 | 3 | 0.71 | 0.43 | 0.036 |
| Ögmundsdóttir et al. [38] | 2009 | R | 45 | 1 | 2.22 | 0.14 | 0.012 |
| Carbone et al. [39] | 2009 | R | 808 | 15 | 1.86 | 0.43 | 0.035 |
| Oliveira et al. [40] | 2010 | R | 110 | 0 | 0.00 | - | - |
| Zyada et al. [41] | 2010 | R | 43 | 3 | 6.98 | - | - |
| Thongprasom et al. [42] | 2010 | R | 533 | 1 | 0.19 | 0.01 | 0.001 |
| Bajaj et al. [43] | 2010 | R | 95 | 0 | 0.00 | - | - |
| Bermejo-Fenoll et al. [44] | 2010 | R | 550 | 5 | 0.91 | - | - |
| Torrente-Castells et al. [45] | 2010 | R | 65 | 2 | 3.08 | - | - |
| Bombeccari et al. [75] | 2011 | P | 327 | 8 | 2.45 | 0.75 | 0.062 |
| Jaafari-Ashkavandi et al. [46] | 2011 | R | 217 | 2 | 0.92 | - | - |
| Warnakulasuriya et al. [47] | 2011 | R | 607 | 6 | 0.99 | - | - |
| Brzak et al. [48] | 2012 | R | 538 | 2 | 0.37 | - | - |
| Kaplan et al. [49] | 2012 | R | 171 | 10 | 5.85 | - | - |
| Shen et al. [50] | 2012 | R | 518 | 5 | 0.97 | 0.16 | 0.014 |
| Bardellini et al. [51] | 2013 | R | 204 | 2 | 0.98 | 0.24 | 0.020 |
| Gümrü et al. [52] | 2013 | R | 330 | 1 | 0.30 | 0.15 | 0.013 |
| Tovaru et al. [53] | 2013 | R | 633 | 6 | 0.95 | - | - |
| Budimir et al. [54] | 2014 | R | 563 | 4 | 0.71 | 0.09 | 0.008 |
| Radochová et al. [55] | 2014 | R | 171 | 0 | 0.00 | 0.00 | 0.000 |
| Wang et al. [56] | 2014 | R | 381 | 2 | 0.52 | 0.78 | 0.065 |
| Casparis et al. [57] | 2015 | R | 505 | 12 | 2.38 | 1.50 | 0.125 |
| Mostafa et al. [58] | 2015 | R | 64 | 2 | 3.13 | - | - |
| Lauritano et al. [59] | 2016 | R | 87 | 1 | 1.15 | 0.23 | 0.019 |
| Irani et al. [60] | 2016 | R | 112 | 1 | 0.89 | 3.57 | 0.298 |
| Varghese et al. [61] | 2016 | R | 122 | 0 | 0.00 | 0.00 | 0.000 |
| Yahalom et al. [76] | 2016 | P | 30 | 3 | 10.00 | 3.70 | 0.308 |
| Bandyopadhyay et al. [62] | 2017 | R | 143 | 2 | 1.40 | 0.40 | 0.033 |
| Gonzalez-Moles et al. [63] | 2017 | R | 102 | 4 | 3.92 | 4.84 | 0.403 |
| Rimkevičius et al. [64] | 2017 | R | 133 | 3 | 2.26 | - | - |
| Park et al. [65] | 2018 | R | 113 | 0 | 0.00 | 0.00 | 0.000 |
| Rock et al. [5] | 2018 | P | 73 | 6 | 8.22 | 2.58 | 0.215 |
| Laniosz et al. [66] | 2019 | R | 303 | 7 | 2.31 | - | - |
| Shearston et al. [19] | 2019 | R | 281 | 4 | 1.42 | 0.31 | 0.026 |
| Guan et al. [72] | 2020 | R | 829 | 23 | 2.77 | 0.65 | 0.054 |
| Arduino et al. [67] | 2021 | R | 3173 | 82 | 2.58 | - | - |
| Radochová et al. [68] | 2021 | R | 271 | 2 | 0.74 | 0.14 | 0.011 |
| Tsushima et al. [69] | 2021 | R | 565 | 4 | 0.71 | 0.16 | 0.013 |
| Zotti et al. [70] | 2021 | R | 100 | 8 | 8.00 | 3.04 | 0.253 |
| Cai et al. [71] | 2022 | R | 3568 | 10 | 0.28 | 0.06 | 0.005 |
| Total | 24,277 | 331 | 1.36 | 0.32 | 0.027 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-W.; Li, K.Y.; Chan, B.W.A.; McGrath, C.P.; Zheng, L.-W. Rate of Malignant Transformation Differs Based on Diagnostic Criteria for Oral Lichenoid Conditions: A Systematic Review and Meta-Analysis of 24,277 Patients. Cancers 2023, 15, 2537. https://doi.org/10.3390/cancers15092537
Li J-W, Li KY, Chan BWA, McGrath CP, Zheng L-W. Rate of Malignant Transformation Differs Based on Diagnostic Criteria for Oral Lichenoid Conditions: A Systematic Review and Meta-Analysis of 24,277 Patients. Cancers. 2023; 15(9):2537. https://doi.org/10.3390/cancers15092537
Chicago/Turabian StyleLi, Jing-Wen, Kar Yan Li, Bik Wan Amy Chan, Colman Patrick McGrath, and Li-Wu Zheng. 2023. "Rate of Malignant Transformation Differs Based on Diagnostic Criteria for Oral Lichenoid Conditions: A Systematic Review and Meta-Analysis of 24,277 Patients" Cancers 15, no. 9: 2537. https://doi.org/10.3390/cancers15092537
APA StyleLi, J.-W., Li, K. Y., Chan, B. W. A., McGrath, C. P., & Zheng, L.-W. (2023). Rate of Malignant Transformation Differs Based on Diagnostic Criteria for Oral Lichenoid Conditions: A Systematic Review and Meta-Analysis of 24,277 Patients. Cancers, 15(9), 2537. https://doi.org/10.3390/cancers15092537






