Occurrence of Hepatoblastomas in Patients with Beckwith–Wiedemann Spectrum (BWSp)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
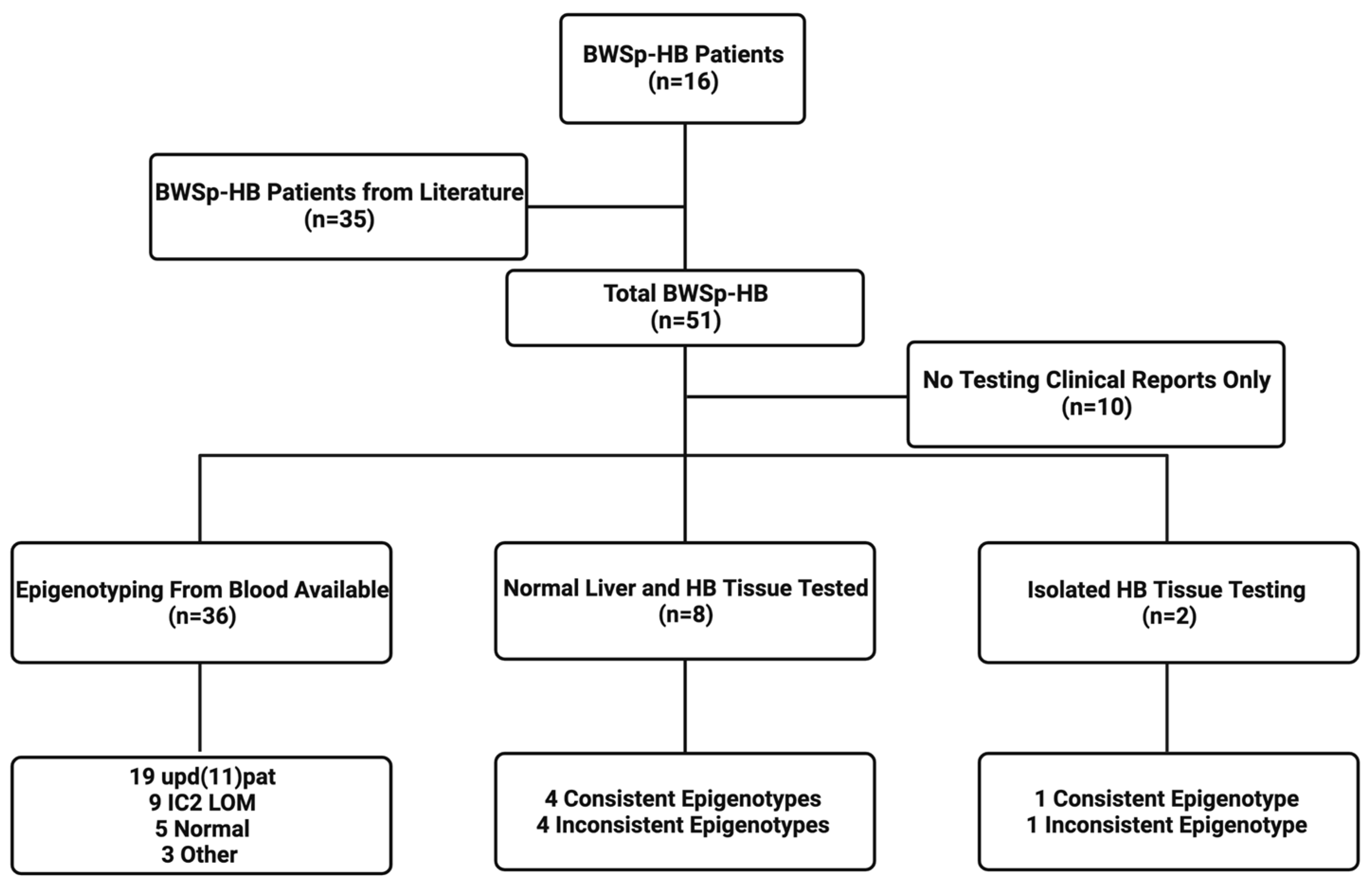
2.1. Patient Cohort
2.2. Molecular Testing
3. Results
3.1. Demographics, Epigenotypes, and Pathologic Types of Patients with BWSp-HB
3.2. BWS Clinical Features
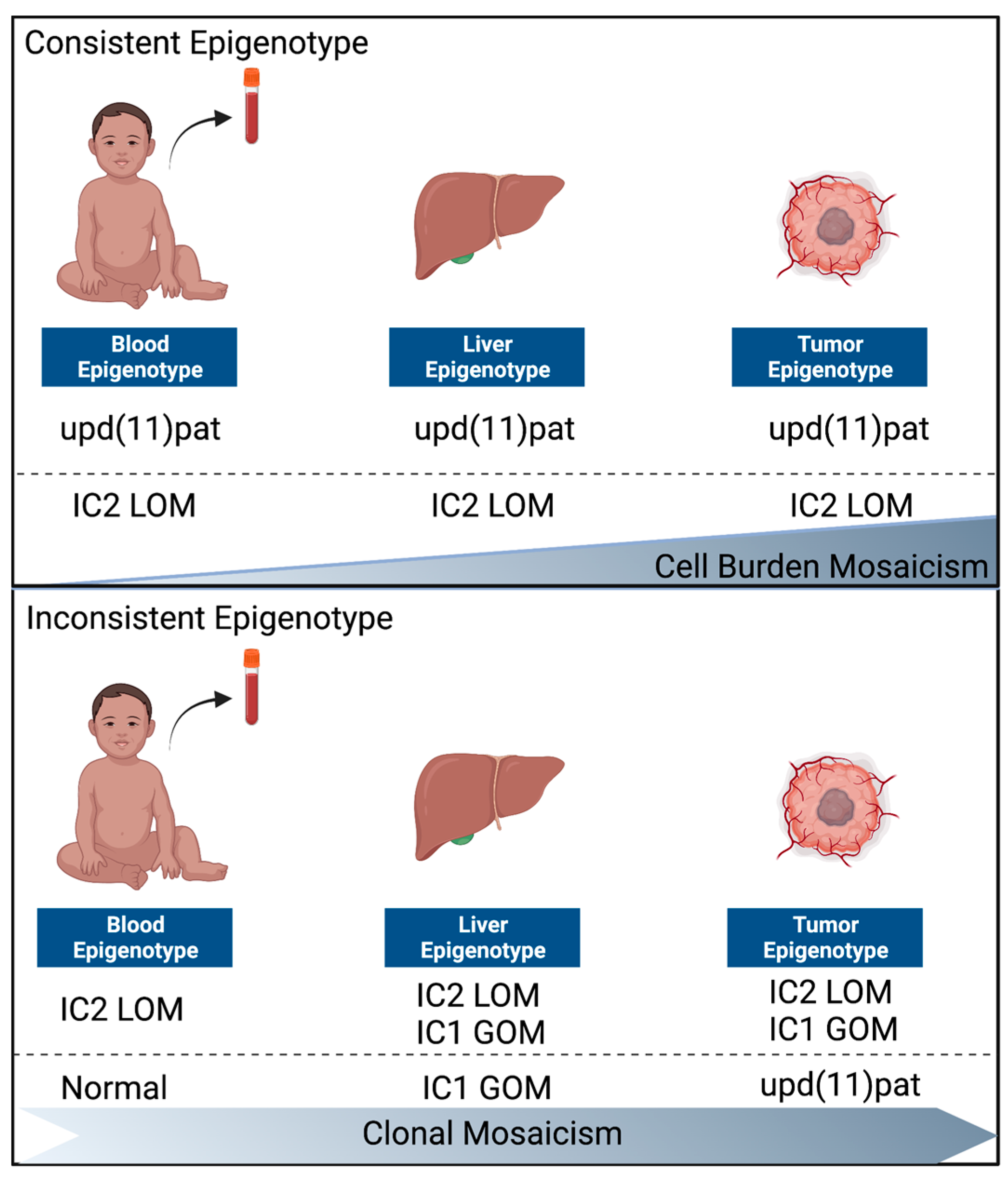
3.3. Normal Liver and Tumor Methylation Analysis
3.4. Solid Tumor Panel Analysis
4. Discussion
4.1. Risk Stratification Based on Blood Genotypes in BWS-HBs
4.2. Two Distinct Groups of BWS-HBs
4.3. The Role of CTNNB1 in BWS-HBs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahla, J.A.; Siegel, D.A.; Dai, S.; Lupo, P.J.; Foster, J.H.; Scheurer, M.E.; Heczey, A.A. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr. Blood Cancer 2022, 10, e29763. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guan, Q.; Guo, H.; Miao, L.; Zhuo, Z. The Genetic Changes of Hepatoblastoma. Front. Oncol. 2021, 11, 690641. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Birch, J. The epidemiology of hepatoblastoma. Pediatr. Blood Cancer 2012, 59, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, G.E.; Kappler, R. Genetics and epigenetics of hepatoblastoma. Pediatr. Blood Cancer 2012, 59, 785–792. [Google Scholar] [CrossRef]
- Goudie, C.; Witkowski, L.; Cullinan, N.; Reichman, L.; Schiller, I.; Tachdjian, M.; Armstrong, L.; Blood, K.A.; Brossard, J.; Brunga, L.; et al. Performance of the McGill Interactive Pediatric OncoGenetic Guidelines for Identifying Cancer Predisposition Syndromes. JAMA Oncol. 2021, 7, 1806–1814. [Google Scholar] [CrossRef]
- Grant, C.N.; Rhee, D.; Tracy, E.T.; Aldrink, J.H.; Baertschiger, R.M.; Lautz, T.B.; Glick, R.D.; Rodeberg, D.A.; Ehrlich, P.F.; Christison-Lagay, E. Pediatric solid tumors and associated cancer predisposition syndromes: Workup, management, and surveillance. A summary from the APSA Cancer Committee. J. Pediatr. Surg. 2022, 57, 430–442. [Google Scholar] [CrossRef]
- Huber, S.; Schimmel, M.; Dunstheimer, D.; Nemes, K.; Richter, M.; Streble, J.; Vollert, K.; Walden, U.; Frühwald, M.C.; Kuhlen, M. The need for tumor surveillance of children and adolescents with cancer predisposition syndromes: A retrospective cohort study in a tertiary-care children’s hospital. Eur. J. Pediatr. 2022, 181, 1585–1596. [Google Scholar] [CrossRef]
- Kalish, J.M.; Doros, L.; Helman, L.J.; Hennekam, R.C.; Kuiper, R.P.; Maas, S.M.; Maher, E.; Nichols, K.E.; Plon, S.E.; Porter, C.C.; et al. Surveillance Recommendations for Children with Overgrowth Syndromes and Predisposition to Wilms Tumors and Hepatoblastoma. Clin. Cancer Res. 2017, 23, e115–e122. [Google Scholar] [CrossRef]
- Achatz, M.I.; Porter, C.C.; Brugières, L.; Druker, H.; Frebourg, T.; Foulkes, W.D.; Kratz, C.P.; Kuiper, R.P.; Hansford, J.R.; Hernandez, H.S.; et al. Cancer Screening Recommendations and Clinical Management of Inherited Gastrointestinal Cancer Syndromes in Childhood. Clin. Cancer Res. 2017, 23, e107–e114. [Google Scholar] [CrossRef]
- Brioude, F.; Kalish, J.M.; Mussa, A.; Foster, A.C.; Bliek, J.; Ferrero, G.B.; E Boonen, S.; Cole, T.; Baker, R.; Bertoletti, M.; et al. Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: An international consensus statement. Nat. Rev. Endocrinol. 2018, 14, 229–249. [Google Scholar] [CrossRef]
- Duffy, K.A.; Cielo, C.M.; Cohen, J.L.; Gonzalez-Gandolfi, C.X.; Griff, J.R.; Hathaway, E.R.; Kupa, J.; Taylor, J.A.; Wang, K.H.; Ganguly, A.; et al. Characterization of the Beckwith-Wiedemann spectrum: Diagnosis and management. Am. J. Med Genet. Part C: Semin. Med Genet. 2019, 181, 693–708. [Google Scholar] [CrossRef]
- Fiala, E.M.; Ortiz, M.V.; Kennedy, J.A.; Glodzik, D.; Fleischut, M.H.; Duffy, K.A.; Hathaway, E.R.; Heaton, T.; Gerstle, J.T.; Steinherz, P.; et al. 11p15.5 epimutations in children with Wilms tumor and hepatoblastoma detected in peripheral blood. Cancer 2020, 126, 3114–3121. [Google Scholar] [CrossRef]
- Duffy, K.A.; Getz, K.D.; Hathaway, E.R.; Byrne, M.E.; MacFarland, S.P.; Kalish, J.M. Characteristics Associated with Tumor Development in Individuals Diagnosed with Beckwith–Wiedemann Spectrum: Novel Tumor-(epi)Genotype-Phenotype Associations in the BWSp Population. Genes 2021, 12, 1839. [Google Scholar] [CrossRef]
- Sotelo-Avila, C.; Gonzalez-Crussi, F.; Fowler, J.W. Complete and incomplete forms of Beckwith-Wiedemann syndrome: Their oncogenic potential. J. Pediatr. 1980, 96, 47–50. [Google Scholar] [CrossRef]
- DeBaun, M.R.; Tucker, M.A. Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J. Pediatr. 1998, 132, 398–400. [Google Scholar] [CrossRef]
- Bliek, J.; Gicquel, C.; Maas, S.; Gaston, V.; le Bouc, Y.; Mannens, M. Epigenotyping as a tool for the prediction of tumor risk and tumor type in patients with Beckwith-Wiedemann syndrome (BWS). J. Pediatr. 2004, 145, 796–799. [Google Scholar] [CrossRef]
- Maas, S.M.; Vansenne, F.; Kadouch, D.J.M.; Ibrahim, A.; Bliek, J.; Hopman, S.; Mannens, M.M.; Merks, J.H.M.; Maher, E.R.; Hennekam, R.C. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am. J. Med Genet. Part A 2016, 170, 2248–2260. [Google Scholar] [CrossRef]
- Weksberg, R.; Nishikawa, J.; Caluseriu, O.; Fei, Y.-L.; Shuman, C.; Wei, C.; Steele, L.; Cameron, J.; Smith, A.; Ambus, I.; et al. Tumor development in the Beckwith-Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alterations including imprinting defects of KCNQ1OT1. Hum. Mol. Genet. 2001, 10, 2989–3000. [Google Scholar] [CrossRef]
- Mussa, A.; Molinatto, C.; Baldassarre, G.; Riberi, E.; Russo, S.; Larizza, L.; Riccio, A.; Ferrero, G.B. Cancer Risk in Beckwith-Wiedemann Syndrome: A Systematic Review and Meta-Analysis Outlining a Novel (Epi)Genotype Specific Histotype Targeted Screening Protocol. J. Pediatr. 2016, 176, 142–149.e1. [Google Scholar] [CrossRef]
- Duffy, K.A.; Deardorff, M.A.; Kalish, J.M. The utility of alpha-fetoprotein screening in Beckwith-Wiedemann syndrome. Am. J. Med Genet. Part A 2017, 173, 581–584. [Google Scholar] [CrossRef]
- Mussa, A.; Russo, S.; de Crescenzo, A.; Freschi, A.; Calzari, L.; Maitz, S.; Macchiaiolo, M.; Molinatto, C.; Baldassarre, G.; Mariani, M.; et al. Fetal growth patterns in Beckwith-Wiedemann syndrome. Clin. Genet. 2016, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.W.; Duffy, K.A.; Richards-Yutz, J.; Deardorff, M.A.; Kalish, J.M.; Ganguly, A. Improved molecular detection of mosaicism in Beckwith-Wiedemann Syndrome. J. Med Genet. 2021, 58, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Conlin, L.K.; Thiel, B.D.; Bonnemann, C.G.; Medne, L.; Ernst, L.; Zackai, E.H.; Deardorff, M.A.; Krantz, I.D.; Hakonarson, H.; Spinner, N.B. Mechanisms of mosaicism, chimerism and uniparental disomy identified by single nucleotide polymorphism array analysis. Hum. Mol. Genet. 2010, 19, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Calton, E.A.; Temple, I.K.; Mackay, D.J.; Lever, M.; Ellard, S.; Flanagan, S.E.; Davies, J.H.; Hussain, K.; Gray, J.C. Hepatoblastoma in a child with a paternally-inherited ABCC8 mutation and mosaic paternal uniparental disomy 11p causing focal congenital hyperinsulinism. Eur. J. Med Genet. 2013, 56, 114–117. [Google Scholar] [CrossRef]
- Little, M.; Thomson, D.B.; Hayward, N.K.; Smith, P.J. Loss of alleles on the short arm of chromosome 11 in a hepatoblastoma from a child with Beckwith-Wiedemann syndrome. Hum. Genet. 1988, 79, 186–189. [Google Scholar] [CrossRef]
- Bachmann, N.; Crazzolara, R.; Bohne, F.; Kotzot, D.; Maurer, K.; Enklaar, T.; Prawitt, D.; Bergmann, C. Novel deletion in 11p15.5 imprinting center region 1 in a patient with Beckwith-Wiedemann syndrome provides insight into distal enhancer regulation and tumorigenesis. Pediatr. Blood Cancer 2017, 64, e26241. [Google Scholar] [CrossRef]
- Haas, O.A.; Zoubek, A.; Grümayer, E.R.; Gadner, H. Constitutional interstitial deletion of 11p11 and pericentric inversion of chromosome 9 in a patient with Wiedemann-Beckwith syndrome and hepatoblastoma. Cancer Genet. Cytogenet. 1986, 23, 95–104. [Google Scholar] [CrossRef]
- Kiruthiga, K.G.; Ramakrishna, B.; Saha, S.; Sen, S. Histological and immunohistochemical study of hepatoblastoma: Correlation with tumour behaviour and survival. J. Gastrointest. Oncol. 2018, 9, 326–337. [Google Scholar] [CrossRef]
- Aronson, D.C.; Meyers, R.L. Malignant tumors of the liver in children. Semin. Pediatr. Surg. 2016, 25, 265–275. [Google Scholar] [CrossRef]
- López-Terrada, D.; Alaggio, R.; de Dávila, M.T.; Czauderna, P.; Hiyama, E.; Katzenstein, H.; Leuschner, I.; Malogolowkin, M.; Meyers, R.; Ranganathan, S.; et al. Towards an international pediatric liver tumor consensus classification: Proceedings of the Los Angeles COG liver tumors symposium. Mod. Pathol. 2014, 27, 472–491. [Google Scholar] [CrossRef]
- Naveh, N.S.S.; Traxler, E.M.; Duffy, K.A.; Kalish, J.M. Molecular networks of hepatoblastoma predisposition and oncogenesis in Beckwith-Wiedemann syndrome. Hepatol. Commun. 2022, 6, 2132–2146. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, S. Mutation Hotspots in the beta-Catenin Gene: Lessons from the Human Cancer Genome Databases. Mol. Cells 2019, 42, 8–16. [Google Scholar]
- Larsen, L.J.; Møller, L.B. Crosstalk of Hedgehog and mTORC1 Pathways. Cells 2020, 9, 2316. [Google Scholar] [CrossRef]
- Duffy, K.A.; Hathaway, E.R.; Klein, S.D.; Ganguly, A.; Kalish, J.M. Epigenetic mosaicism and cell burden in Beckwith–Wiedemann syndrome due to loss of methylation at imprinting control region 2. Cold Spring Harb. Mol. Case Stud. 2021, 7, a006115. [Google Scholar] [CrossRef]
| This Report | The Literature | Total | |
|---|---|---|---|
| Total | 16 | 35 | 51 |
| Male | 5 (31%) | 16 (46%) | 21 (41%) |
| Female | 11 (69%) | 19 (54%) | 30 (59%) |
| Age of diagnosis min | Birth | in utero | in utero |
| Age of diagnosis max | 25 months | 22 years | 22 years |
| Epigenotype from Blood | 15 | 20 | 35 |
| upd(11)pat | 7 (47%) | 13 (65%) | 20 (57%) |
| IC2 LOM | 5 (33%) | 4 (20%) | 9 (26%) |
| Normal | 3 (20%) | n/a | 3 (9%) |
| Other | 0 | 3 (15%) | 3 (9%) |
| Pathology | 14 | 16 | 30 |
| Mixed epithelial | 14 (100%) | 16 (100%) | 30 (100%) |
| +Mesenchymal | 4 (29%) | 1 (6%) | 5 (17%) |
| +Cholangioblastic | 1 (7%) | 1 (6%) | 2 (7%) |
| Clinical Features | # of Patients (n) | % |
|---|---|---|
| Lateralized overgrowth | 14 (16) | 88% |
| Nevus simplex | 8 (10) | 80% |
| Macroglossia | 11 (15) | 80% |
| Ear creases | 8 (10) | 73% |
| Umbilical hernia/diastasis recti | 5 (8) | 55% |
| Hypoglycemia | 8 (15) | 53% |
| Hyperinsulinism | 5 (13) | 38% |
| Placental mesenchymal dysplasia | 1 (3) | 33% |
| Omphalocele | 3 (13) | 23% |
| Nephromegaly | 3 (13) | 21% |
| Hepatomegaly | 2 (13) | 15% |
| ID | BWS Clinical Score | BWS Blood Results | BWS Liver Results | BWS HB Results | CTNNB1 Mutation |
|---|---|---|---|---|---|
| 1 | 9 | upd(11)pat | upd(11)pat | upd(11)pat | c.101G>T, c.98C>T |
| 2 | 10 | upd(11)pat | upd(11)pat | upd(11)pat | c.99_101dup |
| 3 | 8 | upd(11)pat | \ | upd(11)pat | c. 65_100del |
| 4 | 10 | upd(11)pat | \ | \ | c.100G>A |
| 5 | 10 | upd(11)pat | \ | \ | \ |
| 6 | 10 | upd(11)pat | \ | \ | \ |
| 7 | 3 | upd(11)pat | \ | \ | \ |
| 8 | 12 | IC2 LOM | IC2 LOM | IC2 LOM | \ |
| 9 | 6 | IC2 LOM | IC2 LOM | IC2 LOM | c.85_102del |
| 10 | 5 | IC2 LOM | IC2 LOM/IC1 GOM | IC2 LOM/IC1 GOM | c.122C>T |
| 11 | 6 | IC2 LOM | \ | \ | \ |
| 12 | 10 | IC2 LOM | \ | \ | \ |
| 13 | 3 | Normal | \ | IC2 LOM | c.14-28_125del |
| 14 | 2 | \ | IC1GOM | upd(11)pat | c.87_241+71del |
| 15 | 4 | Normal | IC1 GOM | upd(11)pat | c.95A>T |
| 16 | 6 | Normal | IC1 GOM | upd(11)pat | \ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, S.D.; DeMarchis, M.; Linn, R.L.; MacFarland, S.P.; Kalish, J.M. Occurrence of Hepatoblastomas in Patients with Beckwith–Wiedemann Spectrum (BWSp). Cancers 2023, 15, 2548. https://doi.org/10.3390/cancers15092548
Klein SD, DeMarchis M, Linn RL, MacFarland SP, Kalish JM. Occurrence of Hepatoblastomas in Patients with Beckwith–Wiedemann Spectrum (BWSp). Cancers. 2023; 15(9):2548. https://doi.org/10.3390/cancers15092548
Chicago/Turabian StyleKlein, Steven D., Madison DeMarchis, Rebecca L. Linn, Suzanne P. MacFarland, and Jennifer M. Kalish. 2023. "Occurrence of Hepatoblastomas in Patients with Beckwith–Wiedemann Spectrum (BWSp)" Cancers 15, no. 9: 2548. https://doi.org/10.3390/cancers15092548
APA StyleKlein, S. D., DeMarchis, M., Linn, R. L., MacFarland, S. P., & Kalish, J. M. (2023). Occurrence of Hepatoblastomas in Patients with Beckwith–Wiedemann Spectrum (BWSp). Cancers, 15(9), 2548. https://doi.org/10.3390/cancers15092548






