Management of Advanced Prostate Cancer in the Precision Oncology Era
Abstract
Simple Summary
Abstract
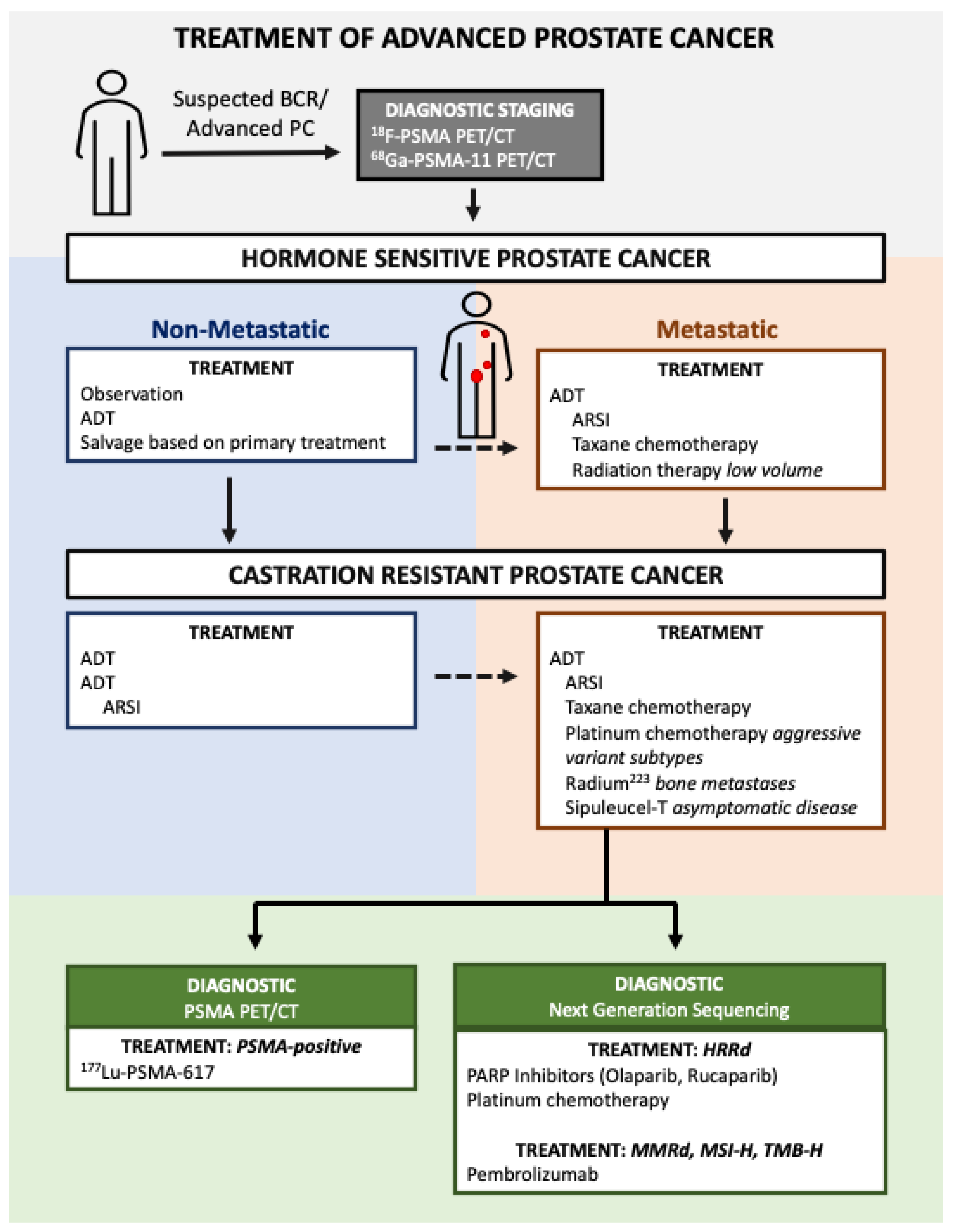
1. Introduction
2. Advances in Molecularly Selected Therapies
2.1. Immunotherapy for Mismatch Repair Deficiency, Microsatellite Instability, and Elevated Tumor Mutational Burden
2.2. Theraputics Indicated for Homologous Recombination Repair Deficiency
2.2.1. Poly (ADP-Ribose) Polymerase (PARP) Inhibitors
2.2.2. Platinum-Based Chemotherapeutics
2.3. Sipuleucel-T Cellular Immunotherapy
3. Theranostics in Prostate Cancer
3.1. Diagnostics
3.2. Therapeutics
3.2.1. Radium-223 Dichloride
3.2.2. 177Lu-PSMA-617
3.2.3. 177Lu-PSMA I&T
3.2.4. 225Actinium-PSMA-617
4. Conclusions
5. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Cancer of the Prostate—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 16 November 2022).
- Denmeade, S.R.; Isaacs, J.T. Overview of Regulation of Systemic Androgen Levels. In Holland-Frei Cancer Medicine; PMPH: Beijing, China, 2003. [Google Scholar]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar] [PubMed]
- Catalona, W.J.; Partin, A.W.; Sanda, M.G.; Wei, J.T.; Klee, G.G.; Bangma, C.H.; Slawin, K.M.; Marks, L.S.; Loeb, S.; Broyles, D.L.; et al. A Multi-Center Study of [−2]Pro-Prostate-Specific Antigen (PSA) in Combination with PSA and Free PSA for Prostate Cancer Detection in the 2.0 to 10.0 Ng/ML PSA Range. J. Urol. 2011, 185, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-L.; Kyprianou, N. Role of Androgens and the Androgen Receptor in Epithelial-Mesenchymal Transition and Invasion of Prostate Cancer Cells. FASEB J. 2010, 24, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.M.; Srinivas, S.; Barocas, D. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Prostate Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 11 January 2023).
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Ikeda, S.; Elkin, S.K.; Tomson, B.N.; Carter, J.L.; Kurzrock, R. Next-Generation Sequencing of Prostate Cancer: Genomic and Pathway Alterations, Potential Actionability Patterns, and Relative Rate of Use of Clinical-Grade Testing. Cancer Biol. Ther. 2019, 20, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Seed, G.; Bertan, C.; Rescigno, P.; Dolling, D.; Figueiredo, I.; Miranda, S.; Nava Rodrigues, D.; Gurel, B.; Clarke, M.; et al. Genomics of Lethal Prostate Cancer at Diagnosis and Castration Resistance. J. Clin. Investig. 2020, 130, 1743–1751. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Gulati, R.; Beightol, M.; Konnick, E.Q.; Cheng, H.H.; Klemfuss, N.; De Sarkar, N.; Yu, E.Y.; Montgomery, R.B.; Nelson, P.S.; et al. Clinical Determinants for Successful Circulating Tumor DNA Analysis in Prostate Cancer. Prostate 2019, 79, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ritch, E.; Fu, S.Y.F.; Herberts, C.; Wang, G.; Warner, E.W.; Schönlau, E.; Taavitsainen, S.; Murtha, A.J.; Vandekerkhove, G.; Beja, K.; et al. Identification of Hypermutation and Defective Mismatch Repair in CtDNA from Metastatic Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Fishel, R. Mismatch Repair. J. Biol. Chem. 2015, 290, 26395–26403. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Shaukat, F.; Isaacsson Velho, P.; Kaur, H.; Shenderov, E.; Pardoll, D.M.; Lotan, T.L. Clinical Features and Therapeutic Outcomes in Men with Advanced Prostate Cancer and DNA Mismatch Repair Gene Mutations. Eur. Urol. 2019, 75, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Fishel, R.; Lescoe, M.K.; Rao, M.R.; Copeland, N.G.; Jenkins, N.A.; Garber, J.; Kane, M.; Kolodner, R. The Human Mutator Gene Homolog MSH2 and Its Association with Hereditary Nonpolyposis Colon Cancer. Cell 1993, 75, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Loeb, L.A. Mutator Phenotype May Be Required for Multistage Carcinogenesis. Cancer Res. 1991, 51, 3075–3079. [Google Scholar]
- Latham, A.; Srinivasan, P.; Kemel, Y.; Shia, J.; Bandlamudi, C.; Mandelker, D.; Middha, S.; Hechtman, J.; Zehir, A.; Dubard-Gault, M.; et al. Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 286–295. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 162, 454. [Google Scholar] [CrossRef]
- Matsushita, H.; Vesely, M.D.; Koboldt, D.C.; Rickert, C.G.; Uppaluri, R.; Magrini, V.J.; Arthur, C.D.; White, J.M.; Chen, Y.-S.; Shea, L.K.; et al. Cancer Exome Analysis Reveals a T Cell Dependent Mechanism of Cancer Immunoediting. Nature 2012, 482, 400–404. [Google Scholar] [CrossRef]
- Graham, L.S.; Schweizer, M.T. Mismatch Repair Deficiency and Clinical Implications in Prostate Cancer. Prostate 2022, 82, S37–S44. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A. Releasing the Brakes on Cancer Immunotherapy. N. Engl. J. Med. 2015, 373, 1490–1492. [Google Scholar] [CrossRef] [PubMed]
- Cutting the Brake Lines: Unlocking Cancer Immunity? Available online: https://www.scienceintheclassroom.org/research-papers/cutting-brake-lines-unlocking-cancer-immunity (accessed on 27 February 2023).
- McGranahan, N.; Furness, A.J.S.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal Neoantigens Elicit T Cell Immunoreactivity and Sensitivity to Immune Checkpoint Blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Wang, S.; Wei, H.; Yu, J. Immune Checkpoint Inhibitor (ICI) Combination Therapy Compared to Monotherapy in Advanced Solid Cancer: A Systematic Review. J. Cancer 2021, 12, 1318–1333. [Google Scholar] [CrossRef]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.M.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus Placebo after Radiotherapy in Patients with Metastatic Castration-Resistant Prostate Cancer That Had Progressed after Docetaxel Chemotherapy (CA184-043): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Fléchon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499.e3. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Dou, C.-X.; Luo, M.-R.; Zhang, K.; Liu, Y.; Zhou, J.-W.; Huang, Z.-P.; Xue, K.-Y.; Liang, H.-Y.; Ouyang, A.-R.; et al. Plasma Cell Subtypes Analyzed Using Artificial Intelligence Algorithm for Predicting Biochemical Recurrence, Immune Escape Potential, and Immunotherapy Response of Prostate Cancer. Front. Immunol. 2022, 13, 241776592. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch-Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.S.; Montgomery, B.; Cheng, H.H.; Yu, E.Y.; Nelson, P.S.; Pritchard, C.; Erickson, S.; Alva, A.; Schweizer, M.T. Mismatch Repair Deficiency in Metastatic Prostate Cancer: Response to PD-1 Blockade and Standard Therapies. PLos ONE 2020, 15, e0233260. [Google Scholar] [CrossRef] [PubMed]
- Barata, P.; Agarwal, N.; Nussenzveig, R.; Gerendash, B.; Jaeger, E.; Hatton, W.; Ledet, E.; Lewis, B.; Layton, J.; Babiker, H.; et al. Clinical Activity of Pembrolizumab in Metastatic Prostate Cancer with Microsatellite Instability High (MSI-H) Detected by Circulating Tumor DNA. J. Immunother. Cancer 2020, 8, e001065. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Fountain, J.; Isaacsson Velho, P.; Lim, S.J.; Wang, H.; Nizialek, E.; Rathi, N.; Nussenzveig, R.; Maughan, B.L.; Velez, M.G.; et al. Tumor Frameshift Mutation Proportion Predicts Response to Immunotherapy in Mismatch Repair-Deficient Prostate Cancer. Oncologist 2021, 26, e270–e278. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Yi-Mi, W.; Cieslik, M.; Lonigro, R.J.; Pankaj, V.; Reimers, M.A.; Xuhong, C.; Yu, N.; Lisha, W.; Kunju, L.P.; de Sarkar, N.; et al. Inactivation of CDK12 Delineates a Distinct Immunogenic Class of Advanced Prostate Cancer. Cell 2018, 173, 1770–1782.e14. [Google Scholar] [CrossRef]
- Anscher, M.S.; Chang, E.; Gao, X.; Gong, Y.; Weinstock, C.; Bloomquist, E.; Adeniyi, O.; Charlab, R.; Zimmerman, S.; Serlemitsos-Day, M.; et al. FDA Approval Summary: Rucaparib for the Treatment of Patients with Deleterious BRCA-Mutated Metastatic Castrate-Resistant Prostate Cancer. Oncologist 2021, 26, 139–146. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research; FDA. Approves Olaparib for HRR Gene-Mutated Metastatic Castration-Resistant Prostate Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer (accessed on 4 January 2023).
- FDA. Approves New Diagnostic Imaging Agent to Detect Recurrent Prostate Cancer. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-diagnostic-imaging-agent-detect-recurrent-prostate-cancer (accessed on 10 April 2023).
- Center for Drug Evaluation and Research; FDA. Approves Second PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-second-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 13 March 2023).
- Center for Drug Evaluation and Research; FDA. Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 13 March 2023).
- Center for Drug Evaluation and Research; FDA. D.I.S.C.O.: Burst Edition: FDA Approval of Pluvicto (Lutetium Lu 177 Vipivotide Tetraxetan) for the Treatment of Adult Patients with Prostate-Specific Membrane Antigen-Positive Metastatic Castration-Resistant Prostate Cancer Who Have Been Treated with Androgen Receptor Pathway Inhibition and Taxane-Based Chemotherapy. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-pluvicto-lutetium-lu-177-vipivotide-tetraxetan-treatment-adult (accessed on 13 March 2023).
- Wright, W.D.; Shah, S.S.; Heyer, W.-D. Homologous Recombination and the Repair of DNA Double-Strand Breaks. J. Biol. Chem. 2018, 293, 10524–10535. [Google Scholar] [CrossRef]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R.; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR Substrate Analysis Reveals Extensive Protein Networks Responsive to DNA Damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wan, J.; Lam, F.C.; Tang, H.; Marley, A.R.; Song, Y.; Miller, C.; Brown, M.; Han, J.; Adeboyeje, G. A Comprehensive Literature Review and Meta-Analysis of the Prevalence of Pan-Cancer BRCA Mutations, Homologous Recombination Repair Gene Mutations, and Homologous Recombination Deficiencies. Environ. Mol. Mutagen. 2022, 63, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.H.; Pritchard, C.C.; Boyd, T.; Nelson, P.S.; Montgomery, B. Biallelic Inactivation of BRCA2 in Platinum-Sensitive Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2016, 69, 992–995. [Google Scholar] [CrossRef]
- Schmid, S.; Omlin, A.; Higano, C.; Sweeney, C.; Martinez Chanza, N.; Mehra, N.; Kuppen, M.C.P.; Beltran, H.; Conteduca, V.; Vargas Pivato de Almeida, D.; et al. Activity of Platinum-Based Chemotherapy in Patients With Advanced Prostate Cancer With and Without DNA Repair Gene Aberrations. JAMA Netw. Open 2020, 3, e2021692. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA Repair Defect in BRCA Mutant Cells as a Therapeutic Strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP Inhibitors: Synthetic Lethality in the Clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Duan, Y.; Zhao, Y.; Li, Y.; Liu, J.; Zhang, C.; He, S. PARP Inhibitors in Breast and Ovarian Cancer with BRCA Mutations: A Meta-Analysis of Survival. Aging 2021, 13, 8975–8988. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Campbell, D.; Patnaik, A.; Shapiro, J.D.; Sautois, B.; Vogelzang, N.J.; Voog, E.G.; Bryce, A.H.; McDermott, R.; Ricci, F.; et al. Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U.; et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Cheng, H.H.; Nelson, P.S.; Montgomery, R.B. Two Steps Forward and One Step Back for Precision in Prostate Cancer Treatment. J. Clin. Oncol. 2020, 38, 3740–3742. [Google Scholar] [CrossRef]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.; Loredo, E.; Procopio, G.; de Menezes, J.; Girotto, G.; Arslan, C.; et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid. 2022, 1, EVIDoa2200043. [Google Scholar] [CrossRef]
- Kwon, D.H.; Booth, C.M.; Prasad, V. Untangling the PROfound Trial for Advanced Prostate Cancer: Is There Really a Role for Olaparib? Eur. Urol. 2021, 79, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Rathkopf, D.E.; Smith, M.R.; Efstathiou, E.; Attard, G.; Olmos, D.; Lee, J.Y.; Small, E.J.; Gomes, A.J.; Roubaud, G.; et al. Phase 3 MAGNITUDE Study: First Results of Niraparib (NIRA) with Abiraterone Acetate and Prednisone (AAP) as First-Line Therapy in Patients (Pts) with Metastatic Castration-Resistant Prostate Cancer (MCRPC) with and without Homologous Recombination Repair (HRR) Gene Alterations. J. Clin. Oncol. 2022, 40, 12. [Google Scholar] [CrossRef]
- Hussain, M.; Daignault-Newton, S.; Twardowski, P.W.; Albany, C.; Stein, M.N.; Kunju, L.P.; Siddiqui, J.; Wu, Y.-M.; Robinson, D.; Lonigro, R.J.; et al. Targeting Androgen Receptor and DNA Repair in Metastatic Castration-Resistant Prostate Cancer: Results From NCI 9012. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 991–999. [Google Scholar] [CrossRef]
- De Sarkar, N.; Dasgupta, S.; Chatterjee, P.; Coleman, I.; Ha, G.; Ang, L.S.; Kohlbrenner, E.A.; Frank, S.B.; Nunez, T.A.; Salipante, S.J.; et al. Genomic Attributes of Homology-Directed DNA Repair Deficiency in Metastatic Prostate Cancer. JCI Insight 2021, 6, e152789. [Google Scholar] [CrossRef] [PubMed]
- Sztupinszki, Z.; Diossy, M.; Krzystanek, M.; Borcsok, J.; Pomerantz, M.; Tisza, V.; Spisak, S.; Rusz, O.; Csabai, I.; Freedman, M.; et al. Detection of Molecular Signatures of Homologous Recombination Deficiency in Prostate Cancer with or without BRCA1/2 Mutations. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2673–2680. [Google Scholar] [CrossRef]
- Swisher, E.M.; Lin, K.K.; Oza, A.M.; Scott, C.L.; Giordano, H.; Sun, J.; Konecny, G.E.; Coleman, R.L.; Tinker, A.V.; O’Malley, D.M.; et al. Rucaparib in Relapsed, Platinum-Sensitive High-Grade Ovarian Carcinoma (ARIEL2 Part 1): An International, Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 75–87. [Google Scholar] [CrossRef]
- Davies, H.; Glodzik, D.; Morganella, S.; Yates, L.R.; Staaf, J.; Zou, X.; Ramakrishna, M.; Martin, S.; Boyault, S.; Sieuwerts, A.M.; et al. HRDetect Is a Predictor of BRCA1 and BRCA2 Deficiency Based on Mutational Signatures. Nat. Med. 2017, 23, 517–525. [Google Scholar] [CrossRef]
- Aparicio, A.M.; Harzstark, A.L.; Corn, P.G.; Wen, S.; Araujo, J.C.; Tu, S.-M.; Pagliaro, L.C.; Kim, J.; Millikan, R.E.; Ryan, C.; et al. Platinum-Based Chemotherapy for Variant Castrate-Resistant Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 3621–3630. [Google Scholar] [CrossRef]
- Hager, S.; Ackermann, C.J.; Joerger, M.; Gillessen, S.; Omlin, A. Anti-Tumour Activity of Platinum Compounds in Advanced Prostate Cancer-a Systematic Literature Review. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 975–984. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Petrylak, D.P.; Sartor, O.; Witjes, J.A.; Demkow, T.; Ferrero, J.-M.; Eymard, J.-C.; Falcon, S.; Calabrò, F.; James, N.; et al. Multinational, Double-Blind, Phase III Study of Prednisone and Either Satraplatin or Placebo in Patients with Castrate-Refractory Prostate Cancer Progressing after Prior Chemotherapy: The SPARC Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Wang, Y.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; Xie, Y. Prevalence of BRCA1 Mutations and Responses to Neoadjuvant Chemotherapy among BRCA1 Carriers and Non-Carriers with Triple-Negative Breast Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 523–528. [Google Scholar] [CrossRef]
- Pomerantz, M.M.; Spisák, S.; Jia, L.; Cronin, A.M.; Csabai, I.; Ledet, E.; Sartor, A.O.; Rainville, I.; O’Connor, E.P.; Herbert, Z.T.; et al. The Association between Germline BRCA2 Variants and Sensitivity to Platinum-Based Chemotherapy among Men with Metastatic Prostate Cancer. Cancer 2017, 123, 3532–3539. [Google Scholar] [CrossRef]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Anassi, E.; Ndefo, U.A. Sipuleucel-T (Provenge) Injection. Pharm. Ther. 2011, 36, 197–202. [Google Scholar]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjug. Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Kumar, U. Positron Emission Tomography: An Overview. J. Med. Phys. Assoc. Med. Phys. India 2006, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Relative Efficacy of 225Ac-PSMA-617 and 177Lu-PSMA-617 in Prostate Cancer Based on Subcellular Dosimetry. Mol. Imaging Radionucl. Ther. 2022, 31, 1–6. [Google Scholar] [CrossRef]
- Radium-223 for Advanced Prostate Cancer-NCI. Available online: https://www.cancer.gov/types/prostate/research/radium-223-improves-survival (accessed on 1 December 2022).
- Wright, G.L.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of Prostate-Specific Membrane Antigen in Normal, Benign, and Malignant Prostate Tissues. Urol. Oncol. 1995, 1, 18–28. [Google Scholar] [CrossRef]
- Cunha, A.C.; Weigle, B.; Kiessling, A.; Bachmann, M.; Rieber, E.P. Tissue-Specificity of Prostate Specific Antigens: Comparative Analysis of Transcript Levels in Prostate and Non-Prostatic Tissues. Cancer Lett. 2006, 236, 229–238. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate Specific Membrane Antigen Expression in Prostatic Intraepithelial Neoplasia and Adenocarcinoma: A Study of 184 Cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-Specific Membrane Antigen Expression in Normal and Malignant Human Tissues. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Farsad, M.; Schiavina, R.; Franceschelli, A.; Sanguedolce, F.; Castellucci, P.; Bertaccini, A.; Brunocilla, E.; Manferrari, F.; Concetti, S.; Garofalo, M.; et al. Positron-Emission Tomography in Imaging and Staging Prostate Cancer. Cancer Biomark. Sect. Dis. Markers 2008, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Saule, L.; Radzina, M.; Liepa, M.; Roznere, L.; Lioznovs, A.; Ratniece, M.; Mamis, E.; Vjaters, E. Recurrent Prostate Cancer Diagnostics with 18F-PSMA-1007 PET/CT: A Systematic Review of the Current State. Diagnostics 2022, 12, 3176. [Google Scholar] [CrossRef]
- Hara, T.; Kosaka, N.; Kishi, H. PET Imaging of Prostate Cancer Using Carbon-11-Choline. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1998, 39, 990–995. [Google Scholar]
- Welle, C.L.; Cullen, E.L.; Peller, P.J.; Lowe, V.J.; Murphy, R.C.; Johnson, G.B.; Binkovitz, L.A. 11C-Choline PET/CT in Recurrent Prostate Cancer and Nonprostatic Neoplastic Processes. RadioGraphics 2016, 36, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Parent, E.E.; Schuster, D.M. Update on 18F-Fluciclovine PET for Prostate Cancer Imaging. J. Nucl. Med. 2018, 59, 733. [Google Scholar] [CrossRef]
- Marcus, C.; Abiodun-Ojo, O.A.; Jani, A.B.; Schuster, D.M. Clinical Utility of 18F-Fluciclovine PET/CT in Recurrent Prostate Cancer with Very Low (≤0.3 Ng/ML) Prostate-Specific Antigen Levels. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 406–414. [Google Scholar]
- Kaushik, A.K.; Vareed, S.K.; Basu, S.; Putluri, V.; Putluri, N.; Panzitt, K.; Brennan, C.A.; Chinnaiyan, A.M.; Vergara, I.A.; Erho, N.; et al. Metabolomic Profiling Identifies Biochemical Pathways Associated with Castration-Resistant Prostate Cancer. J. Proteome Res. 2014, 13, 1088–1100. [Google Scholar] [CrossRef]
- Sun, J.; Bok, R.A.; DeLos Santos, J.; Upadhyay, D.; DeLos Santos, R.; Agarwal, S.; Van Criekinge, M.; Vigneron, D.B.; Aggarwal, R.; Peehl, D.M.; et al. Resistance to Androgen Deprivation Leads to Altered Metabolism in Human and Murine Prostate Cancer Cell and Tumor Models. Metabolites 2021, 11, 139. [Google Scholar] [CrossRef]
- Zhou, R.; Choi, H.; Cao, J.; Pantel, A.; Gupta, M.; Lee, H.; Mankoff, D. 18F-Fluciclovine PET Imaging of Glutaminase Inhibition in Breast Cancer Models. J. Nucl. Med. 2022, 64, 131–136. [Google Scholar] [CrossRef]
- Schuster, D.M.; Nieh, P.T.; Jani, A.B.; Amzat, R.; Bowman, F.D.; Halkar, R.K.; Master, V.A.; Nye, J.A.; Odewole, O.A.; Osunkoya, A.O.; et al. Anti-3-[18F]FACBC Positron Emission Tomography-Computerized Tomography and 111In-Capromab Pendetide Single Photon Emission Computerized Tomography-Computerized Tomography for Recurrent Prostate Carcinoma: Results of a Prospective Clinical Trial. J. Urol. 2014, 191, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Abiodun-Ojo, O.A.; Akintayo, A.A.; Akin-Akintayo, O.O.; Tade, F.I.; Nieh, P.T.; Master, V.A.; Alemozaffar, M.; Osunkoya, A.O.; Goodman, M.M.; Fei, B.; et al. 18F-Fluciclovine Parameters on Targeted Prostate Biopsy Associated with True Positivity in Recurrent Prostate Cancer. J. Nucl. Med. 2019, 60, 1531–1536. [Google Scholar] [CrossRef]
- Werner, R.A.; Derlin, T.; Lapa, C.; Sheikbahaei, S.; Higuchi, T.; Giesel, F.L.; Behr, S.; Drzezga, A.; Kimura, H.; Buck, A.K.; et al. 18F-Labeled, PSMA-Targeted Radiotracers: Leveraging the Advantages of Radiofluorination for Prostate Cancer Molecular Imaging. Theranostics 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Rowe, S.P.; Shih, J.H.; Lindenberg, L.; Turkbey, B.; Fourquet, A.; Lin, F.I.; Adler, S.; Eclarinal, P.; McKinney, Y.L.; et al. Predictors of 18F-DCFPyL PET/CT Positivity in Patients with Biochemical Recurrence of Prostate Cancer After Local Therapy. J. Nucl. Med. 2022, 63, 1184–1190. [Google Scholar] [CrossRef]
- Morigi, J.J.; Stricker, P.D.; van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Urso, L.; Bianconi, F.; Palumbo, B.; Marzola, M.C.; Evangelista, L.; Schillaci, O. Radiomics and Theranostics with Molecular and Metabolic Probes in Prostate Cancer: Toward a Personalized Approach. Expert Rev. Mol. Diagn. 2023, 23, 243–255. [Google Scholar] [CrossRef]
- Double-Blind, Randomised, Multiple Dose, Phase III, Multicentre Study of Alpharadin in the Treatment of Patients with Symptomatic Hormone Refractory Prostate Cancer With Skeletal Metastases. Available online: https://clinicaltrials.gov/ct2/show/NCT00699751 (accessed on 30 November 2022).
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical Therapy in Cancer: Clinical Advances and Challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Sartor, O.; Baghian, A. Prostate Specific Membrane Antigen Binding Radiopharmaceuticals: Current Data and New Concepts. Front. Med. 2022, 9, 1060922. [Google Scholar] [CrossRef]
- ASCO 2021: VISION Study Results-Phase III Study of Lutetium-177-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer. Available online: https://www.urotoday.com/conference-highlights/asco-2021/asco-2021-prostate-cancer/130060-asco-2021-vision-study-results-phase-iii-study-of-lutetium-177-psma-617-in-patients-with-metastatic-castration-resistant-prostate-cancer.html (accessed on 23 January 2023).
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer (TheraP): A Randomised, Open-Label, Phase 2 Trial. Lancet Lond. Engl. 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Michalski, K.; Ruf, J.; Goetz, C.; Seitz, A.K.; Buck, A.K.; Lapa, C.; Hartrampf, P.E. Prognostic Implications of Dual Tracer PET/CT: PSMA Ligand and [18F]FDG PET/CT in Patients Undergoing [177Lu]PSMA Radioligand Therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2024–2030. [Google Scholar] [CrossRef]
- Sartor, A.O.; Morris, M.J.; Chi, K.N.; De Bono, J.S.; Shore, N.D.; Crosby, M.; Kreisl, T.N.; Fizazi, K. PSMAfore: A Phase 3 Study to Compare 177Lu-PSMA-617 Treatment with a Change in Androgen Receptor Pathway Inhibitor in Taxane-Naïve Patients with Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2022, 40, TPS211. [Google Scholar] [CrossRef]
- Novartis PluvictoTM Shows Statistically Significant and Clinically Meaningful Radiographic Progression-Free Survival Benefit in Patients with PSMA–Positive Metastatic Castration-Resistant Prostate Cancer. Available online: https://www.novartis.com/news/media-releases/novartis-pluvictotm-shows-statistically-significant-and-clinically-meaningful-radiographic-progression-free-survival-benefit-patients-psma-positive-metastatic-castration-resistant-prostate-cancer (accessed on 20 March 2023).
- Sartor, A.O.; Tagawa, S.T.; Saad, F.; De Bono, J.S.; Feng, F.Y.; Fizazi, K.; Sakharova, O.V.; Morris, M.J. PSMAddition: A Phase 3 Trial to Compare Treatment with 177Lu-PSMA-617 plus Standard of Care (SOC) versus SOC Alone in Patients with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2022, 40, TPS210. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals An Open-Label, Randomized, Phase III Study Comparing 177Lu-PSMA-617 in Combination With Standard of Care, Versus Standard of Care Alone, in Adult Male Patients with Metastatic Hormone Sensitive Prostate Cancer (MHSPC). Available online: https://clinicaltrials.gov/ct2/show/NCT04720157 (accessed on 3 April 2023).
- Schuchardt, C.; Zhang, J.; Kulkarni, H.R.; Chen, X.; Müller, D.; Baum, R.P. Prostate-Specific Membrane Antigen Radioligand Therapy Using 177Lu-PSMA I&T and 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: Comparison of Safety, Biodistribution, and Dosimetry. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 1199–1207. [Google Scholar] [CrossRef]
- Privé, B.M.; Janssen, M.J.R.; van Oort, I.M.; Muselaers, C.H.J.; Jonker, M.A.; van Gemert, W.A.; de Groot, M.; Westdorp, H.; Mehra, N.; Verzijlbergen, J.F.; et al. Update to a Randomized Controlled Trial of Lutetium-177-PSMA in Oligo-Metastatic Hormone-Sensitive Prostate Cancer: The BULLSEYE Trial. Trials 2021, 22, 768. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in Chemotherapy-Naive Patients with Advanced Prostate Cancer: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Endocyte AcTION: A Phase I Study of [225Ac]Ac-PSMA-617 in Men with PSMA-Positive Prostate Cancer with or without Prior [177Lu]Lu-PSMA-617 Radioligand Therapy. Available online: https://clinicaltrials.gov/ct2/show/NCT04597411 (accessed on 22 January 2023).
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-Resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholomä, M.; Ezziddin, S. 225Ac-PSMA-617/177Lu-PSMA-617 Tandem Therapy of Metastatic Castration-Resistant Prostate Cancer: Pilot Experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef]
| Agent | Year FDA Approval | Supporting Clinical Trial | Indication |
|---|---|---|---|
| Pembrolizumab | 2017 [11] | NCT02628067 (KEYNOTE-158) | Tissue-agnostic approval for unresectable or metastatic microsatellite-high (MSI-H) or mismatch repair deficiency (MMRd) solid tumors following prior treatment with no satisfactory alternative treatment available |
| 2020 [35] | NCT02628067 (KEYNOTE-158) | Tissue-agnostic approval for unresectable or metastatic cancer with tumor mutational burden-high (TMB-H) | |
| Rucaparib | 2020 [41] | NCT02952534 (TRITION2) | Patients with mCRPC with deleterious BRCA1/2 mutation who have progressed following androgen receptor-directed therapy and taxane chemotherapy |
| Olaparib | 2020 [42] | NCT02987543 (PROfound) | Patients with mCRPC with deleterious or suspected deleterious BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, fANCL, PALB2, RAD51B, RAD51C, RAD51D, and RAD54L mutations who have progressed following treatment with enzalutamide or abiraterone |
| 18F-fluciclovine PET/CT | 2016 [43] | NCT02578940 (FALCON) | Patients with suspected biochemically recurrent prostate cancer |
| 18F-DCFPyL-PSMA PET/CT | 2021 [44] | NCT02981368 (OSPREY) | Patients with suspected prostate cancer metastasis who are potentially curable via surgery or other therapy |
| 2021 [44] | NCT03739684 (CONDOR) | Suspected biochemical recurrence indicated by prostate-specific antigen levels | |
| 68Ga-PSMA-11 PET/CT | 2020 [45] | NCT03368547 | Patients with suspected prostate cancer metastasis who are potentially curable with surgery or radiation |
| 2020 [45] | NCT02940262 | Suspected biochemical recurrence indicated by prostate-specific antigen levels | |
| Radium223 | 2013 | NCT00699751 (ALSYMPCA) | Patients with metastatic prostate cancer with symptomatic bone metastasis |
| 177Lu-PSMA-617 | 2022 [46] | NCT03511664 (VISION) | Patients with PSMA-positive metastatic castration-resistant prostate cancer who have progressed following androgen receptor pathway inhibition and taxane chemotherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gillette, C.M.; Yette, G.A.; Cramer, S.D.; Graham, L.S. Management of Advanced Prostate Cancer in the Precision Oncology Era. Cancers 2023, 15, 2552. https://doi.org/10.3390/cancers15092552
Gillette CM, Yette GA, Cramer SD, Graham LS. Management of Advanced Prostate Cancer in the Precision Oncology Era. Cancers. 2023; 15(9):2552. https://doi.org/10.3390/cancers15092552
Chicago/Turabian StyleGillette, Claire M., Gabriel A. Yette, Scott D. Cramer, and Laura S. Graham. 2023. "Management of Advanced Prostate Cancer in the Precision Oncology Era" Cancers 15, no. 9: 2552. https://doi.org/10.3390/cancers15092552
APA StyleGillette, C. M., Yette, G. A., Cramer, S. D., & Graham, L. S. (2023). Management of Advanced Prostate Cancer in the Precision Oncology Era. Cancers, 15(9), 2552. https://doi.org/10.3390/cancers15092552





