Dietary Choline and Betaine Intake and Risk of Colorectal Cancer in an Iranian Population
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
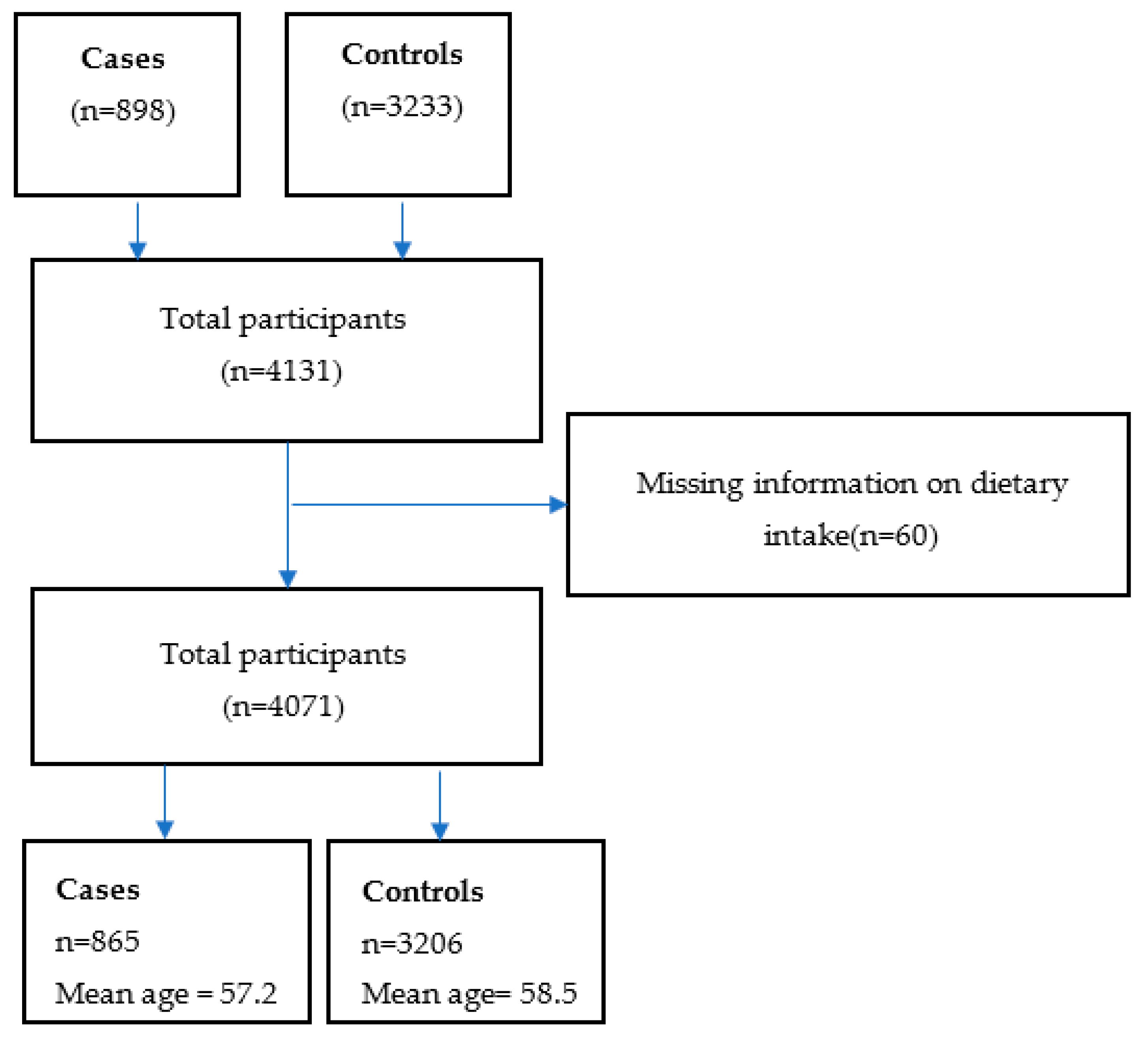
2.1. Study Design and Population
2.2. Anthropometry and Lifestyle Factor
2.3. Dietary Factors
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | The body mass index |
| CRC | Colorectal cancer |
| CI | Confidence interval |
| FCT | Food composition table |
| FINJEM | Finland Job Exposure Matrix |
| FFQ | Food Frequency Questionnaire |
| GPC | Glycerophosphocholine |
| ORs | Odds ratios |
| PtdCho | Phosphatidylcholine |
| PPWL | Physical activity workload |
| Pcho | Phosphocholine |
| SM | Sphingomyelin |
| SD | Standard deviations |
| SES | Socioeconomic status |
| TMA | Trimethylamine |
| USDA | US Department of Agriculture |
Appendix A
| List of Choline Type | Source 1 | Source 2 | Source 3 | Source 4 | Source 5 |
|---|---|---|---|---|---|
| Total choline | Eggs | Yogurt | Bread | Chicken | Lamb products and red meat |
| Free choline | Onion | Tomatoes | Lamb products and red meat | Tea | Milk |
| GPC | Yogurt | Milk | Doogh | Lamb products and red meat | Rice |
| Pcho | Yogurt | Tomatoes | Milk | Onion | Chicken |
| PtdCho | Eggs | Chicken | Lamb products and red meat | Apples | Lentil, Mung, Bean |
| SM | Chicken | Eggs | Yogurt | Lamb products and red meat | Cheese |
| Betaine | Bread | Pasta and noodles | Tea | Beets, turnips | Green leafy vegetables |
References
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- WHO Cancer. Available online: https://gco.iarc.fr/today/home (accessed on 14 July 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Roshandel, G.; Ferlay, J.; Ghanbari-Motlagh, A.; Partovipour, E.; Salavati, F.; Aryan, K.; Mohammadi, G.; Khoshaabi, M.; Sadjadi, A.; Davanlou, M.; et al. Cancer in Iran 2008 to 2025: Recent incidence trends and short-term predictions of the future burden. Cancer Epidemiol. 2021, 149, 594–605. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.E.; Metcalfe, A.; Hillier, T.W.; King, W.D.; Lee, S.; Pader, J.; Brenner, D.R. Combinations of modifiable lifestyle behaviours in relation to colorectal cancer risk in Alberta’s Tomorrow Project. Sci. Rep. 2020, 10, 20561. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Shin, Y.J. Role of Choline in Ocular Diseases. Int. J. Mol. Sci. 2021, 22, 4733. [Google Scholar] [CrossRef] [PubMed]
- Van Parys, A.; Karlsson, T.; Vinknes, K.J.; Olsen, T.; Øyen, J.; Dierkes, J.; Nygård, O.; Lysne, V. Food Sources Contributing to Intake of Choline and Individual Choline Forms in a Norwegian Cohort of Patients with Stable Angina Pectoris. Front. Nutr. 2021, 8, 676026. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Parikh, V. Choline transporters, cholinergic transmission and cognition. Nat. Rev. Neurosci. 2005, 6, 48–56. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef]
- Patterson, K.Y.; Bhagwat, S.A.; Williams, J.R.; Howe, J.C.; Holden, J.M. USDA Database for the Choline Content of Common Foods; USDA: Washington, DC, USA, 2008. [Google Scholar]
- Zhou, R.F.; Chen, X.L.; Zhou, Z.G.; Zhang, Y.J.; Lan, Q.Y.; Liao, G.C.; Chen, Y.M.; Zhu, H.L. Higher dietary intakes of choline and betaine are associated with a lower risk of primary liver cancer: A case-control study. Sci. Rep. 2017, 7, 679. [Google Scholar] [CrossRef]
- Sun, S.; Li, X.; Ren, A.; Du, M.; Du, H.; Shu, Y.; Zhu, L.; Wang, W. Choline and betaine consumption lowers cancer risk: A meta-analysis of epidemiologic studies. Sci. Rep. 2016, 6, 35547. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.S.; Fang, Y.J.; Pan, Z.Z.; Zhong, X.; Zheng, M.C.; Chen, Y.M.; Zhang, C.X. choline and betaine intake and colorectal cancer risk in Chinese population: A case-control study. PLoS ONE 2015, 10, e0118661. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Giovannucci, E.; Fuchs, C.S.; Willett, W.C.; Zeisel, S.H.; Cho, E. Choline and betaine intake and the risk of colorectal cancer in men. Cancer Epidemiol. Biomark. Prev. 2010, 19, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Hadji, M.; Rashidian, H.; Marzban, M.; Gholipour, M.; Naghibzadeh-Tahami, A.; Mohebbi, E.; Ebrahimi, E.; Hosseini, B.; Haghdoost, A.A.; Rezaianzadeh, A.; et al. The Iranian Study of Opium and Cancer (IROPICAN): Rationale, Design, and Initial Findings. Arch. Iran Med. 2021, 24, 167–176. [Google Scholar] [CrossRef]
- Kauppinen, T.; Toikkanen, J.; Pukkala, E. From cross-tabulations to multipurpose exposure information system: A new job-exposure matrix. Am. J. Ind. Med. 1998, 33, 409–417. [Google Scholar] [CrossRef]
- Kauppinen, T.; Heikkilä, P.; Plato, N.; Woldbæk, T.; Lenvik, K.; Hansen, J.; Kristjansson, V.; Pukkala, E. Construction of job-exposure matrices for the Nordic occupational cancer study. Acta Oncol. 2009, 48, 791–800. [Google Scholar] [CrossRef]
- Mohebbi, E.; Hadji, M.; Rashidian, H.; Rezaianzadeh, A.; Marzban, M.; Haghdoost, A.A.; Naghibzadeh Tahami, A.; Moradi, A.; Gholipour, M.; Najafi, F.; et al. Opium use and the risk of head and neck squamous cell carcinoma. Int. J. Cancer 2021, 148, 1066–1076. [Google Scholar] [CrossRef]
- Poustchi, H.; Eghtesad, S.; Kamangar, F.; Etemadi, A.; Keshtkar, A.A.; Hekmatdoost, A.; Mohammadi, Z.; Mahmoudi, Z.; Shayanrad, A.; Roozafzai, F.; et al. Prospective Epidemiological Research Studies in Iran (the PERSIAN Cohort Study): Rationale, Objectives, and Design. Am. J. Epidemiol. 2018, 187, 647–655. [Google Scholar] [CrossRef]
- Seyyedsalehi, M.S.; Collatuzzo, G.; Huybrechts, I.; Hadji, M.; Rashidian, H.; Safari-Faramani, R.; Alizadeh-Navaei, R.; Kamangar, F.; Etemadi, A.; Pukkala, E.; et al. Association between dietary fat intake and colorectal cancer: A multicenter case-control study in Iran. Front. Nutr. 2022, 9, 1017720. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Ahuja, J.K.C.; Wu, X.; Somanchi, M.; Nickle, M.; Nguyen, Q.A.; Roseland, J.M.; Williams, J.R.; Patterson, K.Y.; Li, Y.; et al. USDA National Nutrient Database for Standard Reference. Legacy Release, Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, Haytowitz. 2019. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 25 May 2020).
- US Department of Agriculture (USDA) ARSFC. Foundation Foods. Version Current: October 2020. Available online: https://fdc.nal.usda.gov (accessed on 25 May 2020).
- US Department of Agriculture (USDA) ARSFC. Agricultural Research Service. FoodData Central, Agricultural Research Service. USDA Food and Nutrient Database for Dietary Studies 2017–2018. Food Surveys Research Group Home Page. Available online: http://wwwarsusdagov/nea/bhnrc/fsrg (accessed on 25 May 2020).
- US Department of Agriculture (USDA) ARS, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Legacy. 2018. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=349687 (accessed on 25 May 2020).
- Food composition tables for the Near East. FAO Food Nutr. Pap. 1982, 26, 1–265.
- Musaiger, A.O. Food Composition Tables for Kingdom of Bahrain; Arab Center for Nutrition: Manama, Bahrain, 2011. [Google Scholar]
- Rossi, M.; Khalifeh, M.; Fiori, F.; Parpinel, M.; Serraino, D.; Pelucchi, C.; Negri, E.; Giacosa, A.; Crispo, A.; Collatuzzo, G.; et al. Dietary Choline and Sphingomyelin Choline moiety Intake and Risk of Colorectal Cancer: A Case Control Study. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Lewis, E.D.; Kosik, S.J.; Zhao, Y.Y.; Jacobs, R.L.; Curtis, J.M.; Field, C.J. Total choline and choline-containing moieties of commercially available pulses. Plant Foods Hum. Nutr. 2014, 69, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Lewis, E.; Zhao, Y.-; Asomaning, J.; Jacobs, R.; Field, C. Measurement of the total choline content in 48 commercial dairy products or dairy alternatives. J. Food Compos. Anal. 2016, 45, 1–8. [Google Scholar] [CrossRef]
- FAO/INFOODS. Guidelines for food Matching Version 1.2.; FAO: Rome, Italy, 2012. [Google Scholar]
- Seyyedsalehi, M.S.; Collatuzzo, G.; Rashidian, H.; Hadji, M.; Gholipour, M.; Mohebbi, E.; Kamangar, F.; Pukkala, E.; Huybrechts, I.; Gunter, M.J.; et al. Dietary Ruminant and Industrial Trans-Fatty Acids Intake and Colorectal Cancer Risk. Nutrients 2022, 14, 4912. [Google Scholar] [CrossRef]
- Cho, E.; Willett, W.C.; Colditz, G.A.; Fuchs, C.S.; Wu, K.; Chan, A.T.; Zeisel, S.H.; Giovannucci, E.L. Dietary choline and betaine and the risk of distal colorectal adenoma in women. J. Natl. Cancer Inst. 2007, 99, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Cho, E.; Lee, J.E. Association of choline and betaine levels with cancer incidence and survival: A meta-analysis. Clin. Nutr. 2019, 38, 100–109. [Google Scholar] [CrossRef]
- Guertin, K.A.; Li, X.S.; Graubard, B.I.; Albanes, D.; Weinstein, S.J.; Goedert, J.J.; Wang, Z.; Hazen, S.L.; Sinha, R. Serum Trimethylamine Noxide, Carnitine, Choline, and Betaine in Relation to Colorectal Cancer Risk in the Alpha Tocopherol, Beta Carotene Cancer Prevention Study. Cancer Epidemiol. Biomark. Prev. 2017, 26, 945–952. [Google Scholar] [CrossRef]
- Myte, R.; Gylling, B.; Schneede, J.; Ueland, P.M.; Häggström, J.; Hultdin, J.; Hallmans, G.; Johansson, I.; Palmqvist, R.; Van Guelpen, B. Components of One-carbon Metabolism Other than Folate and Colorectal Cancer Risk. Epidemiology 2016, 27, 787–796. [Google Scholar] [CrossRef]
- Vance, D.E.; Ridgway, N.D. The methylation of phosphatidylethanolamine. Prog. Lipid Res. 1988, 27, 61–79. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Natker, E.; Zeisel, S.H.; Zelman, K.M. Choline: The Underconsumed and Underappreciated Essential Nutrient. Nutr. Today 2018, 53, 240–253. [Google Scholar] [CrossRef]
- Craig, S.A.S. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Sonkar, K.; Ayyappan, V.; Tressler, C.M.; Adelaja, O.; Cai, R.; Cheng, M.; Glunde, K. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed. 2019, 32, e4112. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Serkova, N.J. Therapeutic targets and biomarkers identified in cancer choline phospholipid metabolism. Pharmacogenomics 2006, 7, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.L.; Carroll, D.; Day, S.; Gray, H.; Sadarangani, P.; Murthi, S.; Job, C.; Baggett, B.; Raghunand, N.; Gillies, R.J. Characterization of breast cancers and therapy response by MRS and quantitative gene expression profiling in the choline pathway. NMR Biomed. 2009, 22, 114–127. [Google Scholar] [CrossRef]
- Newman, A.C.; Maddocks, O.D.K. One-carbon metabolism in cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Nishimura, Y.; Ono, H.; Sakura, N. Betaine and homocysteine concentrations in foods. Pediatr. Int. 2002, 44, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Slow, S.; Donaggio, M.; Cressey, P.; Lever, M.; George, P.; Chambers, S. The betaine content of New Zealand foods and estimated intake in the New Zealand diet. J. Food Compos. Anal. 2005, 18, 473–485. [Google Scholar] [CrossRef]
- Vesper, H.; Schmelz, E.M.; Nikolova-Karakashian, M.N.; Dillehay, D.L.; Lynch, D.V.; Merrill, A.H., Jr. Sphingolipids in Food and the Emerging Importance of Sphingolipids to Nutrition. J. Nutr. 1999, 129, 1239–1250. [Google Scholar] [CrossRef]
| Controls | Cases | |||
|---|---|---|---|---|
| Colorectal * | Colon | Rectum | ||
| Overall | 3206 | 865 | 434 | 404 |
| Age, years, N (%) | ||||
| <30 | 21 (0.66%) | 8 (0.92%) | 3 (0.69%) | 5 (1.24%) |
| ≥30 & <40 | 227 (7.08%) | 60 (6.94%) | 32 (7.37%) | 27 (6.68%) |
| ≥40 & <50 | 503 (15.69%) | 126 (14.57%) | 64 (14.75%) | 58 (14.36%) |
| ≥50 & <60 | 993 (30.97%) | 242 (27.98%) | 112 (25.81%) | 123 (30.45%) |
| ≥60 & <70 | 1020 (31.82%) | 258 (29.83%) | 137 (31.57%) | 112 (27.72%) |
| ≥70 | 442 (13.79%) | 171 (19.77%) | 86 (19.82%) | 79 (19.55%) |
| Gender, N (%) | ||||
| Women | 1003 (31.28%) | 368 (42.54%) | 193 (44.47%) | 169 (41.83%) |
| Men | 2203 (68.71%) | 497 (57.46%) | 241 (55.53%) | 235 (58.17%) |
| Province, N (%) | ||||
| Tehran | 816 (25.45%) | 165 (19.08%) | 101 (23.27%) | 64 (15.84%) |
| Fars | 943 (29.41%) | 248 (28.67%) | 93 (21.43%) | 155(38.37%) |
| Kerman | 525 (16.38%) | 100 (11.56%) | 49 (11.29%) | 51 (12.62%) |
| Golestan | 373 (11.63%) | 149 (17.23%) | 89 (20.51%) | 53(13.12%) |
| Mazandaran | 136 (4.24%) | 59 (6.82%) | 34 (7.83%) | 25 (6.19%) |
| Kermanshah | 251 (7.83%) | 68 (7.86%) | 31(7.14%) | 35 (8.66%) |
| Mashhad | 162 (5.05%) | 76 (8.79%) | 37(8.53%) | 21 (5.20%) |
| BMI, kg/m2, mean (±SD) | 26.59 (±4.72) | 26.93 (±4.99) | 26.91 (±5.07) | 26.83 (±4.85) |
| BMI, N (%) | ||||
| Underweight (<18.5) | 90 (2.81%) | 28 (3.24%) | 14 (3.23%) | 14 (3.47%) |
| Normal weight (≥18.5 & <25) | 1121 (34.97%) | 261 (30.17%) | 135 (31.11%) | 119 (29.46%) |
| Overweight (≥25 & <30) | 1311 (40.89%) | 371 (42.89%) | 184 (42.40%) | 177 (43.81%) |
| Obese (≥30) | 684 (21.33%) | 205 (23.70%) | 101 (23.27%) | 94 (23.27%) |
| Work-related physical activity, N (%) | ||||
| Sedentary | 1034 (32.27%) | 287 (33.18%) | 147 (33.87%) | 132 (32.67%) |
| Moderate | 701 (21.88%) | 155 (17.92%) | 78 (17.97%) | 72 (17.82%) |
| Heavy | 694 (21.66%) | 184 (21.27%) | 87 (20.05%) | 87 (21.53%) |
| Unknown | 775 (24.19%) | 239 (27.63%) | 122 (28.11%) | 113 (27.97%) |
| SES, N (%) | ||||
| Low | 861 (26.86%) | 337 (38.27%) | 159 (36.64%) | 161 (39.85%) |
| Moderate | 1078 (33.62%) | 234 (27.05%) | 118 (27.19%) | 109 (26.98%) |
| High | 1267 (39.52%) | 300 (34.68%) | 157 (36.18%) | 134 (33.17%) |
| Tobacco consumption, N (%) | ||||
| Never user | 2153 (67.16%) | 629 (72.72%) | 334 (76.96%) | 274 (67.82%) |
| Ever user | 1053 (32.84%) | 236 (27.28%) | 100 (23.04%) | 130 (32.18%) |
| Opium consumption, N (%) | ||||
| No user | 2646 (82.53%) | 731 (84.51%) | 369 (85.02%) | 340 (84.16%) |
| Regular user | 432 (13.47%) | 88 (10.17%) | 40 (9.22%) | 46 (11.39%) |
| Irregular user | 128 (3.99%) | 46 (5.32%) | 25 (5.76%) | 18 (4.46%) |
| Aspirin use, N (%) | ||||
| No | 2469 (77%) | 709 (81.97%) | 358 (82.49%) | 327 (80.94%) |
| Yes | 737 (22.99%) | 156 (18.03%) | 76 (17.51%) | 77 (19.06%) |
| Family History, N (%) | ||||
| No | 2534 (79.04%) | 653 (75.49%) | 330 (76.04%) | 304 (75.25%) |
| Yes | 672 (20.96%) | 212 (24.51%) | 104 (23.96%) | 100 (24.75%) |
| Dietary items intake, Mean (SD) | ||||
| Total Vegetable (g/day) | 481.07 (5.01) | 475.35 (8.60) | 479.04 (12.69) | 473.54 (12.12) |
| Fiber (g/day) | 24.72 (±11.2) | 25.86 (±12.4) | 25.28 (±12.2) | 26.34 (±12.73) |
| Red Meat(g/day) | 16.67 (±17.3) | 22.13 (±25.1) | 21.79 (±23.3) | 22.16 (±26.82) |
| Folate (mcg/day) | 369.97 (2.83) | 391.70 (6.32) | 388.55 (8.95) | 392.63 (9.31) |
| Calcium (mg/day) | 860.35 (±6.6) | 908.28 (±14.7) | 919.66 (±20.7) | 880.24 (±21.2) |
| Total energy (kcal/day) | 2319.45 (±878.1) | 2405.61 (±1076) | 2387.21 (±1081.9) | 2393.21(±1066.3) |
| Total | Controls | Total Choline | Free Choline | GPC | Pcho | PtdCho | SM | Betaine | |
|---|---|---|---|---|---|---|---|---|---|
| Total choline | 334.80 (151.46) | 329.17 (146.61) | 1.00 | 0.43 * | 0.34 * | 0.35 * | 0.57 * | 0.56 * | 0.03 ** |
| Free choline | 72.06 (32.33) | 71.10 (31.51) | 1.00 | 0.62 * | 0.81 * | 0.50 * | 0.53 * | 0.10 * | |
| GPC | 44.01 (23.05) | 43.03 (22.24) | 1.00 | 0.72 * | 0.34 * | 0.58 * | 0.02 | ||
| Pcho | 16.18 (7.08) | 15.96 (6.90) | 1.00 | 0.33 * | 0.44 * | 0.06 * | |||
| PtdCho | 135.83 (79.66) | 132.77 (77.81) | 1.00 | 0.86 * | 0.06 * | ||||
| SM | 16.15 (8.87) | 15.78 (8.69) | 1.00 | 0.02 | |||||
| Betaine | 74.84 (49.92) | 74.94 (48.39) | 1.00 |
| Choline Type | Q2 | Q3 | Q4 | OR (95% CI) | p-Trend | |||
|---|---|---|---|---|---|---|---|---|
| Mean (Cut-Off) mg/day | OR (95% CI) | Mean (Cut-Off) mg/day | OR (95% CI) | Mean (Cut-Off) mg/day | OR (95% CI) | |||
| Total Choline | 288.9 (61–642) | 1.02 (0.80–1.32) | 303.3 (99–722) | 1.53 (1.20–1.95) | 470.2 (196–1167) | 1.74 (1.36–2.23) | 1.23 (1.13–1.33) | <0.001 |
| Free Choline | 55.5 (13–173) | 0.85 (0.65–1.13) | 71.9 (26–160) | 0.83 (0.60–1.15) | 104.1 (47–250) | 1.18 (0.81–1.73) | 1.0 (0.93–1.19) | 0.40 |
| GPC | 33.9 (8–90) | 1.24 (0.95–1.61) | 45.3 (21–101) | 1.33 (0.97–1.81) | 70.4 (34–236) | 1.52 (1.05–2.21) | 1.13 (1.00–1.27) | 0.03 |
| Pcho | 12.8 (5–35) | 1.07 (0.80–1.45) | 16.5 (9–31) | 0.88 (0.60–1.29) | 24.3 (13–61) | 0.92 (0.57–1.48) | 0.94 (0.80–1.10) | 0.464 |
| PtdCho | 102.3 (15–226) | 0.97 (0.73–1.27) | 122.8 (38–368) | 1.01 (0.75–1.37) | 223.5 (78–600) | 1.08 (0.75–1.54) | 1.03 (0.92–1.15) | 0.559 |
| SM | 12.5 (2–30) | 1.06 (0.80–1.40) | 15.5 (6–40) | 1.30 (0.95–1.79) | 25.2 (9–69) | 1.45 (1.00–2.09) | 1.14 (1.01–1.28) | 0.02 |
| Betaine | 47.3 (7–176) | 0.99 (0.78–1.24) | 73 (24–147) | 0.84 (0.66–1.07) | 144.1 (63–602) | 0.80 (0.61–1.06) | 0.91 (0.83–0.99) | 0.03 |
| Choline Type | Cancer site | Q2 | Q3 | Q4 | OR (95% CI) | p-Trend | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean mg/day | OR (95% CI) | Mean mg/day | OR (95% CI) | Mean mg/day | OR (95% CI) | ||||
| Total choline | Colon | 287.8 | 0.99 (0.70–1.41) | 308.8 | 1.63 (1.18–2.26) | 471.2 | 1.76 (1.26–2.45) | 1.24 (1.12–1.38) | <0.001 |
| Proximal colon | 287.6 | 1.12 (0.63–2.00) | 327.2 | 1.47 (0.84–2.56) | 431.2 | 2.11 (1.23–3.63) | 1.30 (1.09–1.54) | 0.003 | |
| Distal colon | 300.6 | 0.94 (0.57–1.57) | 305.6 | 1.77 (1.11–2.82) | 465.9 | 1.41 (0.86–2.30) | 1.17 (1.00–1.36) | 0.04 | |
| Rectum | 285.1 | 1.11 (0.79–1.57) | 293.1 | 1.52 (1.09–2.13) | 468.6 | 1.87 (1.34–2.61) | 1.24 (1.12–1.38) | <0.001 | |
| GPC | Colon | 33.5 | 1.10 (0.76–1.57) | 44.7 | 1.08 (0.71–1.64) | 70.3 | 1.34 (0.82–2.19) | 1.08 (0.92–1.27 | 0.298 |
| Proximal colon | 33.6 | 0.76 (0.41–1.38) | 45.45 | 0.73 (0.36–1.45) | 66.1 | 1.06 (0.47–2.37) | 1.01 (0.78–1.32) | 0.902 | |
| Distal colon | 34.9 | 1.32 (0.79–2.18) | 44.1 | 1.00 (0.55–1.83) | 68.7 | 1.02 (0.49–2.08) | 0.96 (0.76–1.20) | 0.744 | |
| Rectum | 33.7 | 1.28 (0.89–1.84) | 45.1 | 1.58 (1.04–2.40) | 69.9 | 1.67 (1.00–2.78) | 1.18 (1.00–1.39) | 0.04 | |
| SM | Colon | 12.8 | 1.18 (0.80–1.73) | 15.1 | 1.57 (1.02–2.42) | 25.8 | 1.73 (1.05–2.83) | 1.21 (1.03–1.41) | 0.01 |
| Proximal colon | 13.9 | 0.84 (0.44–1.61) | 15.6 | 1.38 (0.69–2.76) | 25.3 | 1.15 (0.51–2.61) | 1.10 (0.85–1.42) | 0.442 | |
| Distal colon | 11.6 | 1.09 (0.62–1.92) | 15.3 | 1.56 (0.85 2.86) | 25.2 | 1.83 (0.91–3.68) | 1.23 (0.98–1.54) | 0.063 | |
| Rectum | 12.1 | 0.90 (0.61–1.32) | 15.8 | 0.98 (0.64–1.51) | 24.3 | 1.08 (0.66–1.78) | 1.03 (0.88–1.21) | 0.687 | |
| Betaine | Colon | 47.1 | 0.98 (0.72–1.32) | 72.1 | 0.80 (0.58–1.11) | 156.9 | 0.74 (0.51–1.07) | 0.89 (0.79–0.99) | 0.04 |
| Proximal colon | 52.3 | 0.82 (0.50–1.35) | 69 | 0.97 (0.58–1.61) | 141.8 | 0.66 (0.35–1.24) | 0.89 (0.74–1.08) | 0.260 | |
| Distal colon | 44.6 | 1.02 (0.66–1.57) | 72.6 | 0.65 (0.39–1.06) | 161.5 | 0.76 (0.45–1.30) | 0.88 (0.74–1.04) | 0.140 | |
| Rectum | 47.1 | 0.94 (0.68–1.30) | 73.7 | 0.83 (0.60–1.16) | 131.8 | 0.75 (0.51–1.08) | 0.89 (0.79–1.00) | 0.069 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyyedsalehi, M.S.; Rossi, M.; Hadji, M.; Rashidian, H.; Marzban, M.; Parpinel, M.; Fiori, F.; Naghibzadeh-Tahami, A.; Hannun, Y.A.; Luberto, C.; et al. Dietary Choline and Betaine Intake and Risk of Colorectal Cancer in an Iranian Population. Cancers 2023, 15, 2557. https://doi.org/10.3390/cancers15092557
Seyyedsalehi MS, Rossi M, Hadji M, Rashidian H, Marzban M, Parpinel M, Fiori F, Naghibzadeh-Tahami A, Hannun YA, Luberto C, et al. Dietary Choline and Betaine Intake and Risk of Colorectal Cancer in an Iranian Population. Cancers. 2023; 15(9):2557. https://doi.org/10.3390/cancers15092557
Chicago/Turabian StyleSeyyedsalehi, Monireh Sadat, Marta Rossi, Maryam Hadji, Hamideh Rashidian, Maryam Marzban, Maria Parpinel, Federica Fiori, Ahmad Naghibzadeh-Tahami, Yusuf A. Hannun, Chiara Luberto, and et al. 2023. "Dietary Choline and Betaine Intake and Risk of Colorectal Cancer in an Iranian Population" Cancers 15, no. 9: 2557. https://doi.org/10.3390/cancers15092557





