Radioiodine-131 Therapy Used for Differentiated Thyroid Cancer Can Impair Titanium Dental Implants: An In Vitro Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Radioiodine-131 Irradiation Protocol and Sample Preparation
2.2. Electrochemical Measurements
2.3. Scanning Electron Microscopy Assessment
3. Results
3.1. Scanning Electron Microscopy Analysis
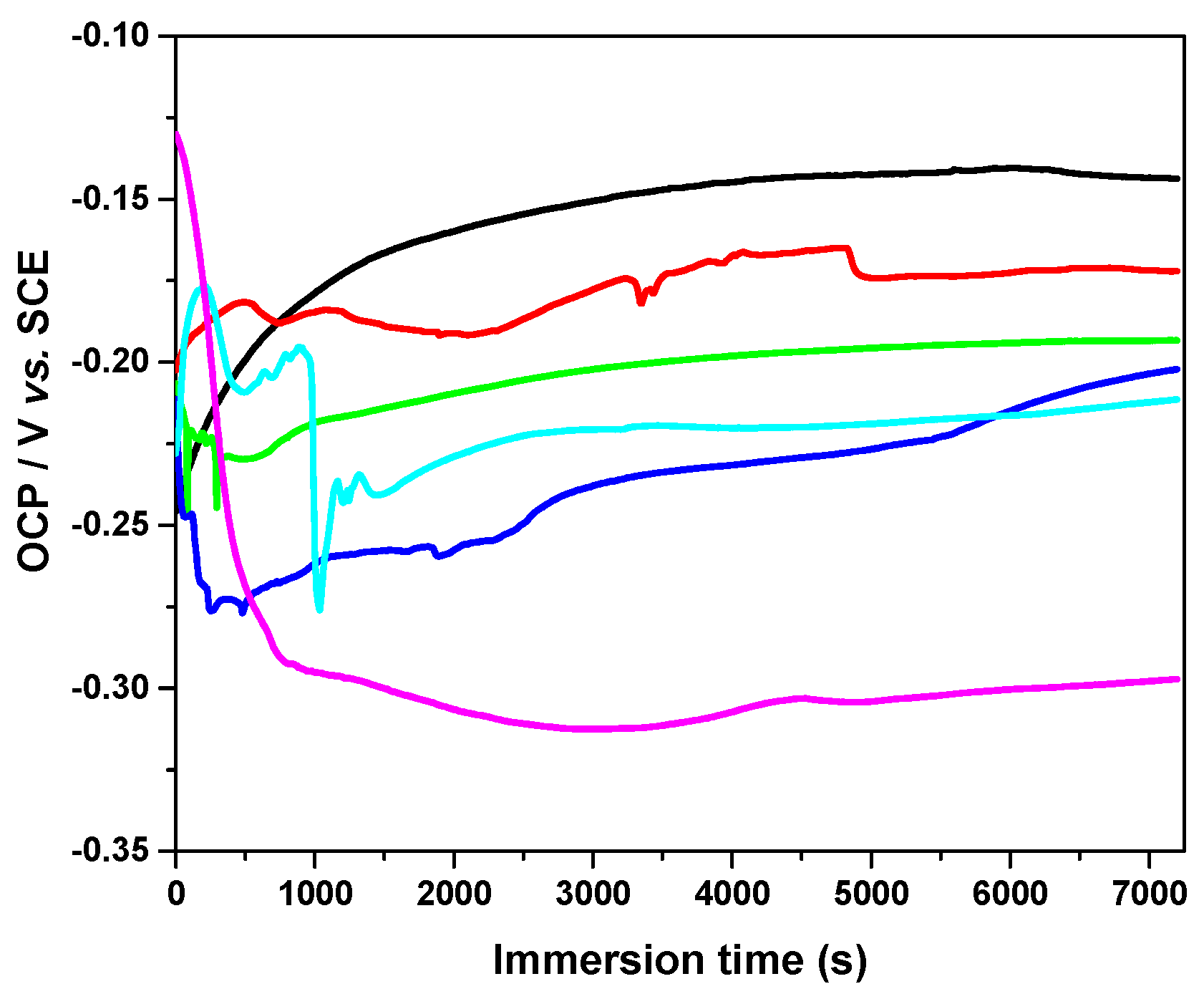
3.2. Open-Circuit Potential Measurements
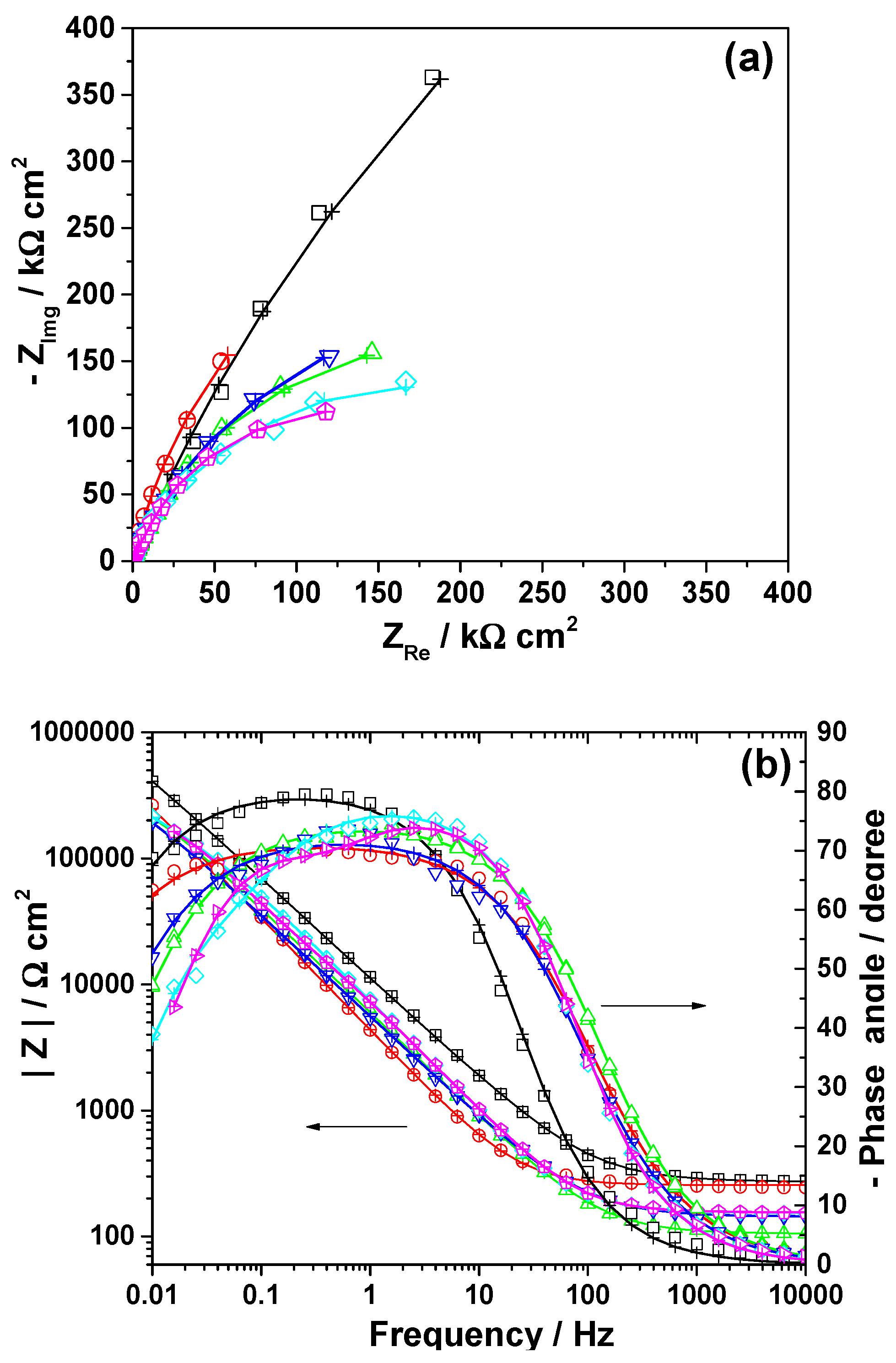
3.3. Electrochemical Impedance Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms; Springer: New York, NY, USA, 2022; Volume 33, ISBN 1202202209. [Google Scholar]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Borda, A.; Zahan, A.-E.; Piciu, D.; Barbuș, E.; Berger, N.; Nechifor-Boilă, A. A 15 year institutional experience of well-differentiated follicular cell-derived thyroid carcinomas; impact of the new 2017 TNM and WHO Classifications of Tumors of Endocrine Organs on the epidemiological trends and pathological characteristics. Endocrine 2020, 67, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.; Sawka, A.; Schlumberger, M.; et al. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2015, 26, 1–133. [Google Scholar] [CrossRef]
- Sugitani, I.; Ito, Y.; Takeuchi, D.; Nakayama, H.; Masaki, C.; Shindo, H.; Teshima, M.; Horiguchi, K.; Yoshida, Y.; Kanai, T.; et al. Indications and Strategy for Active Surveillance of Adult Low-Risk Papillary Thyroid Microcarcinoma: Consensus Statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid 2021, 31, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Baudin, C.; Lussey-Lepoutre, C.; Bressand, A.; Buffet, C.; Menegaux, F.; Soret, M.; Broggio, D.; Bassinet, C.; Huet, C.; Armengol, G.; et al. Salivary Dysfunctions and Consequences After Radioiodine Treatment for Thyroid Cancer: Protocol for a Self-Controlled Study (START Study). JMIR Res. Protoc. 2022, 11, e35565. [Google Scholar] [CrossRef] [PubMed]
- Walshaw, E.G.; Smith, M.; Kim, D.; Wadsley, J.; Kanatas, A.; Rogers, S.N. Systematic review of health-related quality of life following thyroid cancer. Tumori J. 2022, 108, 291–314. [Google Scholar] [CrossRef]
- Auttara-Atthakorn, A.; Sungmala, J.; Anothaisintawee, T.; Reutrakul, S.; Sriphrapradang, C. Prevention of salivary gland dysfunction in patients treated with radioiodine for differentiated thyroid cancer: A systematic review of randomized controlled trials. Front. Endocrinol. 2022, 13, 960265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Zhang, W. Optimal administration time of vitamin C after (131)I therapy in differentiated thyroid cancer based on propensity score matching. Front. Surg. 2022, 9, 993712. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, M.-K.; Graillon, N.; Guyot, L.; Taieb, D.; Galli, P.; Godio-Raboutet, Y.; Chossegros, C.; Foletti, J.-M. Salivary side effects after radioiodine treatment for differentiated papillary thyroid carcinoma: Long-term study. Head Neck 2020, 42, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L. Complications of radioactive iodine treatment of thyroid carcinoma. J. Natl. Compr. Cancer Netw. 2010, 8, 1277–1286, quiz 1287. [Google Scholar] [CrossRef] [PubMed]
- Badam, R.K.; Suram, J.; Babu, D.B.G.; Waghray, S.; Marshal, R.; Bontha, S.C.; Lavanya, R.; Kanth, S. Assessment of Salivary Gland Function Using Salivary Scintigraphy in Pre and Post Radioactive Iodine Therapy in Diagnosed Thyroid Carcinoma Patients. J. Clin. Diagn. Res. 2016, 10, ZC60-2. [Google Scholar] [CrossRef] [PubMed]
- Mester, A.; Moldovan, M.; Taulescu, M.; Sarosi, C.; Petean, I.; Vulpoi, A.; Piciu, A.; Voina-Tonea, A.; Moisescu-Goia, C.; Barbus, E.; et al. The Side Effects of Therapeutic Radioiodine-131 on the Structure of Enamel and Dentin in Permanent Human Teeth. Biology 2021, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Mester, A.; Piciu, A.; Lucaciu, O.; Apostu, D.; Piciu, D.; Voina-Tonea, A. Assessment and Care of Oral Lesions for Patients Who Undergo Radioiodine Treatment for Thyroid Cancer. Am. J. Med. Sci. 2021, 361, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Tabei, F.; Asli, I.N.; Azizmohammadi, Z.; Javadi, H.; Assadi, M. Assessment of radioiodine clearance in patients with differentiated thyroid cancer. Radiat. Prot. Dosim. 2012, 152, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Mester, A.; Piciu, A.; Piciu, D.; Petean, I.; Lucaciu, P.O.; Apostu, D.; Moisescu-Goia, C.; Voina-Tonea, A.; Moldovan, M. Disorders of Dental Hard Tissues Induced by Radioiodine-131 (I-131) Therapy Used in Differentiated Thyroid Cancer: An In Vitro Study. Biomedicines 2020, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Führer, D.; et al. Controversies, Consensus, and Collaboration in the Use of (131)I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecula. Thyroid 2019, 29, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Sunavala-Dossabhoy, G. Radioactive iodine: An unappreciated threat to salivary gland function. Oral Dis. 2018, 24, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Dental implants in irradiated versus nonirradiated patients: A meta-analysis. Head Neck 2016, 38, 448–481. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.B.F.; Soares, C.J.; Limirio, P.H.J.O.; Lara, V.C.; Moura, C.C.G.; Zanetta-Barbosa, D. Biomechanical and morphological changes produced by ionizing radiation on bone tissue surrounding dental implant. J. Appl. Oral Sci. 2020, 28, e20200191. [Google Scholar] [CrossRef] [PubMed]

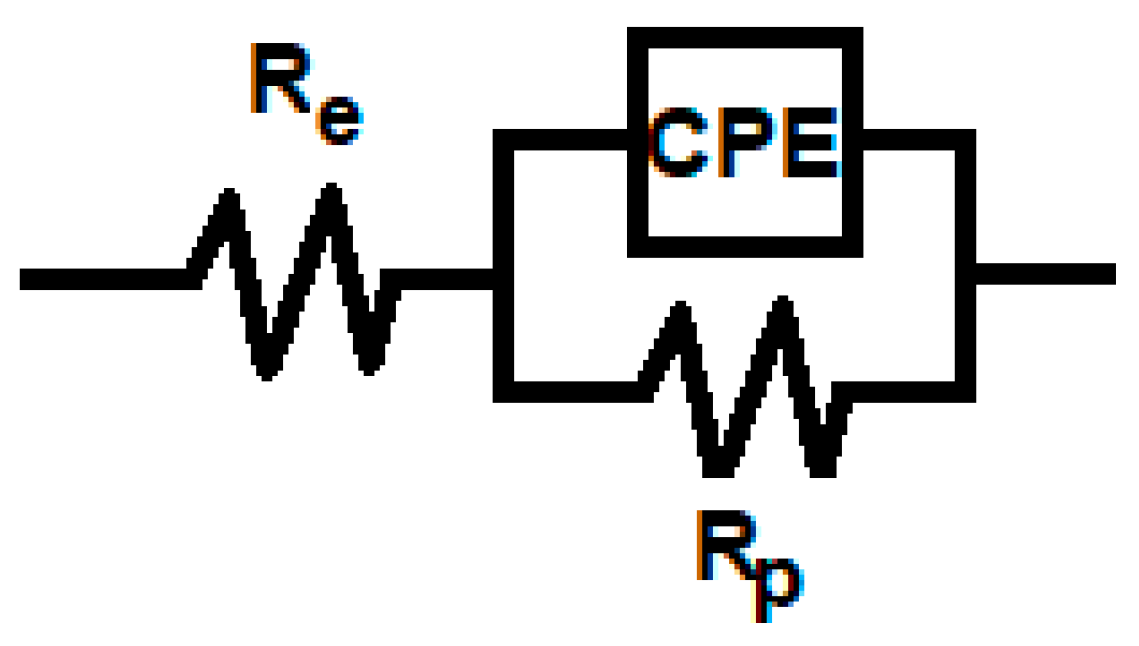
| Irradiation Period (Hours) | Re (Ω cm2) | Rp (kΩ cm2) | Q (μF sn−1 cm−2) | n | C (μF cm−2) |
|---|---|---|---|---|---|
| 0 | 255 | 1100 | 44.9 | 0.893 | 71.7 |
| 6 | 301 | 1090 | 19.5 | 0.797 | 57.2 |
| 12 | 105 | 436.7 | 36.2 | 0.833 | 63.1 |
| 48 | 144 | 532.7 | 40.6 | 0.809 | 84.1 |
| 192 | 153 | 295.3 | 28.7 | 0.861 | 37.6 |
| 384 | 152 | 285.5 | 30.6 | 0.848 | 45.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piciu, D.; Bran, S.; Moldovan, M.; Varvara, S.; Piciu, A.; Cuc, S.; Moisescu-Goia, C.; Barbus, E.; Mester, A.; Onisor, F. Radioiodine-131 Therapy Used for Differentiated Thyroid Cancer Can Impair Titanium Dental Implants: An In Vitro Analysis. Cancers 2023, 15, 2558. https://doi.org/10.3390/cancers15092558
Piciu D, Bran S, Moldovan M, Varvara S, Piciu A, Cuc S, Moisescu-Goia C, Barbus E, Mester A, Onisor F. Radioiodine-131 Therapy Used for Differentiated Thyroid Cancer Can Impair Titanium Dental Implants: An In Vitro Analysis. Cancers. 2023; 15(9):2558. https://doi.org/10.3390/cancers15092558
Chicago/Turabian StylePiciu, Doina, Simion Bran, Marioara Moldovan, Simona Varvara, Andra Piciu, Stanca Cuc, Cristina Moisescu-Goia, Elena Barbus, Alexandru Mester, and Florin Onisor. 2023. "Radioiodine-131 Therapy Used for Differentiated Thyroid Cancer Can Impair Titanium Dental Implants: An In Vitro Analysis" Cancers 15, no. 9: 2558. https://doi.org/10.3390/cancers15092558
APA StylePiciu, D., Bran, S., Moldovan, M., Varvara, S., Piciu, A., Cuc, S., Moisescu-Goia, C., Barbus, E., Mester, A., & Onisor, F. (2023). Radioiodine-131 Therapy Used for Differentiated Thyroid Cancer Can Impair Titanium Dental Implants: An In Vitro Analysis. Cancers, 15(9), 2558. https://doi.org/10.3390/cancers15092558








