Current Approaches for the Curative-Intent Surgical Treatment of Pancreatic Ductal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patient Selection
2.1. Resectable Pancreatic Cancer
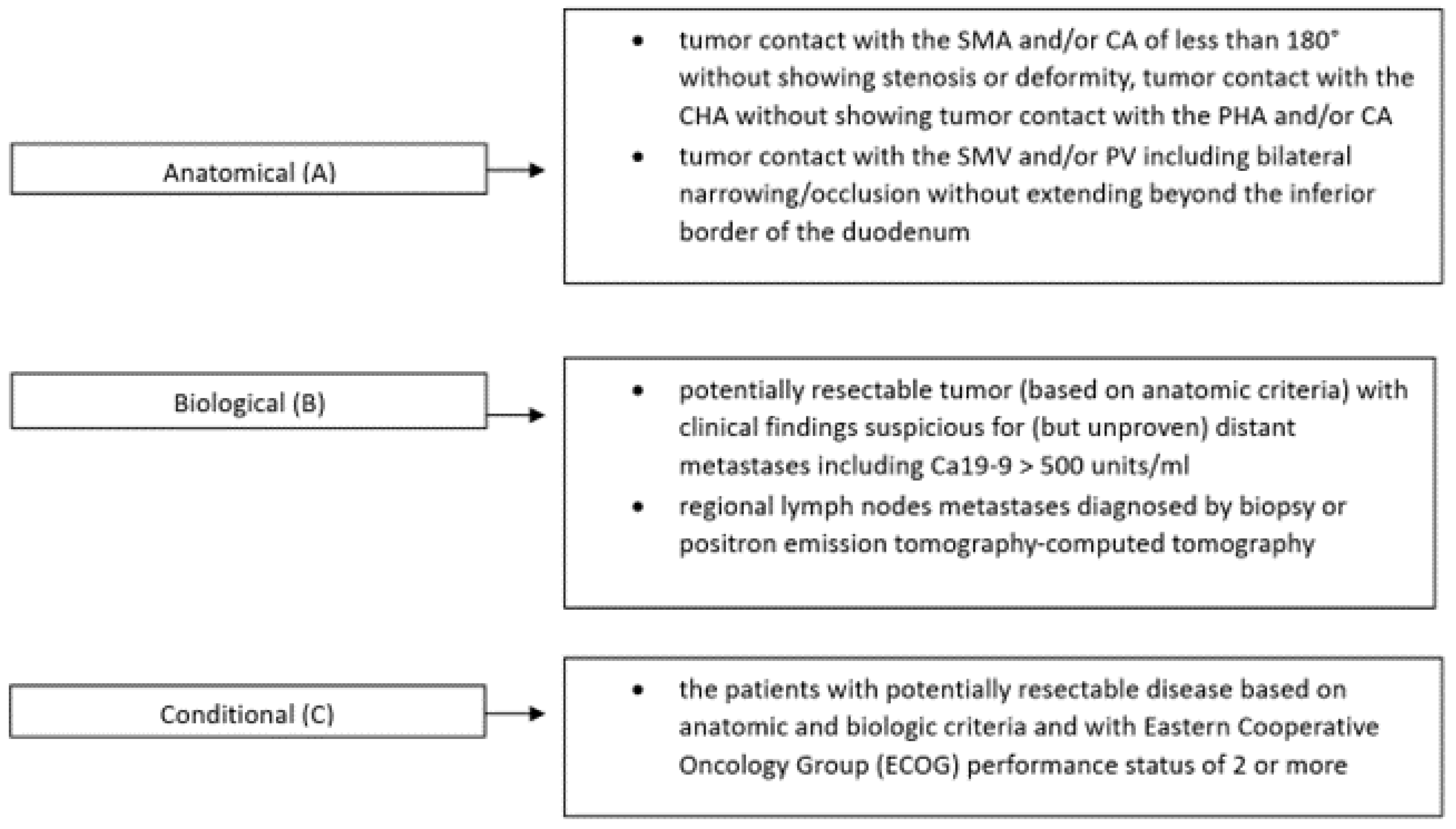
2.2. Borderline Resectable Cancer
2.3. Locally Advanced Pancreatic Cancer
2.4. Metastatic Disease
2.5. Treatment of Recurrent Disease
2.6. Adjuvant Therapy
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Takeda, T.; Sasaki, T.; Inoue, Y.; Okamoto, T.; Mori, C.; Mie, T.; Furukawa, T.; Yamada, Y.; Kasuga, A.; Matsuyama, M.; et al. Early-onset pancreatic cancer: Clinical characteristics and survival outcomes. Pancreatology 2022, 22, 507–515. [Google Scholar] [CrossRef]
- Verma, V.; Li, J.; Lin, C. Neoadjuvant Therapy for Pancreatic Cancer: Systematic Review of Postoperative Morbidity, Mortality, and Complications. Am. J. Clin. Oncol. 2016, 39, 302–313. [Google Scholar] [CrossRef]
- Elsayed, M.; Abdelrahim, M. The Latest Advancement in Pancreatic Ductal Adenocarcinoma Therapy: A Review Article for the Latest Guidelines and Novel Therapies. Biomedicines 2021, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- Howlader, N.N.A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2018; National Cancer Institute: Bethesda, MD, USA, 2021. [Google Scholar]
- Ansari, D.; Althini, C.; Ohlsson, H.; Andersson, R. Early-onset pancreatic cancer: A population-based study using the SEER registry. Langenbecks Arch. Surg. 2019, 404, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Niessen, A.; Hackert, T. State-of-the-art surgery for pancreatic cancer. Langenbecks Arch. Surg. 2022, 407, 443–450. [Google Scholar] [CrossRef]
- Hackert, T.; Klaiber, U.; Pausch, T.; Mihaljevic, A.L.; Büchler, M.W. Fifty Years of Surgery for Pancreatic Cancer. Pancreas 2020, 49, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Kommalapati, A.; Tella, S.H.; Goyal, G.; Ma, W.W.; Mahipal, A. Contemporary Management of Localized Resectable Pancreatic Cancer. Cancers 2018, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Hackert, T.; Strobel, O.; Michalski, C.W.; Mihaljevic, A.L.; Mehrabi, A.; Müller-Stich, B.; Berchtold, C.; Ulrich, A.; Büchler, M.W. The triangle operation—Radical surgery after neoadjuvant treatment for advanced pancreatic cancer: A single arm observational study. HPB 2017, 19, 1001–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Shi, S.; Hua, J.; Xu, J.; Yu, X. Simultaneous resection of the primary tumour and liver metastases after conversion chemotherapy versus standard therapy in pancreatic cancer with liver oligometastasis: Protocol of a multicentre, prospective, randomised phase III control trial (CSPAC-1). BMJ Open 2019, 9, e033452. [Google Scholar] [CrossRef] [Green Version]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Tuveson, D.A.; Neoptolemos, J.P. Understanding metastasis in pancreatic cancer: A call for new clinical approaches. Cell 2012, 148, 21–23. [Google Scholar] [CrossRef] [Green Version]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-Del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.-W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Hayasaki, A.; Isaji, S.; Kishiwada, M.; Fujii, T.; Iizawa, Y.; Kato, H.; Tanemura, A.; Murata, Y.; Azumi, Y.; Kuriyama, N.; et al. Survival Analysis in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Chemoradiotherapy Followed by Surgery According to the International Consensus on the 2017 Definition of Borderline Resectable Cancer. Cancers 2018, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Ushida, Y.; Inoue, Y.; Ito, H.; Oba, A.; Mise, Y.; Ono, Y.; Sato, T.; Saiura, A.; Takahashi, Y. High CA19-9 level in resectable pancreatic cancer is a potential indication of neoadjuvant treatment. Pancreatology 2021, 21, 130–137. [Google Scholar] [CrossRef]
- Søreide, K.; Rangelova, E.; Dopazo, C.; Mieog, S.; Stättner, S. Pancreatic cancer. Eur. J. Surg. Oncol. 2023, 49, 521–525. [Google Scholar] [CrossRef]
- Pelzer, U.; Schwaner, I.; Stieler, J.; Adler, M.; Seraphin, J.; Dörken, B.; Riess, H.; Oettle, H. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: A phase III-study from the German CONKO-study group. Eur. J. Cancer 2011, 47, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef] [Green Version]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Kirkegård, J.; Aahlin, E.K.; Al-Saiddi, M.; Bratlie, S.O.; Coolsen, M.; de Haas, R.J.; den Dulk, M.; Fristrup, C.; Harrison, E.M.; Mortensen, M.B.; et al. Multicentre study of multidisciplinary team assessment of pancreatic cancer resectability and treatment allocation. Br. J. Surg. 2019, 106, 756–764. [Google Scholar] [CrossRef]
- Grossberg, A.J.; Chu, L.C.; Deig, C.R.; Fishman, E.K.; Hwang, W.L.; Maitra, A.; Marks, D.L.; Mehta, A.; Nabavizadeh, N.; Simeone, D.M.; et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J. Clin. 2020, 70, 375–403. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Talar-Wojnarowska, R.; Dąbrowski, A.; Degowska, M.; Durlik, M.; Gąsiorowska, A.; Głuszek, S.; Jurkowska, G.; Kaczka, A.; Lampe, P.; et al. Diagnostic and therapeutic recommendations in pancreatic ductal adenocarcinoma. Recommendations of the Working Group of the Polish Pancreatic Club. Przegląd Gastroenterol. 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Rahman, S.H.; Urquhart, R.; Molinari, M. Neoadjuvant therapy for resectable pancreatic cancer. World J. Gastrointest. Oncol. 2017, 9, 457–465. [Google Scholar] [CrossRef]
- Uson Junior, P.L.S.; Dias E Silva, D.; de Castro, N.M.; da Silva Victor, E.; Rother, E.T.; Araújo, S.E.A.; Borad, M.J.; Moura, F. Does neoadjuvant treatment in resectable pancreatic cancer improve overall survival? A systematic review and meta-analysis of randomized controlled trials. ESMO Open 2023, 8, 100771. [Google Scholar] [CrossRef]
- Dhir, M.; Malhotra, G.K.; Sohal, D.P.S.; Hein, N.A.; Smith, L.M.; O’Reilly, E.M.; Bahary, N.; Are, C. Neoadjuvant treatment of pancreatic adenocarcinoma: A systematic review and meta-analysis of 5520 patients. World J. Surg. Oncol. 2017, 15, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, A.; Ferrone, C.R. Neoadjuvant Therapy for Resectable Pancreatic Cancer: An Evolving Paradigm Shift. Front. Oncol. 2019, 9, 1085. [Google Scholar] [CrossRef] [Green Version]
- Mokdad, A.A.; Minter, R.M.; Zhu, H.; Augustine, M.M.; Porembka, M.R.; Wang, S.C.; Yopp, A.C.; Mansour, J.C.; Choti, M.A.; Polanco, P.M. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J. Clin. Oncol. 2017, 35, 515–522. [Google Scholar] [CrossRef]
- Casolino, R.; Braconi, C.; Malleo, G.; Paiella, S.; Bassi, C.; Milella, M.; Dreyer, S.B.; Froeling, F.E.M.; Chang, D.K.; Biankin, A.V.; et al. Reshaping preoperative treatment of pancreatic cancer in the era of precision medicine. Ann. Oncol. 2021, 32, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, A.C.; Rupji, M.; Switchenko, J.M.; Lee, R.M.; Turgeon, M.K.; Meyer, B.I.; Russell, M.C.; Cardona, K.; Kooby, D.A.; Maithel, S.K.; et al. Optimal timing and treatment strategy for pancreatic cancer. J. Surg. Oncol. 2020, 122, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.D.; Thompson, K.J.; Musselwhite, L.W.; Hwang, J.J.; Baker, E.H.; Martinie, J.B.; Vrochides, D.; Iannitti, D.A.; Ocuin, L.M. The treatment sequence may matter in patients undergoing pancreatoduodenectomy for early stage pancreatic cancer in the era of modern chemotherapy. Am. J. Surg. 2021, 222, 159–166. [Google Scholar] [CrossRef]
- Soreide, K. Neoadjuvant and Adjuvant Therapy in Operable Pancreatic Cancer: Both Honey and Milk (but No Bread?). Oncol. Ther. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.M.; van Dam, R.M. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Kim, R.Y.; Christians, K.K.; Aldakkak, M.; Clarke, C.N.; George, B.; Kamgar, M.; Khan, A.H.; Kulkarni, N.; Hall, W.A.; Erickson, B.A. Total Neoadjuvant Therapy for Operable Pancreatic Cancer. Ann. Surg. Oncol. 2021, 28, 2246–2256. [Google Scholar] [CrossRef]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Total Neoadjuvant Therapy with FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Kunzmann, V.; Siveke, J.T.; Algül, H.; Goekkurt, E.; Siegler, G.; Martens, U.; Waldschmidt, D.; Pelzer, U.; Fuchs, M.; Kullmann, F.; et al. German Pancreatic Cancer Working Group (AIO-PAK) and NEOLAP investigators Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): A multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 128–138. [Google Scholar]
- Unno, M.; Motoi, F.; Matsuyama, Y.; Satoi, S.; Matsumoto, I.; Aosasa, S.; Shirakawa, H.; Wada, K.; Fujii, T.; Yoshitomi, H.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J. Clin. Oncol. 2019, 37, 189. [Google Scholar] [CrossRef]
- van Dam, J.L.; Janssen, Q.P.; Besselink, M.G.; Homs, M.Y.V.; van Santvoort, H.C.; van Tienhoven, G.; de Wilde, R.F.; Wilmink, J.W.; van Eijck, C.H.J.; Groot Koerkamp, B.; et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: A meta-analysis of randomised controlled trials. Eur. J. Cancer 2022, 160, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, C.S.; Leitch, D.; Menon, K.V.; McMahon, M.J.; Guillou, P.J.; Anthoney, A. Redefining the R1 resection in pancreatic cancer. Br. J. Surg. 2006, 93, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I.; Kleeff, J.; Bergmann, F.; Reiser, C.; Herpel, E.; Friess, H.; Schirmacher, P.; Büchler, M.W. Most pancreatic cancer resections are R1 resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar] [CrossRef]
- Campbell, F.; Smith, R.A.; Whelan, P.; Sutton, R.; Raraty, M.; Neoptolemos, J.P.; Ghaneh, P. Classification of R1 resections for pancreatic cancer: The prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009, 55, 277–283. [Google Scholar] [CrossRef]
- Chandrasegaram, M.D.; Goldstein, D.; Simes, J.; Gebski, V.; Kench, J.G.; Gill, A.J.; Samra, J.S.; Merrett, N.D.; Richardson, A.J.; Barbour, A.P. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br. J. Surg. 2015, 102, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Zimmitti, G.; Codignola, C.; Manzoni, A.; Garatti, M.; Sega, V.; Rosso, E. Follow “the superior mesenteric artery”: Laparoscopic approach for total mesopancreas excision during pancreaticoduodenectomy. Surg. Endosc. 2019, 33, 4186–4191. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Strobel, O.; Hackert, T.; Büchler, M.W. Pancreatic resection for cancer-the Heidelberg technique. Langenbecks Arch. Surg. 2019, 404, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Saiura, A.; Yoshioka, R.; Ono, Y.; Takahashi, M.; Arita, J.; Takahashi, Y.; Koga, R. Pancreatoduodenectomy With Systematic Mesopancreas Dissection Using a Supracolic Anterior Artery-first Approach. Ann. Surg. 2015, 262, 1092–1101. [Google Scholar] [CrossRef]
- Diener, M.K.; Mihaljevic, A.L.; Strobel, O.; Loos, M.; Schmidt, T.; Schneider, M.; Berchtold, C.; Mehrabi, A.; Müller-Stich, B.P.; Jiang, K. Periarterial divestment in pancreatic cancer surgery. Surgery 2021, 169, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Mayer, P.; Giannakis, A.; Klauß, M.; Gaida, M.M.; Bergmann, F.; Kauczor, H.U.; Feisst, M.; Hackert, T.; Loos, M. Radiological evaluation of pancreatic cancer: What is the significance of arterial encasement >180 degrees after neoadjuvant treatment? Eur. J. Radiol. 2021, 137, 109603. [Google Scholar] [CrossRef]
- Tol, J.A.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef]
- Farnell, M.B.; Pearson, R.K.; Sarr, M.G.; DiMagno, E.P.; Burgart, L.J.; Dahl, T.R.; Foster, N.; Sargent, D.J.; Pancreas Cancer Working Group. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 2005, 138, 618–628, discussion 628–630. [Google Scholar] [CrossRef]
- Nimura, Y.; Nagino, M.; Takao, S.; Takada, T.; Miyazaki, K.; Kawarada, Y.; Miyagawa, S.; Yamaguchi, A.; Ishiyama, S.; Takeda, Y.; et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: Long-term results of a Japanese multicenter randomized controlled trial. J. Hepatobiliary Pancreat. Sci. 2012, 19, 230–241. [Google Scholar] [CrossRef]
- Yamamoto, J.; Kudo, H.; Kyoden, Y.; Ajiro, Y.; Hiyoshi, M.; Okuno, T.; Kawasaki, H.; Nemoto, M.; Yoshimi, F. An anatomical review of various superior mesenteric artery-first approaches during pancreatoduodenectomy for pancreatic cancer. Surg. Today 2021, 51, 872–879. [Google Scholar] [CrossRef]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Büchler, M.; Charnley, R.M.; et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Mihaljevic, A.L.; Hackert, T.; Loos, M.; Hinz, U.; Schneider, M.; Mehrabi, A.; Hoffmann, K.; Berchtold, C.; Müller-Stich, B.P.; Diener, M.; et al. Not all Whipple procedures are equal: Proposal for a classification of pancreatoduodenectomies. Surgery 2021, 169, 1456–1462. [Google Scholar] [CrossRef]
- Van Buren, G., 2nd; Vollmer, C.M., Jr. The Landmark Series: Mitigation of the Postoperative Pancreatic Fistula. Ann. Surg. Oncol. 2021, 28, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzoli, S. Surgical treatment of pancreatic cancer: Currently debated topics on morbidity, mortality, and lymphadenectomy. Surg. Oncol. 2022, 45, 101858. [Google Scholar] [CrossRef]
- Mahipal, A.; Frakes, J.; Hoffe, S.; Kim, R. Management of borderline resectable pancreatic cancer. World J. Gastrointest. Oncol. 2015, 7, 241–249. [Google Scholar] [CrossRef]
- Traub, B.; Link, K.H.; Kornmann, M. Curing pancreatic cancer. Semin. Cancer Biol. 2021, 76, 232–246. [Google Scholar] [CrossRef]
- Boone, B.A.; Steve, J.; Zenati, M.S.; Hogg, M.E.; Singhi, A.D.; Bartlett, D.L.; Zureikat, A.H.; Bahary, N.; Zeh, H.J. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 4351–4358. [Google Scholar] [CrossRef]
- Dimitrokallis, N.; Karachaliou, G.S.; Moris, D. New NCCN Guidelines for Locally Advanced Pancreatic Cancer: New Horizons in Extending Resectability. J. BUON 2020, 25, 2125–2126. [Google Scholar] [PubMed]
- Ghaneh, P.; Palmer, D.; Cicconi, S.; Jackson, R.; Halloran, C.M.; Rawcliffe, C.; Sripadam, R.; Mukherjee, S.; Soonawalla, Z.; Wadsley, J.; et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): A four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef] [PubMed]
- Dua, M.M.; Tran, T.B.; Klausner, J.; Hwa, K.J.; Poultsides, G.A.; Norton, J.A.; Visser, B.C. Pancreatectomy with vein reconstruction: Technique matters. HPB 2015, 17, 824–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younan, G.; Tsai, S.; Evans, D.B.; Christians, K.K. Techniques of Vascular Resection and Reconstruction in Pancreatic Cancer. Surg. Clin. N. Am. 2016, 96, 1351–1370. [Google Scholar] [CrossRef] [PubMed]
- Li, A.Y.; Visser, B.C.; Dua, M.M. Surgical Indications and Outcomes of Resection for Pancreatic Neuroendocrine Tumors with Vascular Involvement. Cancers 2022, 14, 2312. [Google Scholar] [CrossRef]
- Tucker, O.N.; Rela, M. Controversies in the management of borderline resectable proximal pancreatic adenocarcinoma with vascular involvement. HPB Surg. 2008, 2008, 839503. [Google Scholar] [CrossRef] [PubMed]
- Balaban, E.P.; Mangu, P.B.; Yee, N.S. Locally Advanced Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Summary. J. Oncol. Pract. 2017, 13, 265–269. [Google Scholar] [CrossRef]
- Rosso, E.; Zimmitti, G.; Iannelli, A.; Garatti, M. The ‘TRIANGLE Operation’ by Laparoscopy: Radical Pancreaticoduodenectomy with Major Vascular Resection for Borderline Resectable Pancreatic Head Cancer. Ann. Surg. Oncol. 2020, 27, 1613–1614. [Google Scholar] [CrossRef]
- Klotz, R.; Hackert, T.; Heger, P.; Probst, P.; Hinz, U.; Loos, M.; Berchtold, C.; Mehrabi, A.; Schneider, M.; Müller-Stich, B.P. The TRIANGLE operation for pancreatic head and body cancers: Early postoperative outcomes. HPB 2022, 24, 332–341. [Google Scholar] [CrossRef]
- Wei, K.; Klotz, R.; Kalkum, E.; Holze, M.; Probst, P.; Hackert, T. Safety and efficacy of TRIANGLE operation applied in pancreatic surgery: A protocol of the systematic review and meta-analysis. BMJ Open 2022, 12, e059977. [Google Scholar] [CrossRef]
- Kolbeinsson, H.; Hoppe, A.; Bayat, A.; Kogelschatz, B.; Mbanugo, C.; Chung, M.; Wolf, A.; Assifi, M.M.; Wright, G.P. Recurrence patterns and postrecurrence survival after curative intent resection for pancreatic ductal adenocarcinoma. Surgery 2021, 169, 649–654. [Google Scholar] [CrossRef]
- Tachezy, M.; Gebauer, F.; Janot, M.; Uhl, W.; Zerbi, A.; Montorsi, M.; Perinel, J.; Adham, M.; Dervenis, C.; Agalianos, C. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery 2016, 160, 136–144. [Google Scholar] [CrossRef]
- Andreou, A.; Knitter, S.; Klein, F.; Malinka, T.; Schmelzle, M.; Struecker, B.; Schmuck, R.B.; Noltsch, A.R.; Lee, D.; Pelzer, U.; et al. The role of hepatectomy for synchronous liver metastases from pancreatic adenocarcinoma. Surg. Oncol. 2018, 27, 688–694. [Google Scholar] [CrossRef]
- Aussilhou, B.; Ftériche, F.S.; Bouquot, M.; Lesurtel, M.; Sauvanet, A.; Dokmak, S. Laparoscopic pancreatic enucleation: Cystic lesions and proximity to the Wirsung duct increase postoperative pancreatic fistula. Surg. Endosc. 2023, 37, 544–555. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Valente, R.; Tanaka, K.; Satoi, S.; Del Chiaro, M. Surgical treatment of metastatic pancreatic ductal adenocarcinoma: A review of current literature. Pancreatology 2019, 19, 672–680. [Google Scholar] [CrossRef]
- Hackert, T.; Niesen, W.; Hinz, U.; Tjaden, C.; Strobel, O.; Ulrich, A.; Michalski, C.W.; Büchler, M.W. Radical surgery of oligometastatic pancreatic cancer. Eur. J. Surg. Oncol. 2017, 43, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Dolay, K.; Malya, F.U.; Akbulut, S. Management of pancreatic head adenocarcinoma: From where to where? World J. Gastrointest. Surg. 2019, 11, 143–154. [Google Scholar] [CrossRef]
- Buanes, T.A. Role of surgery in pancreatic cancer. World J. Gastroenterol. 2017, 23, 3765–3770. [Google Scholar] [CrossRef] [PubMed]
- Nienhuser, H.; Buchler, M.W.; Schneider, M. Resection of Recurrent Pancreatic Cancer: Who Can Benefit? Visc. Med. 2022, 38, 42–48. [Google Scholar] [CrossRef]
- Encyclopædia Britannica. Leaf-nosed bat. In Encyclopædia Britannica Online; Encyclopædia Britannica: Chicago, IL, USA, 2009. [Google Scholar]
- Neoptolemos, J.P.; Dunn, J.A.; Stocken, D.D.; Almond, J.; Link, K.; Beger, H.; Bassi, C.; Falconi, M.; Pederzoli, P.; Dervenis, C.; et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet 2001, 358, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zuelke, C.; Burkart, C.; et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007, 297, 267–277. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA 2010, 304, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Pelzer, U.; O’Reilly, E.M.; Winter, J.; Oh, D.Y.; Li, C.P.; Tortora, G.; Chang, H.M.; Lopez, C.D.; Bekaii-Saab, T.; et al. Adjuvant nab-Paclitaxel + Gemcitabine in Resected Pancreatic Ductal Adenocarcinoma: Results From a Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2022, 41, JCO2201134. [Google Scholar] [CrossRef] [PubMed]
- Sinn, M.; Bahra, M.; Liersch, T.; Gellert, K.; Messmann, H.; Bechstein, W.; Waldschmidt, D.; Jacobasch, L.; Wilhelm, M.; Rau, B.M.; et al. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J. Clin. Oncol. 2017, 35, 3330–3337. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słodkowski, M.; Wroński, M.; Karkocha, D.; Kraj, L.; Śmigielska, K.; Jachnis, A. Current Approaches for the Curative-Intent Surgical Treatment of Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 2584. https://doi.org/10.3390/cancers15092584
Słodkowski M, Wroński M, Karkocha D, Kraj L, Śmigielska K, Jachnis A. Current Approaches for the Curative-Intent Surgical Treatment of Pancreatic Ductal Adenocarcinoma. Cancers. 2023; 15(9):2584. https://doi.org/10.3390/cancers15092584
Chicago/Turabian StyleSłodkowski, Maciej, Marek Wroński, Dominika Karkocha, Leszek Kraj, Kaja Śmigielska, and Aneta Jachnis. 2023. "Current Approaches for the Curative-Intent Surgical Treatment of Pancreatic Ductal Adenocarcinoma" Cancers 15, no. 9: 2584. https://doi.org/10.3390/cancers15092584
APA StyleSłodkowski, M., Wroński, M., Karkocha, D., Kraj, L., Śmigielska, K., & Jachnis, A. (2023). Current Approaches for the Curative-Intent Surgical Treatment of Pancreatic Ductal Adenocarcinoma. Cancers, 15(9), 2584. https://doi.org/10.3390/cancers15092584





